15. If 2.00 moles of NO gas occupies 10.0 L at 295 K, what is the pressure of the gas in
atmospheres?
Answers
The pressure of the gas in atmospheres is 4.84 atm
Solution:
PV = nRT
Solve for P
R = 0.0821 L x atm/ mol x K (constant)
If volume and temperature are held constant, the ideal gas law can be reconstructed to show that the pressure of a gas sample is directly proportional to the number of moles of gas present. At constant temperature and pressure, the volume of gas is directly proportional to the number of moles of gas.
At constant temperature and volume, the pressure of a gas is directly proportional to the number of moles of gas. Atmospheric pressure is the pressure caused by most of our gaseous atmosphere. Atmospheric pressure Mercury density Gravitational acceleration Height of mercury column It can be measured using mercury.
Learn more about The pressure here:-https://brainly.com/question/25736513
#SPJ1
Related Questions
a sample of unknown material weighs 500 n in air and 200 n when immesersed in alcholol with a specfic gravity of 0.7 what is the mass density
Answers
Answer: The mass density is 1166.36 \(kg/m^{3}\).
Explanation:
Given: Weight of sample in air \((F_{air})\) = 500 N
Weight of sample in alcohol \((F_{alc})\) = 200 N
Specific gravity = 0.7 = \(0.7 \times 1000 = 700 kg/m^{3}\)
Formula used to calculate Buoyant force is as follows.
\(F_{B} = F_{air} - F_{alc}\\= 500 - 200 \\= 300 N\)
Hence, volume of the material is calculated as follows.
\(V = \frac{F_{B}}{\rho \times g}\)
where,
\(F_{B}\) = Buoyant force
\(\rho\) = specific gravity
g = acceleration due to gravity = 9.81
Substitute the values into above formula.
\(V = \frac{F_{B}}{\rho \times g}\\= \frac{300}{700 \times 9.81}\\= \frac{300}{6867}\\= 0.0437 m^{3}\)
Now, mass of the material is calculated as follows.
\(mass = \frac{F_{air}}{g}\\= \frac{500 N}{9.81}\\= 50.97 kg\)
Therefore, density of the material or mass density is as follows.
\(Density = \frac{mass}{volume}\\= \frac{50.97 kg}{0.0437 m^{3}}\\= 1166.36 kg/m^{3}\)
Thus, we can conclude that the mass density is 1166.36 \(kg/m^{3}\).
how many ơ and À bonds are found in 3-butyn-2-one?
Answers
In 3-butyn-2-one, there is 1 triple bond (Æ bond) and 1 double bond (€ bond).
In 3-butyn-2-one, there are three types of bonds:
single bonds, double bonds, and triple bonds.
Let's break down the molecule to identify the number of each type of bond.
The molecular formula for 3-butyn-2-one is C₄H₆O. This tells us that it contains 4 carbon atoms, 6 hydrogen atoms, and 1 oxygen atom.
Now, let's focus on the carbon-carbon bonds. The molecule has a triple bond between the second and third carbon atoms, which is a total of 1 triple bond (represented by a Æ bond).
Next, let's look at the carbon-oxygen bond. The molecule has a double bond between the first carbon atom and the oxygen atom, which is a total of 1 double bond (represented by an € bond).
There is 1 triple bond and 1 double bond .
Learn more about molecule from:
https://brainly.com/question/475709
#SPJ11
The temperatures varies from 100 degrees celsius to -170 degrees celsius
neptune
pluto
jupiter
the moon
Answers
How are renewable and nonrenewable resources different?
Renewable resources can be replaced, but nonrenewable resource are recycled.
There are a lot of nonrenewable resources and very few renewable resources.
Nonrenewable resources cannot be replaced, whereas renewable resources can be recycled.
Renewable resources can be replaced in a reasonable amount of time, but nonrenewable resources cannot.
Answers
Renewable resources can be replaced, while nonrenewable resources cannot be replaced.
What are some examples of renewable resources?Some examples of renewable resources include solar energy, wind energy, hydropower, geothermal energy, and biomass.
Why is it important to prioritize the use of renewable resources over nonrenewable resources?It is important to prioritize the use of renewable resources over nonrenewable resources because nonrenewable resources are finite and their depletion can have significant negative impacts on the environment and human society. In contrast, renewable resources can be replenished and their use can help reduce our dependence on fossil fuels and mitigate climate change.
Learn more about Renewable resources here:
https://brainly.com/question/19604560
#SPJ1
Which molecule has five atoms per molecule?
a.
H2O
b.
NH3
c.
CH4
d.
O2
Answers
Answer:
CH4, methane
Explanation:
CH4 has 1 C (carbon) and 4 H (hydrogen) atoms, for a total of 5 atoms in one molecule.
I have four neutrons and I'm metallic. Who am
I?
Answers
According to the electronic configuration, beryllium has four neutrons and is metallic in nature.
What is electronic configuration?Electronic configuration is defined as the distribution of electrons which are present in an atom or molecule in atomic or molecular orbitals.It describes how each electron moves independently in an orbital.
Knowledge of electronic configuration is necessary for understanding the structure of periodic table.It helps in understanding the chemical properties of elements.
Elements undergo chemical reactions in order to achieve stability. Main group elements obey the octet rule in their electronic configuration while the transition elements follow the 18 electron rule. Noble elements have valence shell complete in ground state and hence are said to be stable.
Learn more about electronic configuration,here:
https://brainly.com/question/29757010
#SPJ5
a flask containing only n2o4 at an initial pressure of 4.5 atm is allowed to reach equilibrium. calculate the equilibrium partial pressures of the gases. b. a flask containing only no at an initial pressure of 2 9.0 atm is allowed to reach equilibrium. calculat
Answers
a) The equilibrium partial pressures of the gases is 1.5 atm. b)The equilibrium partial pressures of NO, \(O_{2}\), and \(NO_{2}\) will be 2.9 atm, 0.5 × (1.3 × \(10^{-11}\)) atm, and (1.3 × \(10^{-11}\)) atm, respectively.
a. In order to calculate the equilibrium partial pressures of the gases in the flask containing only \(N_{2} O_{4}\) at an initial pressure of 4.5 atm,
we first need to write the balanced chemical equation for the reaction that takes place. \(N_{2} O_{4}\) (g) ⇌ 2\(NO_{2}\) (g) The equilibrium constant expression for this reaction is Kc = [NO2]2/[\(N_{2} O_{4}\)]
Now, let x be the amount of \(N_{2} O_{4}\) that decomposes to form \(NO_{2}\) at equilibrium. The equilibrium concentrations of\(N_{2} O_{4}\) and \(NO_{2}\)will be (4.5 - x) atm and (2x) atm, respectively.
Substituting these values in the equilibrium constant expression gives us Kc = (2x)2/(4.5 - x) = 4x2/(4.5 - x)
At equilibrium, this expression must be equal to the value of the equilibrium constant, which is known to be 0.25.
Therefore, we can write the equation 4x2/(4.5 - x) = 0.25 Solving this equation gives us x = 0.75 atm.
Therefore, the equilibrium partial pressures of \(N_{2} O_{4}\) and \(NO_{2}\) will be (4.5 - 0.75) = 3.75 atm and (2 × 0.75) = 1.5 atm, respectively.
b. In the flask containing only NO at an initial pressure of 2.9 atm, the reaction NO (g) + 1/2\(O_{2}\) (g) ⇌ \(NO_{2}\)(g) takes place at equilibrium.
The balanced chemical equation for this reaction shows that the number of moles of \(NO_{2}\) formed is equal to the number of moles of NO consumed.
Therefore, let x be the amount of NO that reacts to form \(NO_{2}\) at equilibrium. The equilibrium concentrations of NO, O2, and \(NO_{2}\) will be (2.9 - x) atm, (0.5x) atm, and (x) atm, respectively.
The equilibrium constant expression for this reaction is Kc = [\(NO_{2}\)]/[NO][\(O_{2}\)]
Substituting the equilibrium concentrations in this expression gives us
Kc = (x)/[(2.9 - x) × (0.5x)]
Simplifying this expression, we get Kc = 2x2/(2.9 - x)
At equilibrium, this expression must be equal to the value of the equilibrium constant,
which is known to be 4.6 × \(10^{-31}\).
Therefore, we can write the equation 2x2/(2.9 - x) = 4.6 × \(10^{-31}\)
Solving this equation gives us x = 1.3 × \(10^{-11}\)atm.
This is a very small value, indicating that the reaction does not proceed to a significant extent. T
Therefore, the equilibrium partial pressures of NO, \(O_{2}\), and\(NO_{2}\) will be 2.9 atm, 0.5 × (1.3 × \(10^{-11}\)) atm, and (1.3 × \(10^{-11}\)) atm, respectively.
Know more about equilibrium here:
https://brainly.com/question/517289
#SPJ11
CONNECT IT
Relate how a household sponge and water can be used to model
the concept of an unsaturated solution, a saturated solution, and a supersaturated
solution.
Answers
The sponge is unsaturated when it is taking in more water. It becomes saturated when it stops taking in water. It is supersaturated when water starts oozing out from the sponge.
A saturated solution contains just as much solute as it normally hold at a particular temperature. An unsaturated solution contains less solute than it can normally hold at a particular temperature. A supersaturated solution contains more solute than it can normally hold at a particular temperature.
We can use a sponge to model these three scenario as follows;
Water continues to enter into the sponge when it is unsaturated, this continues until the sponge becomes saturated with water and takes in no more water. When the sponge becomes supersaturated, water begins to ooze out from the sponge because it can no longer hold more water.
Learn more: https://brainly.com/question/1527403
How many grams are in 0.133 moles of NH4CIO3?

Answers
Answer:
53.49146
Explanation:
A buffer solution is prepared by adding NH4CIto a solution of NH3 (ammonia).NH3(aq) + H2O(0) = NH4+ (aq) + OH-(aq)What happens if HCl is added?

Answers
ANSWER
The addition of HCl will shift to reactants
EXPLANATION
When some strong acid is added to a buffer, the equilibrium is shifted to the left, and the hydrogen ion concentration increases by less than expected for the amount of strong acid added. Buffer solution helps in adjusting the pH of a substance.
Since the HCl is a strong acid, it will shift to the left (reactant sides)
True or false? The ground state electron configuration for manganese is [Ar]4 s
2
4 d
5
. True or false? The ground state electron configuration for calcium is 1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
. How many valences electron dose Si(z=14) continue? How many orbitals are in the 4 s sublevel? How can I calculate the values of JJ (total angular momentum) for a particular term, for instance,
3
P ?
Answers
The ground state electron configuration for manganese is False. The ground state electron configuration for calcium is True.
The ground state electron configuration for manganese is False. The correct ground state electron configuration for manganese (Z = 25) is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁵
The ground state electron configuration for calcium is True. The correct ground state electron configuration for calcium (Z = 20) is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
For silicon (Z = 14), the electron configuration is:
1s² 2s² 2p⁶ 3s² 3p²
Therefore, silicon has 4 valence electrons.
The 4s sublevel can hold a maximum of 2 electrons. It consists of one orbital.
To calculate the values of JJ (total angular momentum) for a particular term, you need to consider the electron configuration and Hund's rule.
For the 3P term, the electron configuration would be:
3s² 3p³
To calculate the values of JJ, you need to consider the total number of electrons in the term. In this case, there are 5 electrons. According to Hund's rule, the maximum value of J is determined by the total number of unpaired electrons. Since there are 3 unpaired electrons in the 3P term, J can have values ranging from 3 - 1 to 3 + 1, which are 2 and 4. Therefore, for the 3P term, the possible values of JJ are 2 and 4.
To know more about electronic configuration:
https://brainly.com/question/31812229
#SPJ4
Nitrogen (N) has one more proton than Carbon (C) where did the proton come from in this reaction?
What type of reaction is depicted in the equation above?
Answers
Answer:
I'm sorry, but I don't see an equation in your question to provide a specific answer. However, I can answer your general question.
Nitrogen has one more proton than Carbon because it has one more positively charged particle (proton) in its nucleus. This difference in the number of protons determines the atomic number and identity of the element.
Protons can only come from the nucleus of another atom, through a nuclear reaction. In nature, nitrogen is usually created by nuclear fusion in stars, where lighter elements combine under high pressure and temperature to form heavier elements.
Regarding the second part of your question, without the specific equation you are referring to, I cannot determine the type of reaction depicted. There are many types of chemical reactions, including synthesis, decomposition, combustion, acid-base, and redox reactions, among others.
what would be the symptoms for an air-cooled condenser where the air leaving the condenser is hitting a barrier and recirculating?
Answers
One thing that would be the symptoms for an air-cooled condenser where the air leaving the condenser is hitting a barrier and recirculating is: that there is less heat transfer from the refrigerant to the surrounding environment. Hence this will cause heat to accumulate in the condenser, making the cooling system inefficient.
What is a condenser?A condenser is a heat transfer used in heat transfer systems to cool a gaseous component and condense it into a liquid condition. The latent heat is released by the material and redistributed to the surrounding environment as a result.
The condenser's function is to accept high-pressure gas from the compressor and convert it to liquid. It does this by heat transfer or the notion that heat always moves from a warmer to a colder substance.
The condenser coil, evaporator, expansion valve, and compressor are the components. Each is critical to the overall performance of your unit.
Learn mroe about Condenser:
https://brainly.com/question/15563071
#SPJ1
BRAINLIEST! A reaction is set up between magnesium and hydrochloric acid. After 30 seconds, the magnesium had decreased in mass by 45g. What was the rate of this reaction?
Answers
Answer:
Acidic oxides, or acid anhydride, are oxides that react with water to form an acid, or with a base to form a salt. They are oxides of either nonmetals or of metals in high oxidation states.
Explanation:
A molecule with two electron domains will display bond angles of __________ degrees.
Answers
A molecule with two electron domains will display bond angles of 180 degrees.
According to the VSEPR theory, the two-electron density areas will set up shop with a 180° bond angle on either side of the center atom.
The connections between atoms in a linear model are linear. 180 degrees is the bond angles setting. Nitric oxide as well as carbon dioxide are two examples of molecules with a linear structure.
An atom that is bound and a nonbonding pair of electrons are the two different kinds of electron domains. All bonded atoms count as one electron domain on the central atom, regardless of whether they are connected by a single, double, as well as triple bond.
Therefore, a molecule with two electron domains will display bond angles of 180 degrees.
To know more about molecule
https://brainly.com/question/24188418
#SPJ4
How many sig figs are in:
0.0058096
Answers
uyuvhgbjhbngcfhjihugftrdesfgjhkjlhgjfhdgxszxfgvhbjnkbhjgvhcfgd
Answers
hello how are you
Explanation:
have a good day
Answer:
Excuse me are you ok?
Explanation:
I'm calling 911. Jerry stay awake!! Martha the ambulance is coming.
Robert uses a spring scale to measure force.
What is the unit of his measurement?
A). newtons
B). joules
C). volts
D). pascals
Answers
Answer:
Newtons
Explanation:
(Hopefully this helps brainliest?)
Newton- Newton is unit in which force is measured
Joules is measured for energy
volts for potential difference
Pascals for pressure
How do you find how many electrons are in a atom?
Answers
Answer:
To determine how many total electrons there are, add the amount of charge to the atomic number. In this case, there are fewer protons than electrons. For example, N 3- has a -3 charge which means it has 3 more electrons than a neutral nitrogen atom. Nitrogen’s atomic number is 7, therefore this ion has 10 electrons.
HOPE IT HELPS
HAVE A NICE DAY
Explanation:
Chlorofluorocarbons rise to the stratosphere and Multiple Choice interact with UV energy to produce free radicals that react with oxygen to create ozone. interact with UV energy to produce free radicals that destroy ozone. react directly with stratospheric ozone to destroy it. react with free radicals to remove carbon dioxide.
Answers
Chlorofluorocarbons rise to the stratosphere and interact with UV energy to produce free radicals that destroy ozone.
Many manufacturing processes by man are introducing chemicals which are harmful to human and his environment and a major reason for Ozone depletion.
One of such manufactured chemicals is the chlorofluorocarbons (CFCs) present in our refrigerators, CFCs comprises of atoms of chlorine, fluorine, and carbon.When these CFCs findt hier way into the atmosphere up to the stratosphere, they interact with the ultra violet Light which breaks them down into free radicals of chlorine. Chlorine then reacts with ozone and reduces it to oxygen gas. With continuous release into the stratosphere, the reaction keeps repeating itself, degrading the ozone layer.
See more here:https://brainly.com/question/18414838
What is the volume of 5.07 grams of copper? The density of copper is 8.96g/mL
Answers
Explanation
Given:
Mass of copper = 5.07 g
Density of copper = 8.96 g/mL = 8960 g/L
Requested: Volume of copper
Solution
p = m/V where p is the density, m is the mass and V is the volume
m = p x V
V = m/p
V = 5.07 g/8.96 g/mL
V = 0.566 mL
Answer
Volume of copper = 0.566 mL
Use your understanding of the ideal gas law to identify the correct relationships among the variables. Pressure is . Temperature is . Volume is . Moles areUse your understanding of the ideal gas law to identify the correct relationships among the variables. Pressure is . Temperature is . Volume is . Moles are
Answers
Answer:
Pressure is inversely proportional to the volume of a gas at constant temperature and number of moles
Temperature is directly proportional to volume at constant pressure and number of moles
Volume is directly proportional to the number of moles of a gas at constant temperature and pressure
Explanation:
The ideal gas law is derived from a combination of the Boyle's, Charles' and Avogadro's law of gases.
Boyle's law states that at constant temperature, the volume of a given mass of gas (number of moles of gas is constant) is inversely proportional to the pressure exerted by the gas.
V ∝ 1/P (at constant n, T)
Charles'law states that at constant pressure, the volume of a given mass of gas (constant amount, n) is directly proportional to the temperature of the gas; V ∝ T (at constant n, P)
Avogadro’s law states that at constant temperature and pressure, the volume of a given mass of gas is directly proportional to the number of moles of gas present; V ∝ n(at constant T, P)
Combining the three equations gives the following relationship: V ∝ nT/P
By inserting a proportionality constant called the molar gas constant, R, in the relationship above, the ideal gas equation is derived as follows:
V = nRT/P or PV = nRT
Answer:
the third one
Explanation:
if we used 8.7 g sunflower oil and recover 7.8 g fames, what is the approximate number of grams of linoleic acid methyl ester generated from this reaction? report your answer to the nearest tenth of a gram
Answers
As a consequence of the above reaction, probably 2.9 grams of an oleic acid the methyl ester was produced.
What constitutes the linoleic acids content of sunflower oil?Sunflower oil includes about 15% saturated fat, 85% unsaturated fat, and 14-43% oleic and 44-75% linoleic acids, respectively, in its of which are unsaturated concentration.
What exactly is FFA in sunflowers oil?Following neutralizing, the free saturated fat content in raw sunflower oil decreased form 1.1 down 0.24 (% The combination of o acid). The modified oil's residual FFA level were less than the highest specified amount of 0.25% in the Bsi norm for pressed processed sunflower seed oil.
To know more about methyl visit:
https://brainly.com/question/12484834
#SPJ1
Students conducted an analysis of baking soda in chemistry lab. During the activity, they had to calculate the mass in
grams of the baking soda being used. Some student data appears in the table. At the end of the activity, the teacher
told the students that the actual mass of baking soda was 0.45 grams. Which student had the most accurate and
precise data?
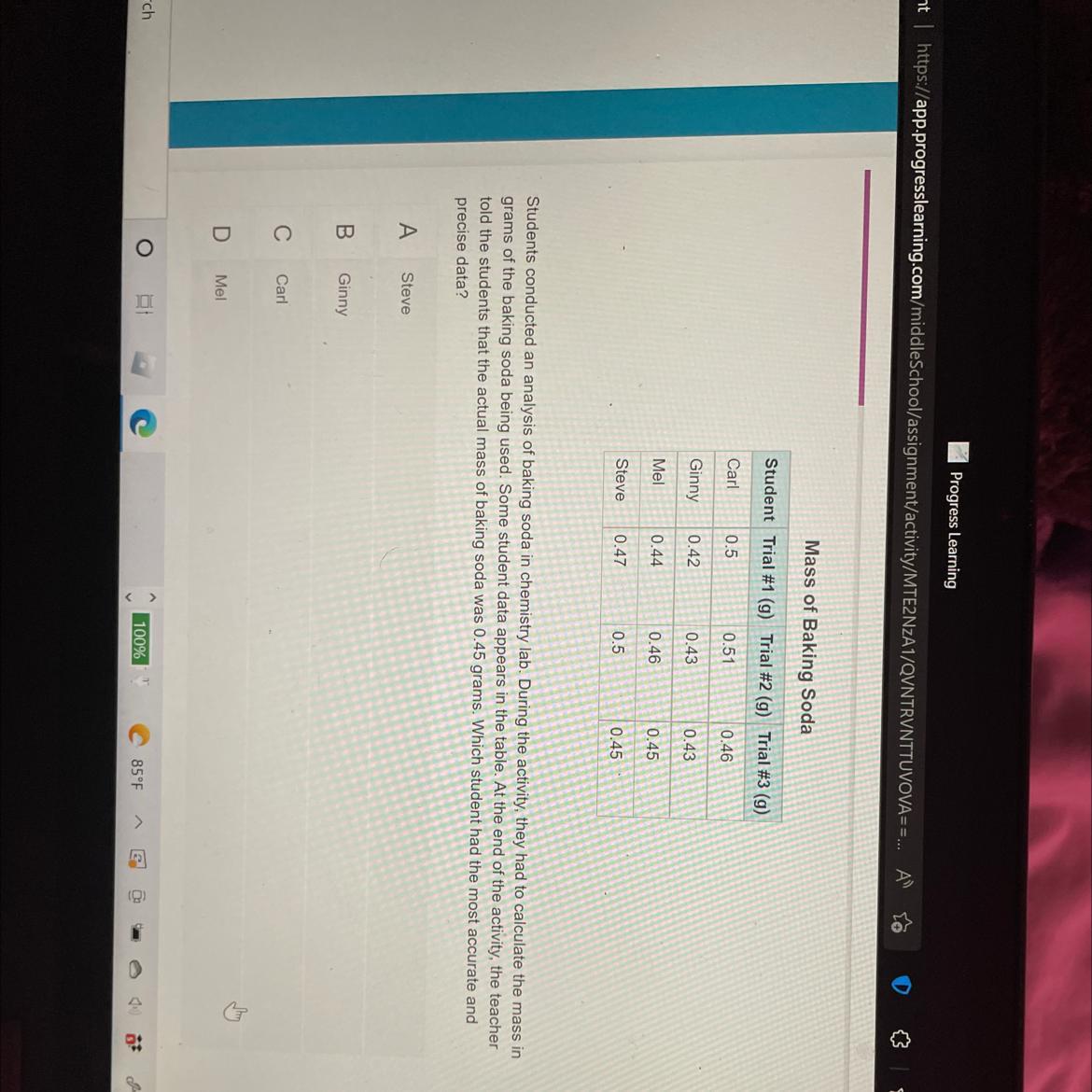
Answers
The student that had the most accurate and precise data is Mel (option D).
What is accuracy and precision?Accuracy is the degree of conformity of a measure to a true or standard value i.e. closeness to a standard or true value.
On the other hand, precision is the ability of a measurement to be reproduced consistently i.e. the closeness of measured values.
According to this question, students calculated the mass in grams of the baking soda being used.
It can be said that Mel, which measured the following values: 0.44g, 0.46g, 0.45g had the most accurate and precise value because they are both close to themselves and the true value.
Learn more about accuracy and precision at: https://brainly.com/question/15276983
#SPJ1
Answer:
D
Explanation:
Mel
I need help with this chemistry question.
If the percent by mass of carbon in sucrose is 42.2%, then how many grams of carbon are in a 30.0 g sample of sucrose?
_______________ g
Use the correct sigfigs in your answer or the computer will mark it incorrect
Answers
Answer:
12.66g
Explanation:
42.2% of 30.0g
=42.2/100 * 30.0
=0.422*30.0
12.66g
What would be the most profitable location for an ethanol manufacturing plant that converts corn into alcohol for use as an additive for gasoline?
Answers
Answer: ear a prime corn-producing area to minimize transportation costs of raw materials.
Explanat hope it helps
1d. draw a specific example (reactant, reagent and product) of the preparation of a lithium acetylide.
Answers
Lithium acetylide is an organic compound that is commonly used as a strong base in organic synthesis. It is prepared by the reaction of acetylene with lithium metal in an inert atmosphere. The reaction is exothermic and requires careful handling.
A specific example of the preparation of lithium acetylide can be illustrated by the reaction between acetylene and lithium in a dry tetrahydrofuran (THF) solvent. The reaction can be written as follows:
C₂H₂ + 2Li → Li₂C₂ + H₂
In this reaction, acetylene acts as the reactant, while lithium metal acts as the reagent. The product of the reaction is lithium acetylide, which is represented by the chemical formula Li₂C₂.
The reaction is usually carried out in an inert atmosphere, such as nitrogen or argon gas, to prevent the reaction of lithium with water or air. The solvent, THF, is used to dissolve the lithium acetylide product and to prevent the formation of side products.
The preparation of lithium acetylide is an important step in organic synthesis, as it can be used as a strong base for various reactions, such as alkylations, acylations, and reductions. The reactivity of lithium acetylide makes it a useful tool for organic chemists.
To know more about organic synthesis, refer to the link below:
https://brainly.com/question/30757552#
#SPJ11
Ozone (o3) in the atmosphere can be converted to oxygen gas by reaction with nitric oxide (no). Nitrogen dioxide is also produced in the reaction. What is the enthalpy change when 8. 50l of ozone at a pressure of 1. 00 atm and 25°c reacts with 12. 00 l of nitric oxide at the same initial pressure and temperature? [δh°f(no) = 90. 4 kj/mol; δh°f(no2) = 33. 85 kj/mol; δh°f(o3) = 142. 2 kj/mol]
Answers
The enthalpy change when 8.50 L of ozone at a pressure of 1.00 atm and 25°C reacts with 12.00 L of nitric oxide at the same initial pressure and temperature is -277.5 kJ/mol.
The enthalpy change when 8.50 L of ozone at a pressure of 1.00 atm and 25°C reacts with 12.00 L of nitric oxide at the same initial pressure and temperature can be calculated by the given equation. The balanced equation for the reaction is:2O3(g) + 2NO(g) → 2NO2(g) + 3O2(g)The enthalpy change for the given reaction can be determined using Hess’s law. Hess’s law states that the enthalpy change of a reaction is independent of the route taken, provided that the initial and final conditions are the same.
Since the given reaction can be expressed as a sum of a series of known reactions, Hess’s law can be used to calculate the enthalpy change.Using the given data, the enthalpy change for the reaction can be calculated as follows:δH° = 2 × [ΔH°f(NO2(g))] + 3 × [ΔH°f(O2(g))] - 2 × [ΔH°f(O3(g))] - 2 × [ΔH°f(NO(g))]δH° = 2 × [33.85 kJ/mol] + 3 × [0 kJ/mol] - 2 × [142.2 kJ/mol] - 2 × [90.4 kJ/mol]δH° = - 277.5 kJ/mol
To know more about enthalpy change visit:-
https://brainly.com/question/28039996
#SPJ11
9. a pot of water is placed on a burner on a gas stove to heat. which part is the source of the activation energy required (the flame/the water), and is this reaction endothermic or exothermic?
Answers
The part that is the source of the activation energy required is the flame and the reaction is endothermic.
Endothermic reactionsIn this scenario, the source of the activation energy required is the flame on the gas stove. The flame provides the heat energy necessary to increase the kinetic energy of the water molecules and cause them to move faster, resulting in an increase in the temperature of the water.
The process of heating the water is an endothermic reaction because energy is being absorbed from the surroundings (in this case, from the flame) in order to increase the temperature of the water.
As the water absorbs heat energy from the flame, the potential energy of the water molecules increases, causing them to move faster and collide more frequently, which leads to an increase in the temperature of the water.
More on endothermic reactions can be found here: https://brainly.com/question/23184814
#SPJ1
1. Which of the following statements is true about seeds?
a. Every plant produces seeds.
c. All seeds are good to eat.
b. All fruits contain many of seeds. d. all seeds has young plant, stored food and a seed coat.
2. Flowers cannot usually produce seeds unless
a. they are visited by insects.
c. they produce nectar.
b. they are on plants growing in good soil. d. the right pollen is placed on their stigmas.
3. Fertilization take place before a seed develops. Which statement describes when fertilization
occurs?
a pollen grain falls on a pistil
c. sperm in a pollen joins with an egg cell in an ovule
b. a pollen tube enters an ovary
d. an ovary becomes a fruit
4. Which of the following is NOT a factor needed by plants?
a. fire
b. nutrients
Please pasagot po ng tanong ko please
c. sunlight
d. water
5. Which is the reproduction in plants where the male and female parts of the flower are involved?
a. asexual
b. sexual
c. rhizome
d. pollination
Answers
Answer:
1 ans: d. all seeds has young plant, stored food and a seed coat.
2 ans: d. the right pollen is placed on their stigmas
3 ans: a. pollen grain falls on a pistil
4 ans: a. fire
5 ans: b. sexual
Hope it helps,
Please mark me as brainliest
Thank you...