Answers
Answer:
C
Explanation:
Related Questions
If the pressure is 0.9 kPa. and the volume is 4.0L, then the pressure changes to 0.20 kPa. What is the new volume?
Answers
Answer:
18 L
Explanation:
boyles law
P1V1 = P2V2
V2 = P1V1/P2
V2 = (.9 * 4)/.2
V2 = 18
This question is based on Boyle's law as temperature is constant. Boyle's law is derived from ideal gas equation only at constant temperature. Therefore, the new pressure is 18L.
What is Boyle's law?Boyle's law is a law which states that at constant temperature, the pressure that is exerted by an ideal gas on the walls of the container is inversely proportional to the volume occupied that gas.
Ideal gas is a gas where there is no forces of attraction or forces of repulsion between the particles . The volume of container is equal to the volume occupied by the gas.
Mathematically, Boyle's law can be written as
P₁V₁ = P₂V₂
P₁=initial pressure
P₂=final pressure
V₁ = initial volume
V₂=final pressure
V₂ = P₁V₁/P₂
V₂ = (0.9 × 4)/0.2
V₂ = 18L
Therefore, the new volume is 18L.
To know more about Boyle's law, here:
https://brainly.com/question/1437490
#SPJ2
When thermal energy is removed from a system, what happens to the temperature and the average kinetic energy of the system?
Temperature decreases, average kinetic energy increases
Temperature decreases, average kinetic energy decreases
Temperature increases, average kinetic energy increases
O Temperature increases, average kinetic energy decreases
Answers
Answer:
When a substance is heated, it gains thermal energy. Therefore, its particles move faster and its temperature rises. When a substance is cooled, it loses thermal energy, which causes its particles to move more slowly and its temperature to drop.
Explanation:
MAKE SURE TO REPHRASE
Please show you work
This is 7th grade science

Answers
2) It will accelerate to the right 8>6
3) It will not move. 15=15
How many liters at STP of Na›SiO; can react with 0.8000 grams of HF?
Answers
The volume of Na2SiO3 that can react with 0.8000 grams of HF at STP is approximately 0.9663 liters.
How many lñiters can react?To calculate the volume of Na2SiO3 (sodium silicate) that can react with 0.8000 grams of HF (hydrofluoric acid) at standard temperature and pressure (STP), we need to use stoichiometry and the ideal gas law.
Write the balanced chemical equation for the reaction between Na2SiO3 and HF:
Na2SiO3 + 6HF → Na2SiF6 + 3H2O
Determine the molar mass of HF:
The molar mass of HF is:
H (hydrogen) = 1.008 g/mol
F (fluorine) = 18.998 g/mol
So, the molar mass of HF = 1.008 + 18.998 = 20.006 g/mol
Convert the given mass of HF to moles:
Mass of HF = 0.8000 g
Molar mass of HF = 20.006 g/mol
Moles of HF = Mass of HF / Molar mass of HF
Moles of HF = 0.8000 g / 20.006 g/mol
Moles of HF = 0.03999 mol (rounded to five decimal places)
Use the stoichiometry of the balanced equation to determine the molar ratio between Na2SiO3 and HF:
From the balanced equation, we can see that 6 moles of HF react with 1 mole of Na2SiO3.
Use the ideal gas law to calculate the volume of Na2SiO3 at STP:
The ideal gas law is given by:
PV = nRT
where:
P = Pressure (at STP, P = 1 atm)
V = Volume
n = Number of moles of gas (in this case, moles of Na2SiO3)
R = Ideal gas constant (0.0821 L atm / mol K)
T = Temperature (at STP, T = 273.15 K)
Rearrange the formula to solve for volume (V):
V = nRT / P
Plugging in the values:
n = 0.03999 mol (from step 3)
R = 0.0821 L atm / mol K
T = 273.15 K (at STP)
P = 1 atm (at STP)
V = 0.03999 mol * 0.0821 L atm / mol K * 273.15 K / 1 atm
V = 0.9663 L (rounded to four decimal places)
Learn more about chemical reactions at:
https://brainly.com/question/11231920
#SPJ1
An atom with 14 protons, 14 neutrons, and 16 electrons is stable, -2 charge
stable, +2 charge
unstable, -2 charge
unstable, no charge *
Answers
We can see that an atom with 14 protons, 14 neutrons, and 16 electrons is unstable, and has a -2 charge.
So the correct option is the third one.
What can we say about the atom?An atom with 14 protons, 14 neutrons, and 16 electrons is not stable. The number of protons in an atom, also known as its atomic number, determines its element and its chemical properties. In this case, the atom has 14 protons, which corresponds to the element silicon (Si) on the periodic table.
For an atom to be stable, it should have a balanced number of protons and electrons. Electrons are negatively charged particles that orbit the nucleus of an atom in energy levels or electron shells. The number of electrons in a stable atom should be equal to the number of protons, resulting in a neutral charge overall.
In this case, the atom has 14 protons and 16 electrons, which means it has two more electrons than protons, resulting in a net charge of -2. This is an example of an ion.
Learn more about atoms.
https://brainly.com/question/17425565
#SPJ1
need help please ,thx

Answers
Answer:
can you get a picture that is in higher quality? we can't read what it's saying
In three to five sentences, predict the bonding activity between Carbon and Chlorine. Explain why they would bond that way in terms of electronegativity and valence electrons.
Answers
Answer:
Carbon has 4 valence electrons and Chlorine has 7 valence electrons. The bonding between the atoms is due to electronegativity difference. The electronegativity of carbon is 2.5 compared to chlorine which is 3.1, so they will bond by sharing the electron pair (valance electron) from each atom in a covalent bond. In addition, Carbon is more electronegative than Chlorine, which means that it takes less energy to remove an electron from Carbon than from Chlorine. This makes it easier for the electrons to be shared between them (since there is less energy required to share an electron).
Explanation:
Why are metals so ductile?
Answers
Answer:
throughout the metallic structure allowing the atoms to slide past each other. This sliding is why metals are ductile and malleable. Ionic compound must break bonds to slide past one another, which causes the ionic material to split and crack.
Explanation:
Hope this helps loves mark me brianlest x
The ____ line on the graph to the right best represents the data table.
Orange
Blue
Green
It was orange
Answers
Answer:
The orange line is the best option
Explanation:
As I was typing my answer I just saw that you have in your question "It was orange" so ig I can't say it is the maroon line :/
The orange line on the graph to the right best represents the data table. Therefore, the correct option is option A among all the given options.
What is graph?In mathematics, a graph is a visual representation or diagram that shows facts or values in an ordered way. The relationships among two or more items are frequently represented by the points on a graph. A bar graph can also be used to display the data. Bar graphs show how many of each supply there are.
Here, as instance, we may plot a graph showing the kind and quantity of school supplies in use by pupils in a class based on the data provided below. Each supply is first counted, and the data is then shown in a table with certain colors in a logical sequence. The orange line on the graph to the right best represents the data table.
Therefore, the correct option is option A among all the given options.
To learn more about graph, here:
https://brainly.com/question/29183673
#SPJ6
CAN SOMEONE PLEASE HELP ME I WILL GIVE BRAINLIEST

Answers
Answer:
9812
Explanation:
i think
Answer:
if im correct its b but im not 100 percent sure
Explanation:
The compound that is not an organic alcohol is:
C 3H 7OH
CH 3CH(OH)CH 3
C 6H 5COOH
(CH 3) 2CHCH(OH)CH 2CH 3
Answers
Answer:
The compound that is not an organic alcohol is C6H5COOH.
C3H7OH is propyl alcohol, CH3CH(OH)CH3 is 2-propanol, and (CH3)2CHCH(OH)CH2CH3 is 3-pentanol, all of which are organic alcohols.
On the other hand, C6H5COOH is benzoic acid, which is not an alcohol but an organic acid. It contains a carboxylic acid (-COOH) functional group and not the -OH functional group of alcohols.
How many Liters are 5.1 grams of Cl2?
Answers
5.1 grams of Cl2 is calculated as 1.610 L. As all the gases that behave ideally have the same number density, they will also have the same molar volume.
What is molar volume?Molar volume of a substance is the volume occupied by one mole of substance at given temperature and pressure. It is equal to the molecular mass of the substance divided by its density at the given temperature and pressure: It has SI unit of cubic meters per mole.
Given 5.1 grams of Cl2
As molar mass of Cl2 = 70.906 g/mol
So, moles = 5.1/70.906
= 0.0719 mol
Volume= moles of cl2 * molar volume of cl2
As molar volume of cl2 = 22.40 L
So, Volume = 0.0719 * 22.40
Volume = 1.610 L
To know more about molar volume, refer
https://brainly.com/question/11676583
#SPJ1
What mass of HF is produced according to the given equation when 2.397 grams of each reactants are combined?
CaF2 + H2SO4 --> CaSO4 + 2HF
Answers
+HF is the answer
Explanation:
A gas sample of 5 moles, has a volume of 95 L. How many moles of the same gas should I add to obtain a volume of 133 L at the same temperature and pressure.
Answers
i don’t know cause i’m in 8th grade
A solution is prepared by dissolving 0.131 g of a substance in 25.4 g of water. The molality of the solution is determined by freezing point
depression to be 0.056 m. What are the moles of the substance?
Answers
The mole of the substance, given the data from the question is 0.0014 mole
What is molality?This is simply defined as the mole of solute per kilogram of water. Mathematically, it is expressed as
Molality = mole / mass (Kg) of water
How to determine the mole of the substanceMass of water = 25.4 g = 25.4 / 1000 = 0.0254 KgMolality = 0.056 mMole of substance =?Mole = molality × mass of water
Mole of substance = 0.056 × 0.0254
Mole of substance = 0.0014 mole
Thus, the mole of the substance is 0.0014 mole
Learn more about Molality:
https://brainly.com/question/4251997
#SPJ1
The Properties of Liquids
Answers
Answer:
Properties of Liquids
Capillary Action. ...
Cohesive and Adhesive Forces. ...
Contact Angles. ...
Surface Tension. ...
Unusual Properties of Water. ...
Vapor Pressure. ...
Viscosity Viscosity is another type of bulk property defined as a liquid's resistance to flow. ...
Wetting Agents
exactly 149.6J will raise the temperature of 10.0g of a metal from 25.0C. what is the specific heat capacity of the metal
Answers
Exactly 149.6J will raise the temperature of 10.0g of a metal from 25.0C. The specific heat capacity of the metal is 5.984 J/g°C.
What is specific heat capacity?The heat capacity of a sample of a substance divided by the mass of the sample yields the specific heat capacity (symbol c), also known as massic heat capacity. Informally, it is the quantity of heat that must be added to one unit of a substance's mass in order to raise its temperature by one unit. The specific heat capacity unit in the SI is the joule per kelvin per kilogram, or Jkg⁻¹K⁻¹. For instance, the specific heat capacity of water is 4184 J kg⁻¹K⁻¹, or the amount of energy needed to raise 1 kilogram of water by 1 K.
The specific heat capacity of the metal can be calculated using the equation Q = m × c ×ΔT.
Q = 149.6J
m = 10.0g
ΔT = (final Temperature - initial Temperature) = (25°C - 0°C) = 25°C
Plugging these values into the equation, we get:
149.6J = 10.0g × c ×25°C
Solving for c, we get:
c = \(\frac{149.6J}{(10.0g *25C)}\)
c = 5.984 J/g°C
Therefore, the specific heat capacity of the metal is 5.984 J/g°C.
To know more about specific heat capacity, visit:
https://brainly.com/question/29766819
#SPJ1
When octane (C8H18) is burned in the presence of oxygen, the yield of products (carbon dioxide and water) is 87%. What mass of carbon dioxide will be produced in this engine when 21.0 g of octane is burned with 19.0 g of oxygen gas
Answers
Answer:
14.5g of CO₂ are produced
Explanation:
The reaction of octane with oxygen is:
C₈H₁₈ + 25/2O₂ → 8CO₂ + 9H₂O
Where 1 mole of octane (Molar mass: 114.23g/mol) reacts with 25/2 moles of O₂ (Molar mass 32g/mol) to produce 8 moles of CO₂ and 9 moles of water.
When 21.0 g of octane is burned with 19.0 g of oxygen gas you need to find limiting reactant to find how many moles of products are formed:
Octane: 21.0g ₓ (1mol / 114.23g) = 0.184 moles octane
Oxygen: 19.0g ₓ (1 mol / 32g) = 0.594 moles oxygen
For a complete reaction of 0.184 moles of octane you will need:
0.184 moles C₈H₁₈ ₓ (25/2 moles O₂ / 1 mole C₈H₁₈) = 2.3 moles of oxygen
As you have just 0.594 moles of oxygen, Oxygen is limiting reactant.
Based on chemical equation, 25/2 of O₂ produce 8 moles of CO₂, that means theoretical yield of CO₂ with 0.594 moles of O₂ is:
0.594 moles O₂ ₓ (8 moles CO₂ / 25/2 moles O₂) = 0.380 moles of CO₂
But, as yield of products is 87%, moles produced of CO₂ are:
0.380 moles of CO₂ ₓ 87% = 0.331 moles CO₂ are produced.
As molar mass of CO₂ is 44g/mol, mass of CO₂ in 0.331 moles is:
0.331 moles CO₂ ₓ (44g / mol) =
14.5g of CO₂ are producedAt a certain temperature Kc = 9.0 for the equilibrium 24() ⇔ 22(). What is
Kc at the same temperature for 2() ⇔ 1/224().
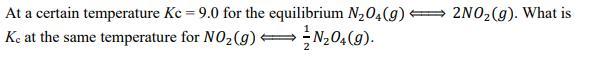
Answers
Answer:
.
Explanation:
.
23) A common reaction that occurs in cells is shown here. In the presence of oxygen, a glucose molecule is combusted to form carbon dioxide and water. If 360 grams of glucose are combusted fully,
calculate how many moles of oxygen gas will be needed to achieve this reaction.
A) 2
B) 8
C)12
D) 18
Answers
Answer:
C
Explanation:
add them together and multiply by 2
The cooling curve for a pure substance as it changes from a liquid to a solid is shows above. The solid and the liquid coexist at
Answers
The solid and the liquid coexist at the melting point, which is the point where the temperature remains constant during the phase transition.
What point does solid and liquid coexist?In the given cooling curve, the melting point is at 50°C, indicated by the flat region on the curve between points C and D. At this point, the energy being released during cooling is used to overcome the energy required for the substance to transition from a liquid to a solid state.
Once all of the substance has solidified, the temperature begins to decrease again.
Learn more about cooling curve:https://brainly.com/question/31069932
#SPJ1
Answer: all points on the curve between Q and S
Explanation: Where the graph looks like <-------->horizontal.
How do you do number thirty?

Answers
What is the rate of a reaction if the value of kis 0.1, [A] is 1 M, and [B] is 2 M?
Rate = K[A]2[B]2
A. 1.6 (mol/L)/s
B. 0.8 (mol/L)/S
C. 0.2 (mol/L)/S
D. 0.4 (mol/L)/S
Answers
Answer:
D. 0.4 (mol/L)/S
Explanation:
You simply have to plug in the given values into the rate law.
Rate = k[A][B]
Rate = (0.1)(1)²(2)²
Rate = (0.1)(1)²(4)²
Rate = 0.4
For the reaction
4PH3(g)↽−−⇀6H2(g)+P4(g)
the equilibrium concentrations were found to be [PH3]=0.250 M, [H2]=0.580 M,
and [P4]=0.750 M.
What is the equilibrium constant for this reaction?
c=
Answers
The equilibrium constant for the reaction given that the equilibrium concentration of [PH₃] = 0.250 M, [H₂] = 0.580 M, and [P₄] = 0.750 M is 7.3
How do I determine the equilibrium constant?From the question given above, the following data were obtained:
Equation: 4PH₃(g) ⇌ 6H₂(g) + P₄(g)Concentration of PH₃, [PH₃] = 0.250 MConcentration of H₂, [H₂] = 0.580 MConcentration of P₄, [P₄] = 0.750 MEquilibrium constant (K) =?The equilibrium constant for the reaction can be obtained as shown below:
Equilibrium constant = [Product]ᵐ / [Reactant]ⁿ
Where
m and n are coefficients of products and reactants respectivelyEquilibrium constant = [H₂]⁶[P₄] / [PH₃]⁴
Equilibrium constant = [(0.580)⁶ × 0.750] / (0.250)⁴
Equilibrium constant = 7.3
Thus, the equilibrium constant for the reaction is 7.3
Learn more about equilibrium constant:
https://brainly.com/question/16589765
#SPJ1
What does it mean to be "moderated by water"?
Answers
water is watching over you
Answer:
it means the higher the water content in the air, the more moderate (less extreme) the climate is.
Stephan’s mother cuts a twig from a rose bush and plants it in the soil. After a few days, Stephan observes a new plant growing. Which characteristic does the growth of the new plant depict?
Answers
The growth of the new plant depicts the asexual reproduction characteristic. The characteristic that describes the growth of the new plant in Stephan's mother cutting a twig from a rose bush and planting it in the soil is asexual reproduction.
Asexual reproduction is the mode of reproduction by which organisms generate offspring that are identical to the parent's without the fusion of gametes. Asexual reproduction is a type of reproduction in which the offspring is produced from a single parent.
The offspring created are clones of the parent plant, meaning they are identical to the parent.The new plant in Stephan’s mother cutting a twig from a rose bush and planting it in the soil depicts the process of asexual reproduction, which is the ability of a plant to reproduce without seeds. In asexual reproduction, plants can reproduce vegetatively by cloning themselves using their roots, bulbs, or stems.
Know more about characteristic here:
https://brainly.com/question/28790299
#SPJ8
what are thetypes of luminous flame
Answers
Types of luminous flames:
1. Yellow Luminous Flame
2. Smoky Luminous Flame
3. Orange Luminous Flame
4. Blue Luminous Flame
Luminous flames are characterized by their visible glow, which is caused by the incomplete combustion of fuel. The presence of soot particles in the flame causes the emission of light. There are different types of luminous flames, which can be classified based on their fuel composition and burning conditions. Here are some common types of luminous flames:
1. Yellow Luminous Flame: This is the most common type of luminous flame, often seen in open fires, candles, and gas stoves. It appears yellow due to the presence of soot particles in the flame. Yellow flames indicate incomplete combustion of hydrocarbon fuels, such as methane, propane, or natural gas. The high carbon content in these fuels leads to the formation of soot, which emits visible light.
2. Smoky Luminous Flame: This type of flame is characterized by a significant amount of black smoke and soot production. It is commonly observed in poorly adjusted or malfunctioning burners or engines. The excessive presence of unburned fuel in the flame results in incomplete combustion and the emission of dark smoke particles.
3. Orange Luminous Flame: An orange flame indicates a higher combustion temperature compared to a yellow flame. It is often seen in more efficient burners or when burning fuels with a higher carbon content, such as oil or diesel. The higher temperature helps in burning more of the carbon particles, reducing the amount of soot and making the flame appear less yellow.
4. Blue Luminous Flame: A blue flame is typically associated with complete combustion. It indicates efficient burning of fuel, resulting in minimal soot formation. Blue flames are commonly observed in gas burners or Bunsen burners. The blue color is a result of the combustion of gases, such as methane, in the presence of sufficient oxygen.
It's important to note that the luminosity of a flame can vary depending on factors such as fuel-air mixture, combustion temperature, and the presence of impurities. Achieving complete combustion and minimizing the production of soot is desirable for efficient and cleaner burning processes.
for more questions on luminous
https://brainly.com/question/27163038
#SPJ8
A geologist conducts an investigation to determine the absolute age of a fossil. She then
repeats the procedure three times. Which BEST explains why she repeated the procedure
several times? (SC.6.N.1.1)
It helps her develop better procedures
It improves the accuracy of the results.
She wants all the results to be different.
She has more than one hypothesis to prove.
Answers
Answer:
Explanation:
Hehs
HELPPP PLZZ
During lunch each day, Alex and Hailey have been noticing the trashcans around the lunchroom are filling rather quickly with cans and bottles. They decide a recycling program would be beneficial for their school and the environment, so they brainstorm ways to get the program started. Before discussing their idea with the principal, they need to decide which of their plans would best solve the problem. Which of their ideas would best solve the problem with the least amount of inconvenience (disturbance) for everyone involved? A) Ask volunteers to separate recyclable materials from the trash. B) Remove all vending machines that sell plastic bottles or aluminum cans. C) Get a petition started to build a recycling center close to the school. D) Place plastic and metal recycling bins next to each lunchroom trash can
Answers
Answer:
c
Explanation:
i think it is C but idek but to me it sounds like the best answers but tell me if im wrong pls give me feedback
The ideas that would best solve the problem with the least amount of inconvenience is to place plastic and metal recycling bins next to each lunchroom trash can.
What is recycling?The term recycling has to do with the reuse of a material after it has passed through certain processes. The idea of recycling is highly beneficial to the environment.
Thus, the ideas that would best solve the problem with the least amount of inconvenience (disturbance) for everyone involved is to place plastic and metal recycling bins next to each lunchroom trash can.
Learn more about recycling:https://brainly.com/question/11861824
#SPJ2
2 dmcube of N2 at a pressure 100kpa and 5dmcube of H2 at pressure of 500kpa are injected into a 10dmcube container, calculate partial pressures of H2 and N2
Answers
The partial pressure of \(N_2\)is 165.6 kPa and the partial pressure of \(H_2\)is 434.4 kPa.
To calculate the partial pressures of \(H_2\)and \(N_2\)in the 10dmcube container, we need to use the ideal gas law equation, which states that the pressure of a gas is directly proportional to its number of moles and temperature, and inversely proportional to its volume.
First, we need to calculate the number of moles for each gas. Since we are given the volume of each gas and the volume of the container, we can use the formula:
Number of moles = Volume / Molar volume
The molar volume is the volume occupied by one mole of a gas at a given temperature and pressure. At standard temperature and pressure (STP), the molar volume is 22.4 L/mol.
For \(N_2\), the number of moles is 2 dmcube / 22.4 L/mol = 0.089 mol
For \(H_2\), the number of moles is 5 dmcube / 22.4 L/mol = 0.223 mol
Next, we can calculate the partial pressures of each gas using the formula:
Partial pressure = (Number of moles / Total number of moles) * Total pressure
The total pressure is the sum of the pressures of each gas:
Total pressure = Pressure of N2 + Pressure of \(H_2\)
Given that the pressure of N2 is 100 kPa and the pressure of \(H_2\)is 500 kPa, we have:
Total pressure = 100 kPa + 500 kPa = 600 kPa
Now we can calculate the partial pressure of \(N_2\):
Partial pressure of \(N_2\)= (0.089 mol / (0.089 mol + 0.223 mol)) * 600 kPa = 165.6 kPa
Similarly, we can calculate the partial pressure of \(H_2\):
Partial pressure of H2 = (0.223 mol / (0.089 mol + 0.223 mol)) * 600 kPa = 434.4 kPa
Therefore, the partial pressure of \(N_2\)is 165.6 kPa and the partial pressure of \(H_2\)is 434.4 kPa.
For more such question on partial pressure visit:
https://brainly.com/question/19813237
#SPJ8
