1: calculate the ph of a 0.25m solution of h3o+
2: calculate the ph of a 6.3x10-8m solution of h3o+
3: look at your answer for 4 and 5 which one is a base?
4: look at 4 and 5 which one is a strong acid
please show your work
Answers
The pH of a 6.3 x \(10^{-8\)M solution of H₃O+ is approximately 7.20.
A 0.25 M solution of H₃O+ is not a strong acid, since it is not a single acid that completely dissociates in water.
A 6.3 x \(10^{-8\) M solution of H₃O+ is not a strong acid, since it is a very weak acid with a very low concentration of H₃O+ ions.
The pH of a 0.25 M solution of H₃O+ can be calculated using the formula:
pH = -log[H₃O+]
where [H₃O+] is the concentration of H₃O+ ions in moles per liter (M).
In this case, [H3O+] = 0.25 M,
pH = -log(0.25) = 0.602
Therefore, the pH of a 0.25 M solution of H₃O+ is approximately 0.602.
The pH of a 6.3 x \(10^{-8\) M solution of H₃O+ can be calculated using the same formula:
pH = -log[H₃O+]
In this case, [H₃O+] = 6.3 x \(10^{-8\)M, so we have:
pH = -log(6.3 x \(10^{-8\)) = 7.20
Therefore, the pH of a 6.3 x \(10^{-8\) M solution of H₃O+ is approximately 7.20.
There is no information given for question 3.
A strong acid is an acid that completely dissociates in water to produce H₃O+ ions. The most common example of a strong acid is hydrochloric acid (HCl).
Looking at the given solutions:
A 0.25 M solution of H₃O+ is not a strong acid, since it is not a single acid that completely dissociates in water.
A 6.3 x \(10^{-8\) M solution of H₃O+ is not a strong acid, since it is a very weak acid with a very low concentration of H₃O+ ions.
Therefore, neither of the given solutions is a strong acid.
To know more about strong acid
https://brainly.com/question/31143763
#SPJ4
Related Questions
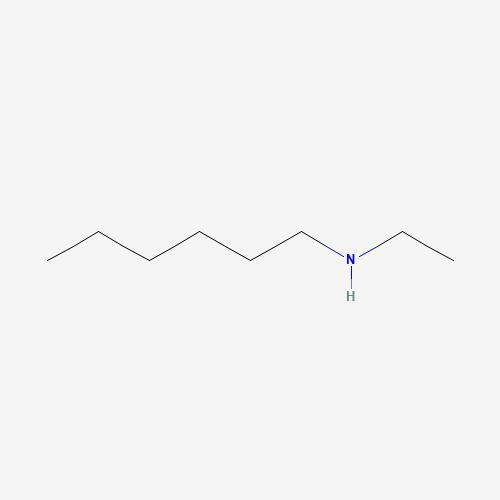
draw the structure of n-ethyl-1-hexanamine or n-ethylhexan-1-amine.
Answers
The structure of n-ethyl-1-hexanamine or n-ethylhexan-1-amine is shown in the image attached below.
N-EthylhexylamineMolecular Formula: The molecular formula for N-Ethylhexylamine is C₈H₁₉N.Synonyms: Some common synonyms for N-Ethylhexylamine are N-Ethylhexan-1-amine, 1-ethylhexylamine, and N-ethyl-1-hexylamine.Molecular Weight: The molecular weight of N-Ethylhexylamine is approximately 129.24 g/mol.Chemical Properties: N-Ethylhexylamine is a colorless to slightly yellow liquid with a strong, unpleasant odor. It is soluble in most organic solvents but has limited solubility in water. As an amine, it is a weak base, meaning it can form salts when reacting with acids. N-Ethylhexylamine has a boiling point of around 175°C and a melting point of around -69°C. It is flammable and can produce toxic fumes when burned.N-Ethylhexylamine is a versatile chemical compound used in various industries. It is used as a reagent or intermediate in chemical synthesis, a surfactant in industrial processes, a solvent in the formulation of paints, coatings, adhesives, and inks, a catalyst in certain chemical reactions, and in gas treatment processes such as removing acid gases from natural gas. It is also used as a pH regulator or stabilizer in various industrial applications.learn more about N-ethyl-1-hexanamine
https://brainly.com/question/31990221
#SPJ11

How many moles of ions are there in 1 mole of iron (III) oxide, Fe2O3?
Answers
Answer:
So one mole of Fe2O3 contains two moles of Fe3+ ions.
Explanation:
As there is an internal mole ratio of 2:3
It means that there are 2moles of iron within every mole of iron(III) oxide.
Iron III oxide is an ionic compound composed of Fe3+ ions and O2- ions. According to the formula, there are three O2- ions for every two Fe3+ ions.
Extra:
One 'unit' of Fe2O3 has two Fe3+ ions and three O2- ions, hence a dozen Fe2O3 contains two dozen Fe3+ ions and three dozen O2- ions, and a mole Fe2O3 contains two moles Fe3+ ions and three moles O2- ions.
A woolly mammoth was found in 1999 buried in the frozen soil of the Siberian tundra. Carbon-14 dating indicated that it had died about 20,000 years ago. Many fossils represent only the partial remains of organisms. However, a complete mammoth with bones, skin, hair, and internal organs intact represented a unique opportunity for scientists to investigate the lifestyle of this animal and the environment in which it lived. The low permeability of the tundra soil helped to preserve the mammoth. Explain why the tundra soil has a low permeability.
Answers
Tundra soils are formed at high latitudes which leaves the tundra always very cold. Tundra soils are generally frozen, and are classifed as Gelisols (this means that permafrost are within 100 cm of the soil surface). These permafrost are as a result of the freezing by winter of the underground water that was accumulated in summer. These soils freeze and thaw alot and as result of that, moisture do not permeate the soil easily. Also, due to this harsh temperature and underground permafrost, most organisms that died in the tundra are preserved within the soil.
me of an Irregular Solid
Use the image to determine the volume of the rock.
Initial volume:
mL
Final volume:
Volume of rock: 20
mL
cm³
Answers
The volume of the irregular solid from the image that have been shown is 12 mL
How do you determine the volume of an irregular solid?To obtain the volume of the irregular solid;
a. Measure the initial volume of water in the cylinder.
b. Carefully lower the irregular solid into the water, ensuring it is fully submerged without trapping any air bubbles.
c. Record the new volume of water in the cylinder.
d. The volume of the irregular solid is equal to the difference between the final and initial water volumes.
The volume is;
32 mL - 20 mL = 12 mL
Learn more about irregular solid:https://brainly.com/question/31829216
#SPJ1

2. During which of the following stato changes would the particles in the matter become more spread out?
changing from liquid to gas?
changing from gas to liquid?
changing from gas to solid?
Answers
Answer
changing from liquid to gas
Explanation
changing from liquid to gas
Maria’s father started a fire in the fireplace. He crumpled some paper, lit a match, and soon the logs in the fireplace were burning. In this case, the stored chemical energy in the logs was changed into
Answers
Answer:
electrical and heat energy
Explanation:
Answer:
Heat and Light
Explanation:
C on usatp
Help with theses two different problems!
1.) 125mL of what is added to 45.3mL of 0.71m NaOH solution
2.) 550mL of water is added to 125mL of 3.01M KOH solution
Answers
1. the final concentration of NaOH after adding 125 mL of water to 45.3 mL of 0.71 M NaOH solution is approximately 0.189 M.
2. the final concentration of KOH after adding 550 mL of water to 125 mL of 3.01 M KOH solution is approximately 0.557 M.
1.) If 125 mL of water is added to 45.3 mL of a 0.71 M NaOH solution, the resulting solution will be a diluted NaOH solution. The addition of water will increase the total volume while reducing the concentration of NaOH. To determine the final concentration of NaOH, we need to consider the conservation of moles.
First, let's calculate the moles of NaOH in the initial solution:
moles of NaOH = volume (in L) × concentration (in M)
moles of NaOH = 0.0453 L × 0.71 M = 0.0321433 moles
After adding 125 mL (0.125 L) of water, the total volume of the solution becomes 0.0453 L + 0.125 L = 0.1703 L.
To find the final concentration, we divide the moles of NaOH by the total volume:
final concentration of NaOH = moles of NaOH / total volume
final concentration of NaOH = 0.0321433 moles / 0.1703 L ≈ 0.189 M
Therefore, the final concentration of NaOH after adding 125 mL of water to 45.3 mL of 0.71 M NaOH solution is approximately 0.189 M.
2.) If 550 mL of water is added to 125 mL of a 3.01 M KOH solution, the resulting solution will also be a diluted solution. Again, we will apply the conservation of moles to determine the final concentration of KOH.
First, calculate the moles of KOH in the initial solution:
moles of KOH = volume (in L) × concentration (in M)
moles of KOH = 0.125 L × 3.01 M = 0.37625 moles
After adding 550 mL (0.55 L) of water, the total volume of the solution becomes 0.125 L + 0.55 L = 0.675 L.
To find the final concentration, divide the moles of KOH by the total volume:
final concentration of KOH = moles of KOH / total volume
final concentration of KOH = 0.37625 moles / 0.675 L ≈ 0.557 M
For more such questions on concentration visit:
https://brainly.com/question/28564792
#SPJ8
: We prepare a solution by mixing 60 g of sugar and 240 g of water. If the total volume of the
solution is 300mL, calculate:
a) The concentration in g/L, the total mass of the solution and the density of the solution. B) The sugar that we are taking if we have a glass of 100 mL from the solution
Answers
a) The concentration of sugar in the solution is 200 g/L, total mass is 0.3kg and density us 1g/mL.
b) We need 20 g of sugar to prepare a glass of 100 mL from the solution.
Concentration in g/L,
Mass of sugar in solution = 60 g
Mass of water in solution = 240 g
Total mass of solution = 60 g + 240 g = 300 g = 0.3 kg
Concentration of sugar = (mass of sugar / total volume of solution) * 1000
= (60 g / 300 mL) * 1000 = 200 g/L
Total mass of the solution = 300 g or 0.3 kg
Density of the solution = total mass of the solution / total volume of the solution
= 300 g / 300 mL = 1 g/mL
The concentration of sugar in the solution is 200 g/L.
The volume of solution needed for a glass of 100 mL is,
Volume of solution = (100 mL / 1000) L = 0.1 L
Mass of sugar in the solution needed for a glass of 100 mL = concentration * volume
= 200 g/L * 0.1 L = 20 g
To know more about sugar, here
brainly.com/question/2513269
#SPJ4
what is ne in the simulation?
Answers
The number of people (or adults) in the population, N, has an evolutionary equivalent called Ne. whereas N determines the ecological effects of population size simulation,
Evolutionary consequences (rates of genetic diversity loss and increase in inbreeding; relative effectiveness of selection) depend on Ne, and Emergency Medical Care Providers in Critical Access Hospitals and ambulance services across the entire state receive technologically advanced simulation training.Ne=(4*Nm*Nf)/(Nm+Nf) is the formula to get the effective population size. The formula Ne = 4NmNf/(Nm + Nf) can be used to calculate the effective population size in this circumstance, where Nm and Nf are the number of men and females, respectively. demonstrates the connection between Ne and Nf in a population of 1,000 reproducing people simulation.
To learn more about simulation please click on below link
https://brainly.com/question/28099331
#SPJ4
PLEASE HELP!!!!!!!
Before the fire, the forest consists of large trees. After the fire, there is only ash. Explain what the law of conservation of matter suggests happened to the rest of the mass of the trees.
Answers
According to law of conservation of matter , the mass of the trees got converted fro one form of matter to another.
What is law of conservation of matter?According to law of conservation of matter, it is evident that matter is neither created nor destroyed rather it is restored at the end of a chemical reaction .
Law of conservation of mass and energy are related as mass and energy are directly proportional which is indicated by the equation E=mc².Concept of conservation of mass is widely used in field of chemistry, fluid dynamics.
Law needs to be modified in accordance with laws of quantum mechanics under the principle of mass and energy equivalence.This law was proposed by Antoine Lavoisier in the year 1789.
Learn more about law of conservation of matter,here:
https://brainly.com/question/9434062
#SPJ2
A balloon with a volume of 2.0 L at 25°C is placed in a hot room at 35°C. The pressure on the balloon is constant at 1.0 atm. How does the volume of the balloon change after moving it to the hot room? ✔ It increases. What is the final volume of the balloon after it has been placed in the hot room?
Answers
Answer:
The volume increases,
Final volume is 2.1L
Explanation:
Based on Boyle's law, the absolute temperature of a gas is directly proportional to the volume of the gas.
As the balloon change increases its temperature, the volume increases
The Boyle's equation is:
V1T2 = V2T1
Where V is volume and T is absolute temperature of 1, initial state and 2, final state of the gas.
Replacing:
V1 = 2.0L
T2 = 35°C + 273.15 = 308.15K
V2 = ?
T1 = 25°C + 273.15 = 298.15K
2.0L*308.15K = V2*298.15K
2.1L = V2
Final volume is 2.1L
Answer:
increase,2.1L
Explanation:
If an atom has 29 electrons and a mass of 64, and it is neutral, what is the name of the atom?
Answers
Explanation:
Copper has an atomic number of 29, so it contains 29 protons and 29 electrons. ... The atomic weight of copper is 64; it has 29 protons and 35 neutrons.
How many moles are equal to 2.4 x 1023 formula units of sodium chloride?
Answers
Answer:
The answer is 0.4 molesExplanation:
To find the number of moles in a substance given it's number of entities we use the formula
\(n = \frac{N}{L} \\ \)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
\(n = \frac{2.4 \times {10}^{23} }{6.02 \times {10}^{23} } = \frac{2.4}{6.02} \\ = 0.398671096...\)
We have the final answer as
0.4 molesHope this helps you
Which equation is balanced? O Mg3N₂ + H₂O → 3MgO + 2NH3 C3H8 +50₂ → H₂O + 3CO₂ Zn + 2HCl → ZnCl₂ + H₂ O 3H₂SO4 + 2Fe → Fe₂(SO4)3 + H₂
Answers
The equation that is balanced is:
3H₂SO4 + 2Fe → Fe₂(SO4)3 + H₂
An equation is considered balanced if it has the same number of atoms of each element on both sides of the equation. In this equation, the number of hydrogen (H), sulfur (S), oxygen (O), and iron (Fe) atoms are equal on both sides.
If there are no inequalities, the chemical equation is said to be balanced. A balanced equation obeys the law of conservation of mass. Here among the given options, the balanced equation is C₃H₈ +50₂ → 4H₂O + 3CO₂. The correct option is B.
What is a balanced equation?A chemical equation in which the number of atoms of reactants and products on both sides of the equation are balanced is defined as the balanced equation. The amount of reactants and products on both sides of the equation will be equal.
The numbers which are added to balance the chemical equation are called the coefficients. The coefficients are added in front of the formula of the chemical compounds.
The balanced equation for the given reaction is:
C₃H₈ +50₂ → 4H₂O + 3CO₂
Here the number of 'C', 'H' and 'O' on both sides of the equation are equal.
Thus the correct option is B.
To know more about balanced equation, visit;
https://brainly.com/question/29769009
#SPJ5
Select the correct structure that
corresponds to the name.
4-chloro-2-hexene
Please help asap

Answers
You’d have to draw out B to check if it’s the same
how much energy is required to melt 64 g of methane at 90 k? (the molar mass of methane is 16 g/mol.)
Answers
The amount of energy required to melt 64 g of methane at 90 k is 3.784 KJ.
The energy required to melt 64 g of methane (CH4) at 90 K can be calculated using the heat of fusion, which is the amount of heat energy required to change the state of a substance from solid to liquid at a constant temperature.
Heat of fusion, also known as the enthalpy of fusion, is the amount of heat energy required to change the state of a substance from solid to liquid at a constant temperature. It is typically expressed in units of joules per mole (J/mol) or kilojoules per mole (kJ/mol).
Heat of fusion is an important physical property of a substance, as it determines the amount of energy required to melt a solid at a given temperature. It is used in many applications, including thermodynamics, process design, and energy storage.
First, we need to convert the amount of methane from grams to moles:
64 g CH4 / 16 g/mol = 4 mol CH4
Next, we can use the heat of fusion to calculate the energy required to melt the methane:
Heat of fusion = moles * heat of fusion per mole
Heat of fusion = 4 mol * 0.946 KJ/mol = 3.784 KJ
Therefore, the energy required to melt 64 g of methane at 90 K is 3.784 KJ.
To know more about heat of fusion refer to:
brainly.com/question/29238773
#SPJ4
A student models convection currents in a laboratory activity. how are the convection currents in the student’s model different from the convection currents in earth’s atmosphere and oceans?
Answers
The warm air in Earth’s atmosphere and the warm water in Earth’s oceans sink instead of rise.
What exactly is the distinction between convection and convection current?The heat energy can be transported by convection by the temperature differential between the two portions of the fluid. Hot fluids tend to ascend due to the temperature differential, but cold fluids prefer to sink. This causes a convection current to form inside the fluid.
Differential heating causes convection currents. Warmer (less dense) stuff rises, whereas cooler (more dense) material sinks. This movement is responsible for the formation of circulation patterns known as convection currents in the Earth’s atmosphere, ocean, and mantle.
To learn more about convection to refer:
https://brainly.com/question/4138428
#SPJ4
Balancing Chemical Equation
Na+Br2=NaBr
Answers
Answer:
2Na + Br2 = 2NaBr
Explanation:
In order to balance a chemical equation you make the make sure both sides have the same number of atoms on each side, you do this by multiplying on both sides as if it was a algebraic equation.
Na+ Br2 = NaBr
Na × 2 = Na2
Na × 2 = Na2
Br × 2 = Br2
2Na + Br2 = 2NaBr
Hope this helps.
Urgent: Suppose that 14.9 mL of HNO3 is neutralized by 53.1 mL of a 0.0048 M solution of KOH in a titration. Calculate the concentration of the HNO3 solution.

Answers
Answer:
0.017 M
Explanation:
The reaction that takes place is:
HNO₃ + KOH → KNO₃ + H₂OFirst we calculate how many KOH moles reacted, using the given volume and concentration:
53.1 mL * 0.0048 M = 0.255 mmolAs 1 mmol of KOH reacts completely with 1 mmol of HNO₃, in 14.9 mL of the HNO₃ solution there were 0.255 HNO₃ mmoles.
With that in mind we can calculate the concentration of the HNO₃ solution using the calculated number of moles and given volume:
0.255 mmol / 14.9 mL = 0.017 MHow will you prepare hydrogen from zinc and dilute acid.
Answers
Dilute Hydro chloric acid is added To zinc granules. The hydrogen produced is then passed through anhydrous calcium chloride or concentrated sulphuric acid. The hydrogen collected by upward delivery because it is lighter than air. If the reaction is too slow Copper (II) Sulphate solution can be added to speed up the solution.
Zn (s)+ 2HCl (aq)———ZnCl2 (aq)+ H2(g).
A chemist used 6.5 moles of water in this reaction. How many grams of water were used?
please go into detail as to why that is the answer PLSSS
Answers
Answer:
\(\boxed {\boxed {\sf 120 \ or \ 117 \ grams \ H_2O \ depending \ on \ significant \ figures }}\)
Explanation:
We want to convert from moles of water to grams of water.
First, find the molar mass of water (H₂O) Look on the Periodic Table for the masses of hydrogen and oxygen.
Hydrogen (H): 1.008 g/molOxygen (O): 15.999 g/molNext, add up the number of each element in water. The subscript of 2 comes after the H, so there are 2 moles of hydrogen.
2 Hydrogen: (1.008 g/mol*2) = 2.016 g/molFinally, add the molar mass of 2 hydrogen and 1 oxygen.
2.016 g/mol (2 Hydrogen) + 15.999 g/mol (1 oxygen)= 18.015 g/molNext, find the grams in 6.5 moles.
Use the molar mass we just found as a ratio.
\(molar \ mass \ ratio: \frac{18.015 \ g \ H_2O}{1 \ mol \ H_2O}\)
We want to find the grams in 6.5 moles. We can multiply the ratio above by 6.5
\(6.5 \ mol \ H_2O * \frac{18.015 \ g \ H_2O}{1 \ mol \ H_2O}\)
Multiply. Note that the moles of H₂O will cancel each other out.
\(6.5 * \frac{18.015 \ g \ H_2O}{1}\)
\(6.5 * {18.015 \ g \ H_2O}\)
\(117.0975 \ g \ H_2O\)
If we want to round to the technically correct significant figures, it would be 2 sig figs. The original measurement, 6.5, has 2 (6 and 5).
\(\approx 120 \ g \ H_2O\)
Potassium metal reacts with oxygen gas to form solid potassium oxide.
Answers
Explanation:
Potassium oxide is an ionic compound formed by combining potassium and oxygen. It carries the chemical formula K2O. Potassium cannot be found free because it is too reactive. It has valency +1 and combines readily with oxygen atoms forming K2O.
Answer:
The Correct answer is
K+O2------>K2O
balancing it will be
4K+O2----->2K2O
15. If I make 94.8 L of O2, how many grams of H2O did I start with? (Show ALL work)

Answers
Answer:
double
Explanation:
.
A chemist mixes ammonium acetate into 500 mL of water. What does the solution contain?
a. The solution is just water. The ammonium acetate will not dissolve and will just sink to the bottom of the solution. b. N3-ions, H+ ions, C4-ions, and O2-ions c. NH4+ and CO32-ions d. NH4+ and C2H302 ions
Answers
Answer:
d. NH4+ and C2H3O2- ions
Explanation:
When ammonium acetate (NH4C2H3O2) is mixed with water, it dissociates into its ions. In this case, it dissociates into NH4+ (ammonium ion) and C2H3O2- (acetate ion). Therefore, the solution will contain NH4+ and C2H3O2- ions dissolved in water.
The solution contains NH4+ and C2H302 ions. Among the given options, the correct answer is option d. It forms a solution containing NH4+ and C2H302 ions that are fully dissolved in the water.
When a chemist mixes ammonium acetate into 500 mL of water, the ammonium acetate (NH4C2H3O2) dissolves and dissociates into its constituent ions. This results in the formation of NH4+ ions (ammonium ions) and C2H3O2- ions (acetate ions) in the solution. Ammonium acetate is a salt that readily dissolves in water, dissociating into NH4+ and C2H302 ions. The aqueous solution will contain NH4+ ions and C2H3O2- ions, which are formed due to the dissociation of ammonium acetate in water. These ions will be dispersed throughout the solution and will not sink to the bottom.
In conclusion, when ammonium acetate is mixed into water, it forms a solution containing NH4+ and C2H302 ions that are fully dissolved in the water.
To know more about ammonium acetate visit:
brainly.com/question/29978998
#SPJ11
What environmental condition is likely to increase the frequency of brightly colored, highly scented flowers in a plant species over many generations?
o Increased shade
o Intense rainfall
o Higher levels of soil nutrients
o Insect pollinators available
Answers
Answer:
the answer is the 3rd one, though I'm not 100% sure on it, sorry
Calculate the molality of potassium chloride (molar mass 79.55 g/mol) in a solution that which contains 25 g of potassium chloride in 120 g of water.
Answers
The molality of potassium chloride in a solution that which contains 25 g of potassium chloride in 120 g of water is 2.618 molal.
Molal concentration is defined as a measure by which concentration of chemical substances which are present in a solution are determined. It is defined in particular reference to solute concentration which is present in a solution . Most commonly used unit for molar concentration is moles/liter.
The molal concentration depends on the change in volume of the solution which is mainly due to thermal expansion. Molal concentration is calculated by the formula, molal concentration=mass/ molar mass ×1/mass of solvent in kg.
On substitution in formula, molal concentration= 25/79.55×1/0.120=2.618 molal.
Thus, the molality is 2.618 molal.
Learn more about molality,here:
https://brainly.com/question/26921570
#SPJ1
How much is required to change 52.2 grams of liquid water into steam from room temperature (25 Celsius) to 115 Celsius
Answers
Heat change, ΔH, required to change 52.2 grams of liquid water into steam from room temperature (25 Celsius) to 115 Celsius is 135915.228 J.
What is the heat change required?
The heat change required to change 52.2 grams of liquid water into steam from room temperature (25 Celsius) to 115 Celsius is determined as follows:
Heat change required, ΔH = ΔH₁ + ΔH₂ + ΔH₃
ΔH₁ is heat required to change liquid water at 25°C to water at 100°C
ΔH₁ = mass * specific heat capacity * temperature change
specific heat capacity of water = 4.184 J/g/K
ΔH₁ = 52.2 * 4.184 * (100 - 25)
ΔH₁ = 16380.36 J
ΔH₂ is the heat required to convert water at 100°C to steam at 100°C
ΔH₂ = mass * latent heat of vaporization of water
latent heat of vaporization of water = 2260 J/g
ΔH₂ = 52.2 * 2260
ΔH₂ = 117972 J
ΔH₃ is the heat required to change steam at 100°C to water at 115°C
ΔH₁ = mass * specific heat capacity * temperature change
specific heat capacity of steam = 1.996 J/g/K
ΔH₁ = 52.2 * 1.996 * (115 - 100)
ΔH₁ = 1562.868 J
Heat change required, ΔH = 16380.36 J + 117972 J + 1562.868 J
Heat change required, ΔH = 135915.228 J
Learn more about heat change at: https://brainly.com/question/24298104
#SPJ1
What can you say about the reactants of photosynthesis and the products of cellular respiration?
pls don't search this up on gòogle
Answers
Answer:
reactants of photosynthesis= carbon dioxide, water, and sunlight.
Products of cellular resperation= water and carbon dioxide and energy
Explanation:
what is the importance of polar covalent and hydrogen bonds in the structure of water?
Answers
Answer:
Water is a remarkable substance, and its unique properties are largely due to the presence of polar covalent bonds and hydrogen bonds in its structure. These characteristics play a crucial role in the physical and chemical properties of water, making it essential for life as we know it.
Explanation:
The polar covalent bonds in water arise from the unequal sharing of electrons between oxygen and hydrogen atoms. This results in the oxygen atom having a partial negative charge (δ-) and the hydrogen atoms having partial positive charges (δ+). These charges create polarity within the water molecule, leading to the formation of hydrogen bonds.
Hydrogen bonds occur when the partially positive hydrogen atom of one water molecule is attracted to the partially negative oxygen atom of another water molecule. These hydrogen bonds are relatively weak individually, but when present in large numbers, they contribute to the cohesion, surface tension, and high boiling point of water.
The importance of these bonds is manifold. The cohesion between water molecules due to hydrogen bonding enables water to form droplets, have a high surface tension, and flow freely, facilitating transport within organisms and in the environment. Additionally, hydrogen bonding leads to the high specific heat capacity and heat of vaporization of water, making it an effective regulator of temperature in living organisms and ensuring stable environmental conditions.
Furthermore, hydrogen bonds play a crucial role in the unique properties of water as a solvent. The polar nature of water allows it to dissolve a wide range of substances, including ionic compounds and polar molecules, facilitating various biological processes such as nutrient transport and chemical reactions in cells.
The half-life of tritium (H-3) is 12.3 years. If 48.0mg of tritium is released from a nuclear power plant during the course of a mishap, what mass of the isotope will remain after 49.2 years?
Answers
Answer:
Explanation:
it is tough question plz give me some time i would give you your ans soon