0.30 moles KBr is dissolved in 0.15 L of solution. What is the concentration in units
of molarity?
2.0 M
0.5 M
0.045 M
1.0 M
Answers
Answer:
2.0 M
Explanation:
To find the concentration in units of molarity (M), we need to calculate the moles of solute (KBr) and divide it by the volume of the solution in liters.
Given:
Moles of KBr = 0.30 moles
Volume of solution = 0.15 L
Concentration (Molarity) = Moles of solute / Volume of solution
Concentration = 0.30 moles / 0.15 L = 2.0 M
Therefore, the concentration of the KBr solution is 2.0 M.
The molarity of 0.30 moles of KBr dissolved in a 0.15 L solution is calculated by the formula for molarity: Moles of solute divided by Liters of solution. Substituting the given values into the formula gives us a molarity of 2.0 M.
Explanation:The subject of this question is related to the concept of molarity in chemistry. Molarity is a measure of the concentration of solutes in a solution, calculated by dividing the moles of solute by the liters of solution. In this case, the solute is potassium bromide (KBr), and we're asked to find its molarity in a 0.15 L solution.
By using the formula for molarity (Moles of solute / Liters of solution = Molarity), we substitute the given numbers into the formula:
0.30 moles KBr / 0.15 L solution = 2.0 M
Therefore, the concentration of KBr in the solution is 2.0 M.
Learn more about Molarity here:https://brainly.com/question/31815736
#SPJ2
Related Questions
How much energy is required to boil 65 grams of 100°C water
And then heat the steam to 150°C?
Answers
Answer:
13598 J
Explanation:
Q = m × c × ∆T
Where;
Q = amount of energy (J)
m = mass (grams)
c = specific heat capacity
∆T = change in temperature
m = 65g, specific heat capacity of water = 4.184J/g°C, initial temperature= 100°C, final temperature = 150°C
Q = 65 × 4.184 × (150 - 100)
Q = 271.96 × 50
Q = 13598 J
Hence, 13598 J of energy is required to boil 65 grams of 100°C water and then heat the steam to 150°C.
Question 13,34 pts Three students are asked to discuss the sources of error that might have affected the outcome of the lab. Select the student that employs correct scientific reasoning: Student 1: If the spot was placed below the solvent level; this would cause the sample to be dissolved into the solvent pool before traveling up the plate; Student 2: If the solvent used has the opposite polarity of the stationary phase this will cause unequal movement of the sample: Student 3: If the developing chamber was closed too quickly than the sample wouldn't be able to travel on the TLC plate_ Student 1 Student 2 Student }
Answers
Based on the given statements, Student 1 employs correct scientific reasoning.
How the best answer was determinedBased on the given statements, Student 1 employs correct scientific reasoning by explaining a possible source of error in terms of the physical properties of the system. They suggest that if the spot was placed below the solvent level, the sample would be dissolved into the solvent pool before it could travel up the plate, leading to inaccurate results. This is a valid explanation based on the principles of chromatography and the physical behavior of the sample and solvent.
Student 2 also provides a valid explanation based on the principle of polarity, but they do not specify how this might lead to unequal movement of the sample on the TLC plate.
Student 3's statement, on the other hand, does not provide a clear explanation of how closing the developing chamber too quickly might affect the outcome of the lab, and it does not demonstrate a clear understanding of the scientific principles involved.
Therefore, based on the given statements, Student 1 employs correct scientific reasoning.
Learn more about solvents here https://brainly.com/question/25326161
#SPJ1
Plzzzzz helpppp meee

Answers
Answer:
2 and 5
Explanation:
What does potential and kinetic energy have in common
Answers
Determine whether the following electron configurations are representative, transition, or noble-gas elements.

Answers
Noble gas configuration for elements is 1. [Ne] 3s² 3p5, 2. [Ne] 3s² 3p⁴, 3. [Ar] 4s² 3d⁵4. noble gas element in period 4 (krypton). 5. [Kr] 5s² 4d¹⁰.
Describe Noble Gas?Noble gases are a group of chemical elements found in group 18 of the periodic table. They are also known as inert gases because they are very stable and non-reactive due to their outermost electron shells being completely filled with electrons. The noble gases include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
Noble gases are odorless, colorless, and tasteless. They have very low boiling points and exist as gases at room temperature and pressure. They are all monatomic, meaning that their atoms exist independently rather than bonded to other atoms in molecules.
Noble gases are used in various industrial applications, such as in lighting (neon lights), welding (argon), and cryogenics (helium). They are also used in medical applications, such as in MRI machines and anesthesia.
This electron configuration represents a non-metal element in group 17 (halogens). The noble gas configuration for this element is [Ne] 3s² 3p5.
This electron configuration represents a non-metal element in group 16 (chalcogens). The noble gas configuration for this element is [Ne] 3s² 3p⁴.
This electron configuration represents a transition metal element in period 4. The noble gas configuration for this element is [Ar] 4s² 3d⁵.
This electron configuration represents a noble gas element in period 4 (krypton).
This electron configuration represents a transition metal element in period 5. The noble gas configuration for this element is [Kr] 5s² 4d¹⁰.
To know more about configuration visit:
https://brainly.com/question/13933772
#SPJ1
What has the lowest ionization erergy?
Fluorine (F)
O Francium (Fr)
O Helium (He)
O lodine (1)
Answers
Answer:
Francium (Fr)
Explanation:
From the given choices, francium will have the lowest ionization energy.
Ionization energy is the energy required to remove the most loosely held electron within an atom.
The magnitude of the ionization energy depends on the characteristics of the atom in relation to its nuclear charge, atomic radius, stability etc.
Generally on the periodic table, ionization energy increases from left to right on the tableAs you go from metals to non-metals and to gases, the value of the ionization energy increases steadily. Down the group, the value reduces. Since Francium is the most metallic of all the given choices, it has the highest ionization energy.a 25.00 ml 0.200 m hcl solution is titrated with 0.2 m naoh. what is the ph after the addition of 25.00 ml of naoh?\
Answers
The pH after the addition of 25.00 ml of NaOH is 7.
To find the pH after the addition of NaOH to the HCl solution, we need to calculate the number of moles of each substance involved and determine the resulting concentration of the remaining acid or base. Then we can use the resulting concentration to determine the pH.
First, let's calculate the number of moles of HCl and NaOH used in the reaction:
Moles of HCl = Volume of HCl solution (in L) × Concentration of HCl (in mol/L)
= 25.00 ml × 0.200 mol/L
= 0.025 L × 0.200 mol/L
= 0.005 mol
Moles of NaOH = Volume of NaOH solution (in L) × Concentration of NaOH (in mol/L)
= 25.00 ml × 0.200 mol/L
= 0.025 L × 0.200 mol/L
= 0.005 mol
Since the stoichiometric ratio between HCl and NaOH is 1:1 (according to the balanced chemical equation), they react completely, leaving no excess HCl or NaOH.
After the reaction, the moles of HCl and NaOH will cancel each other out, resulting in the formation of water (H₂O). Therefore, we are left with only water.
The pH of water is neutral, which is 7.
So, the pH after the addition of 25.00 ml of NaOH is 7.
Learn more about pH from the given link:
https://brainly.com/question/12609985
#SPJ11
4.2 g of lithium (Li) reacts with 2.8 g of nitrogen (N2). Find the simplest molar ratio in which lithium reacts with nitrogen.
Answers
The simplest molar ratio in which lithium reacts with nitrogen is 6 moles of lithium to 1 mol of nitrogen.
What is molar ratio?
A molar ratio examines the ratio that exist between two chemical compounds showing the amounts in moles of the compounds involved in a chemical reaction.
Molar ratio = amount of a constituent in moles ÷ total amount of all constituents in a mixture in moles
Given:
Mass of lithium (Li) = 4.2 g
Mass of nitrogen (N2) = 2.8 g
Total amount of mass = 7g
Moles = molecular weight ÷ mass of water
Moles of lithium (Li) = 4.2 ÷ 7 = 0.60 mol
Moles of nitrogen (N₂) = 2.8 ÷ 7 = 0.10 mol
Therefore, ratio Li : N₂ = 0.60 : 0.10 = 6 : 1
6 Li + N₂
The balanced equation between lithium and nitrogen, to form lithium nitride = 6 Li + N₂ → 2 Li₃N₆L i + N₂ → 2Li₃N
In summary, the simplest molar ratio in which lithium reacts with nitrogen is 6:1
Learn more about molar ratio here: brainly.com/question/14677248
#SPJ1
Which of the following compounds would be expected to have the strongest ionic bonds?
the compound that has the largest ions with the greatest charge
the compound that has the largest ions with the least charge
the compound that has the smallest ions with the greatest charge
the compound that has the smallest ions with the least charge
Answers
Answer:
the compound that has the smallest ions with the greatest charge
The compound that has the smallest ions with the greatest charge will have the strongest ionic bonds. Therefore, option C is correct.
What are ionic compounds?Ionic compounds can be defined as solids formed by closely packed ions of opposite charge. An Ionic compound is commonly formed when metal combines with non-metal.
In ionic compounds, the ions are held together by ionic bonds. The ions are created by accepting or losing electrons in order to get their nearest noble gas configuration. In a reaction, the metals have a tendency to lose electrons to complete their octet while non-metals will gain electrons to fill their octet.
An ionic bond or electrovalent bond is a type of linkage generated from the electrostatic attraction between oppositely charged ions. Such a bond produces when the valence electrons of one atom are transferred permanently to another.
The more the charge on the ions and the smaller the ions stronger the ionic bond will be formed.
Learn more about ionic compounds, here:
brainly.com/question/17217225
#SPJ2
A 285 mL flask contains pure helium at a pressure of 750 torr. A second flask with a volume of 465 mL contains pure argon at a pressure of 722 torr. If we connect the two flasks through a stopcock and open the stopcock, what is the total pressure?
Answers
If we connect the two flasks through a stopcock and open the stopcock, we will obtain the total pressure of 285 torr.
To solve this problem, we will use the formula P₁V₁=P₂V₂, where P stands for pressure and V stands for volume.
The pressure of helium in the first flask is 750 torr and the volume of the first flask is 285 mL.
The pressure of argon in the second flask is 722 torr and the volume of the second flask is 465 mL.
P₁V₁ = P₂V₂ (Boyle's law)
750 torr × 285 mL = 722 torr × 465 mL
213750 torr-mL = 335430 torr-mL
When the two flasks are connected, the volume is now the sum of the volumes of the two flasks, which is 285 mL + 465 mL = 750 mL.
To find the total pressure, we will use the formula P₁V₁ = P₂V₂ = P₃V₃, where P₃ is the total pressure and V₃ is the total volume.
P₁V₁ = P₃V₃
(213750 torr-mL)/750 mL = 285 torr
Therefore, the total pressure is 285 torr.
Learn more about total pressure at https://brainly.com/question/30235826
#SPJ11
B. What do you notice about the density of the sinking objects? Dense objects feel very heavy for their size
Answers
Answer:
The answer is below
Explanation:
The density of an object is its mass per unit of volume. Dense objects feel very heavy for their size, while for objects with low density they are very light for their size.
Density is the ratio of mass to volume; it is given by:
Density (D) = mass (m) / volume (V)
An object sinks in water if the density of the object is greater than the density of water. While an objects floats on water if the density of the object is les than the density of water
When a piece of aluminum is placed in a 25-mL graduated cylinder that contains 10.5 mL of water, the water level rises to 13.5 mL. What is the mass of the aluminum?
Answers
Answer:
Mass of the sample of aluminium = m
Volume of the aluminium = V
Density of the aluminum = d = 2.7 g/mL
Initial level of water mark in cylinder = 10.0 mL
Final level of water after sample is immersed = 18.0 mL
Volume occupied by the sample = V = 18.0 mL - 10.0 mL = 8.0 mL
Explanation:
pls mark me as the brainliest
Answer:
The mass of the aluminium sample is 21.6 grams.
Explanation:
Mass of the sample of aluminium = m
Volume of the aluminium = V
Density of the aluminum = d = 2.7 g/mL
Initial level of water mark in cylinder = 10.0 mL
Final level of water after sample is immersed = 18.0 mL
Volume occupied by the sample = V = 18.0 mL - 10.0 mL = 8.0 mL
The mass of the aluminium sample is 21.6 grams.
Read "The Old Man and his Grandson" by The Brothers Grimm. Then, answer the question that follows.
There was once a very old man, whose eyes had become dim, his ears dull of hearing, his knees trembled, and when he sat at table he could hardly hold the spoon, and spilt the broth upon the table-cloth or let it run out of his mouth. His son and his son's wife were disgusted at this, so the old grandfather at last had to sit in the corner behind the stove, and they gave him his food in an earthenware bowl, and not even enough of it. And he used to look towards the table with his eyes full of tears. Once, too, his trembling hands could not hold the bowl, and it fell to the ground and broke. The young wife scolded him, but he said nothing and only sighed. Then they brought him a wooden bowl for a few half-pence, out of which he had to eat.
They were once sitting thus when the little grandson of four years old began to gather together some bits of wood upon the ground.
"What are you doing there?" asked the father.
"I am making a little trough," answered the child, "for father and mother to eat out of when I am big."
The man and his wife looked at each other for a while, and presently began to cry. Then they took the old grandfather to the table, and henceforth always let him eat with them, and likewise said nothing if he did spill a little of anything.
Which two themes can be supported by details in the text?
Everyone eventually grows old and frail.
Grandparents and children have a lot in common.
A little kindness goes a long way.
Children should be seen and not heard.
A and B
A and C
B and C
C and D
Answers
name the scientist who studied the movement of pollen grains suspended in water through a microscope what is the phenomenon known as
Answers
Answer:
the scientist who studied the movement of pollen grains grains suspended in water through a microscope is Robert Hooke
Being farther from the nucleus and with more electrons shielding the protons results in the valence electrons being held ...
Answers
Answer:
electrons
Explanation:
since they are at the outer part of nucleus
Answer:
Weakly
Explanation:
Being farther from the nucleus and with more electrons shielding the protons results in the valence electrons being held weakly.
The effect of the nuclear pull will not be felt so strongly by the valence electrons. The valence electrons are the outermost electrons that occupies a shell. When the inner orbital electrons are much and the atomic radius is large, the valence electrons are well shielded. This results in electropositivity and a low ionization energy.Two substances were combined and immediately a gas was produced. What type of change is happening?.
Answers
Answer: This is called a chemical reaction.
Explanation: Gas may form, heat may be produced, and color may change. These types of changes indicate that a chemical reaction has occurred.
Hii! Can someone please help me with my chemistry question

Answers
Why does sugar dissolve
Answers
Answer:
Sugar dissolves because energy is given off when the slightly polar sucrose molecules form intermolecular bonds with the polar water molecules.
Explanation:
This diagram would represent the enthalpy changes in which
example?
O endothermic synthesis reaction
Oa melting solid
O a boiling liquid
O a hot flask after mixing two chemicals
Answers
An endothermic reaction is a melting solid
What is an enthalpy change?Enthalpy (H) is a thermodynamic state function that accounts for the internal energy of a system plus the work done by or on the system. When a chemical reaction takes place or a physical change occurs, there may be a change in the enthalpy of the system, represented as ΔH (delta H), which is the difference between the enthalpy of the products and the enthalpy of the reactants.
If ΔH is negative, it means that the reaction or process releases heat energy, and is therefore exothermic. If ΔH is positive, it means that the reaction or process absorbs heat energy, and is therefore endothermic. Enthalpy change is usually measured in joules (J) or kilojoules (kJ) per mole of substance involved in the reaction or process.
Learn more about endothermic reaction:https://brainly.com/question/23184814
#SPJ1
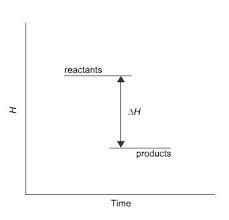
Answer:
A hot flask after mixing two chemicals.
a hypothetical, diprotic, weak acid (h2a) has pka values of 4.5 and 8.5. a. what is the predominate species given a solution of this acid at...
Answers
To determine the predominant species in a solution of a hypothetical diprotic weak acid (H2A) with pKa values of 4.5 and 8.5, we need to consider the acid dissociation constants and the pH of the solution. Here's a step-by-step explanation:
1. Acid Dissociation Constants: The acid dissociation constants (Ka) represent the degree of ionization for each proton (H+) released by the acid.
2. Calculation of pH: The pH of the solution determines the concentration of H+ ions. A low pH indicates a high concentration of H+ ions, while a high pH indicates a low concentration of H+ ions.
3. Predominant Species at Low pH (Acidic Conditions): At low pH, below the first pKa value of 4.5, the acid exists mainly in the protonated form (H2A), since most of the H+ ions remain bound to the acid molecule.
4. Predominant Species between pKa values: Between the two pKa values (4.5 and 8.5), the acid exists in a mixture of protonated (H2A) and singly deprotonated (HA-) forms. The relative concentrations depend on the pH of the solution.
5. Predominant Species at High pH (Basic Conditions): At high pH, above the second pKa value of 8.5, the acid exists mainly in the doubly deprotonated (A2-) form, as the H+ ions have been released.
Therefore, the predominant species in the solution of the hypothetical diprotic weak acid (H2A) at different pH ranges are as follows:
- Low pH: Predominantly H2A
- Between pKa values: Mixture of H2A and HA-
- High pH: Predominantly A2-
Please note that the exact distribution of species will depend on the specific pH of the solution.
Learn more about predominant species from the given link: https://brainly.com/question/31936571
#SPJ11
Select all that happen through stomata (assume this question is about a plant which is actively photosynthesizing during the day).
-Water vapors exit leaves.
-Carbon dioxide enters leaves.
-Oxygen exits leaves.
Answers
The things which happen through stomata during photosynthesis include water vapors exit the leaves, carbon dioxide enters the leaves, and oxygen exits the leaves for the formation of glucose (carbohydrate). Thus, all are correct options.
What are stomata?Stomata are small pores found on the surfaces of leaves, stems, and other plant parts that enable gas exchange between the atmosphere and the interior of the plant. During photosynthesis, stomata are important for regulating the flow of carbon dioxide and oxygen into and out of the plant. They also help to prevent water loss from the plant by controlling the opening and closing of the stomata.
When photosynthesis occurs, the plant uses energy from the sun to combine water and carbon dioxide to create glucose (a sugar) and oxygen. Stomata facilitate the uptake of carbon dioxide and the release of oxygen during photosynthesis. The water produced as a by-product of photosynthesis exits the plant through stomata via transpiration.
Thus, the three things that happen through stomata (assume this question is about a plant that is actively photosynthesizing during the day) are carbon dioxide entering the leaves, water vapors exiting the leaves, and oxygen exiting the leaves.
Therefore, all the options are correct.
Learn more about Stomata here:
https://brainly.com/question/14351755
#SPJ11
how many moles are in 425g of KNO3?
Answers
Answer:
The answer is 101.1032
Explanation:
Yo Brainlies❤️
I love you guys sm and i have to ask something
Whatt is cell membrane And give a sentence
Ahve great DAIESSSS
Answers
Answer:
cell membrains
Explanation:
for a molecule of chlorous acid, the atoms are arranged as hoclo
Answers
Chlorous acid, a weak acid with the formula HClO2, can be synthesized by reacting hydrogen peroxide with hypochlorous acid, generating hydrogen chloride and oxygen as by-products. Chlorous acid is a fundamental compound that has a broad range of applications, including disinfection, wastewater treatment, and food preservation.
For a molecule of chlorous acid, the atoms are arranged as HOClo, as you have mentioned in your question. The hydrogen atom is covalently bonded to the oxygen atom, while the oxygen atom is doubly bonded to one of the two chlorine atoms, which is single bonded to the other chlorine atom. The bond angles between the atoms in chlorous acid are not equivalent, with the oxygen-hydrogen and oxygen-chlorine bonds being roughly 110° and 103°, respectively.
The geometry of chlorous acid can be determined using VSEPR theory. According to VSEPR theory, chlorous acid has a bent shape, with a bond angle of approximately 108°. This is due to the presence of two lone pairs of electrons on the oxygen atom, which repel the bonding pairs and cause the molecule to bend.
The acidity of chlorous acid is due to the ease with which the hydrogen atom dissociates from the molecule to form hydronium ions and chlorite ions. The equilibrium constant for the dissociation of chlorous acid is relatively small (approximately 1.1 x 10^-2), indicating that only a small fraction of the chlorous acid molecules will dissociate in solution.
In summary, chlorous acid is a weak acid with the formula HClO2, and the atoms in a molecule of chlorous acid are arranged as HOClo. The geometry of chlorous acid is bent, with a bond angle of approximately 108°, and its acidity is due to the ease with which the hydrogen atom dissociates from the molecule to form hydronium ions and chlorite ions.
To know more about VSEPR theory visit
https://brainly.com/question/17177984
#SPJ11
Tabulate all of the possible orbitals (by name, i.e. 4s) for n=4 and give the three quantum numbers which define each orbital.
Answers
These are all the possible orbitals for the principal quantum number n=4. For n=4, there are several possible orbitals. I have tabulated them below along with their respective quantum numbers (n, l, and ml):
For n=4, the possible orbitals (by name) are 4s, 4p, 4d, and 4f.
The three quantum numbers that define each orbital are:
1. Principle quantum number (n): This defines the energy level of the orbital and can have a value from 1 to infinity. For n=4, the value of n is fixed.
2. Angular momentum quantum number (l): This defines the shape of the orbital and can have integer values from 0 to n-1. For 4s, l=0; for 4p, l=1; for 4d, l=2; and for 4f, l=3.
3. Magnetic quantum number (m): This defines the orientation of the orbital in space and can have integer values from -l to +l. For 4s, m=0; for 4p, m can have values -1, 0, or 1; for 4d, m can have values -2, -1, 0, 1, or 2; and for 4f, m can have values -3, -2, -1, 0, 1, 2, or 3.
Therefore, for n=4, the possible orbitals (by name) and their corresponding quantum numbers are:
- 4s: n=4, l=0, m=0
- 4p: n=4, l=1, m=-1, 0, or 1
- 4d: n=4, l=2, m=-2, -1, 0, 1, or 2
- 4f: n=4, l=3, m=-3, -2, -1, 0, 1, 2, or 3.
Learn more about orbitals here:
https://brainly.com/question/18914648
#SPJ11
Which of the following statements on HPLC modes is true? A. Increasing the polarity of the mobile phase decreases the elution time of polar compounds in normal-phase HPLC B. A non-polar stationary phase is used in normal-phase HPLC C. Compounds have a lower attraction to the mobile phase than to the stationary phase in displacement development D. A polar stationary phase is used in reversed-phase HPLC E. More polar compounds elute first in normal-phase HPLC
Answers
The following statements on HPLC modes are true is more polar compounds elute first in normal-phase HPLC (Option E).
The liquid chromatography (HPLC) is a technique in analytical chemistry employed for the separation, identification, and quantification of elements. It is considered a highly sensitive method, and it works by separating the components in a mixture with the assistance of a solvent under high pressure.
There are two modes of HPLC: Reversed-Phase HPLC (RP-HPLC) and Normal-Phase HPLC (NP-HPLC). In RP-HPLC, a nonpolar stationary phase, such as C18, is used, and polar solvents, such as water, are used as mobile phases. Polar stationary phases, such as silica gel, are used in NP-HPLC, while nonpolar solvents, such as hexane, are used as mobile phases.
More polar compounds have a greater affinity for the polar stationary phase than less polar compounds, which have a higher affinity for the nonpolar mobile phase in NP-HPLC. As a result, less polar compounds elute first in normal-phase HPLC.
Thus, the correct option is E.
Learn more about HPLC: https://brainly.com/question/13490391
#SPJ11
12.0 grams of Barium nitrate react with 6.0 grams of sodium sulfate in a double replacement reaction.Write out the complete balanced reaction including states of matter: What is the theoretical yield of solid compound that we will be able to produce?B.What is the limiting reactant and excess reactant?C. Calculate the quantity of excess reactant remaining.D. Suppose in the lab you actually collected 9.5 grams of the solid compound. Calculate the percentyield of the reaction
Answers
A chemical equation shows the reactants (left side) and products (right side) in a chemical reaction.
What is chemical reaction?chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products. Substances are either chemical elements or compounds. A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products.Chemical reactions are an integral part of technology, of culture, and indeed of life itself. Burning fuels, smelting iron, making glass and pottery, brewing beer, and making wine and cheese are among many examples of activities incorporating chemical reactions that have been known and used for thousands of years. Chemical reactions abound in the geology of Earth, in the atmosphere and oceans, and in a vast array of complicated processes that occur in all living systems.
To learn more about compounds refer to:
https://brainly.com/question/26487468
#SPJ1
Describe the geologic events that occur at a divergent tectonic plate boundary and the process that causes these events.
Answers
Answer: A divergent boundary occurs when two tectonic plates move away from each other. Along these boundaries, earthquakes are common and magma (molten rock) rises from the Earth's mantle to the surface, solidifying to create new oceanic crust.
yes very good
Sodium reacts with water to produce sodium hydroxide and hydrogen gas. Calculate the volume of hydrogen gas produced at 88. 9 kpa and 34 degrees celsius when 4. 78g of sodium is reacted
Answers
The value of the volume of hydrogen gas produced is 4.5 L.
We can calculate the moles of hydrogen gas produced by using the balanced chemical equation of the reaction.
Sodium + Water → Sodium hydroxide + Hydrogen gas2Na + 2H₂O → 2NaOH + H₂
Molar mass of Na = 23 g/mol
Moles of Na = Mass/Molar mass = 4.78/23 = 0.208 moles
From the above equation, it is evident that 1 mole of sodium produces 1 mole of hydrogen gas.
Therefore, moles of hydrogen gas produced = moles of Na = 0.208 moles
Now, we can use the ideal gas law to calculate the volume of hydrogen gas produced.
PV = nRTV = nRT/P
Where;
R = 8.31 J/K mol
P = 88.9 kPa = 88.9 × 1000 Pa
T = 307 K
N = 0.208 mol
Volume,
V = 0.208 × 8.31 × 307 / (88.9 × 1000)
V = 0.0045 m³ or 4.5 L (rounded to one decimal place)
Learn more about chemical equation at:
https://brainly.com/question/14460953
#SPJ11
Isomers are defined as:_________.
i. atoms with the same number of protons but different numbers of neutrons.
ii. atoms with the same number of protons but different numbers of neutrons.
iii. molecules with different chemical formulas but similar biological functions.
iv. molecules with the same general three-dimensional structures but different chemical formulas.
v. elements with the same number of electrons in the outer shell.
vi. molecules with the same chemical formula but different structures.
Answers
Isomers are defined as molecules with the same chemical formula but different structures. The correct answer is vi.
Isomers are molecules that have the same chemical formula, meaning they have the same types and numbers of atoms, but they differ in their arrangement or connectivity of atoms.
This results in different structural arrangements and, in turn, different chemical and physical properties. Isomers can have different functional groups, spatial arrangements, or bond connectivity while maintaining the same chemical formula.
These differences in structure can lead to variations in reactivity, biological activity, and other properties of the molecules.
Option i and ii are incorrect because they refer to isotopes, which are atoms of the same element with different numbers of neutrons.
Option iii is incorrect as it describes molecules with different chemical formulas but similar biological functions.
Option iv is incorrect as it describes stereoisomers, which have the same three-dimensional structure but differ in spatial arrangement.
Option v is incorrect as it describes elements with the same number of electrons in the outer shell, which are known as isotopes.
Therefore, the correct option is vi. molecules with the same chemical formula but different structures.
To know more about Isomers, refer here:
https://brainly.com/question/32508297#
#SPJ11