4. Calculate the percent ionization of a formic acid solution having the given concentration. a. 1.00 M b. 0.500 M c. 0.100 M d. 0.0500 M
Answers
The percent ionization of a formic acid solution having the concentration (a) 1.00M is 1.37% ; (b) 0.500M is 3.23% ; (c)0.100M is 21.64% ; (d) 0.0500M is 58.73%
The percent ionization of a formic acid solution having a given concentration can be calculated using the formula : Percent ionization = [H+]/[HCOO-] × 100%
The formula of formic acid is HCOOH.
To calculate the percent ionization of a formic acid solution, it is necessary to use the Ka value of formic acid.
Ka for formic acid = 1.8 × 10^-4
Let's consider the reaction: HCOOH + H2O ⇋ H3O+ + HCOO-
Let the degree of ionization of formic acid be x.
Hence, [H+] = x M and [HCOO-] = x M
The concentration of unionized formic acid = (initial concentration - x) M
(a) 1.00 M :
Initial concentration = 1.00 M
Therefore, [HCOOH] = (1.00 - x) M
Ka = [H+][HCOO-]/[HCOOH]
Substituting the given values, we get, 1.8 × 10^-4 = x^2/(1.00 - x)
Thus, x = 0.0135 (Approx.)
Percent ionization = [H+]/[HCOO-] × 100%= x M/(1.00 - x) M × 100%
= 0.0135 M/0.9865 M × 100% = 1.37%
(b) 0.500 M :
Initial concentration = 0.500 M
Therefore, [HCOOH] = (0.500 - x) M
Ka = [H+][HCOO-]/[HCOOH]
Substituting the given values, we get, 1.8 × 10^-4 = x^2/(0.500 - x)
Thus, x = 0.0156 (Approx.)
Percent ionization = [H+]/[HCOO-] × 100%= x M/(0.500 - x) M × 100%
= 0.0156 M/0.4844 M × 100% = 3.23%
(c) 0.100 M :
Initial concentration = 0.100 M
Therefore, [HCOOH] = (0.100 - x) M
Ka = [H+][HCOO-]/[HCOOH]
Substituting the given values, we get, 1.8 × 10^-4 = x^2/(0.100 - x)
Thus, x = 0.0178 (Approx.)
Percent ionization = [H+]/[HCOO-] × 100% = x M/(0.100 - x) M × 100%
= 0.0178 M/0.0822 M × 100% = 21.64%
(d) 0.0500 M:
Initial concentration = 0.0500 M
Therefore, [HCOOH] = (0.0500 - x) M
Ka = [H+][HCOO-]/[HCOOH]
Substituting the given values, we get, 1.8 × 10^-4 = x^2/(0.0500 - x)
Thus, x = 0.0185 (Approx.)
Percent ionization = [H+]/[HCOO-] × 100% = x M/(0.0500 - x) M × 100%
= 0.0185 M/0.0315 M × 100% = 58.73%
Therefore, the percent ionization of a formic acid solution having the given concentrations are:
a. 1.00 M = 1.37%
b. 0.500 M = 3.23%
c. 0.100 M = 21.64%
d. 0.0500 M = 58.73%.
To learn more about percent ionization :
https://brainly.com/question/31358773
#SPJ11
Related Questions
is i-131 and i-133 isotopes of different elements?
Answers
Answer:
These two are nucleotides and isotopes of Iodine having atomic number 53.
Explanation:
Isotopes are the species of the same element having same number of protons and electrons but different number of neutrons.
Atomic number remains same but their atomic masses are different.
write a balanced chemical equation you explored in lab that describes the equilibrium between hexaaquocobalt(ii) and tetrachlorocobalt(ii) complex ions, in which the tetrachlorocobalt(ii) species is the product.
Answers
Co(H₂O)₆²⁺ (aq) + 4Cl⁻ (aq) ⇌ CoCl₄²⁻ (aq) + 6H₂O (l) is the balanced chemical equation of hexaaquocobalt(ii) and tetrachlorocobalt(ii).
The balanced chemical equation that describes the equilibrium between hexaaquocobalt(II) and tetrachlorocobalt(II) complex ions can be written as:
Co(H₂O)₆²⁺ (aq) + 4Cl⁻ (aq) ⇌ CoCl₄²⁻ (aq) + 6H₂O (l)
This equation shows that the hexaaquocobalt(II) ion (Co(H2O)6 2+) reacts with chloride ions (Cl-) to form tetrachlorocobalt(II) complex ion (CoCl4 2-) and water (H2O). The reaction is in a state of dynamic equilibrium, which means that the rates of the forward and reverse reactions are equal.
Learn more about The balanced chemical equation: https://brainly.com/question/29130807
#SPJ11
Calculate the total bond energy of H20. Show your work.
Answers
Cuáles son los efectos de la temperatura, la presión y el volumen en los cambios de estado de la materia. *???
Answers
Soryyyyyy I needed pointsss
15. why might doubling the number of moles of hcl decrease the rate of hcl production? select the acid convertase enzyme is converting hcl back into h and cl- select cannot be determined select no more h or cl- exists to be converted select the acid convertase enzyme has become inactive
Answers
When doubling the number of the moles of the HCl decrease the rate of HCl production because the acid convertase enzyme is converting HCl back into H⁺ and Cl⁻.
The reaction is as follows :
H⁺ + Cl⁻ ⇄ HCl
If we double the number of the moles of the HCl , it decreases the rate of the HCl production because of the reason that the acid convertase enzyme is converting the HCl back into the H⁺ and Cl⁻. The purpose of the enzyme is to allow the conversion of the reactant to the product and the product back to the reactant
To learn more about moles here
https://brainly.com/question/29724957
#SPJ4
What would you predict, the solubility of KHT (solid) in pure water compared with the solubility of KHT (solid) in a 0.1 M KCl solution, which one will be higher? Explain your answer.
Answers
The solubility of KHT (solid) in pure water compared with the solubility of KHT (solid) in a 0.1 M KCl solution is predicted to be higher in the 0.1 M KCl solution. This is because the KCl solution has a higher ionic strength, increasing the solubility of ionic compounds like KHT.
Let's understand this in detail:
What is solubility?
Solubility is defined as the ability of a substance to dissolve in a particular solvent under certain conditions. It measures the maximum amount of solute that can be dissolved in a given amount of solvent at a particular temperature, pressure, and other conditions.
Solubility of KHT in pure water:
KHT (Potassium hydrogen tartrate) is a weak acid salt that has low solubility in pure water. The solubility of KHT in pure water is affected by various factors such as temperature, pH, and pressure. The solubility of KHT in pure water is around 4.4 g/L at room temperature.
Solubility of KHT in 0.1 M KCl solution: The solubility of KHT in a 0.1 M KCl solution is predicted to be higher than in pure water. KCl is an ionic salt dissociating in water to produce K+ and Cl- ions. The presence of KCl increases the ionic strength of the solution. This ionic strength improves the solubility of other ionic compounds, such as KHT. KHT has a higher solubility in a 0.1 M KCl solution than in pure water due to this reason.
#SPJ11
Learn more about solubility: Explain how you would find the solubility of a solute https://brainly.com/question/23946616
each member of the following set of compounds is an alcohol; that is, each contains an (hydroxyl group, section 1.3a). which structural formulas represent the same compound? which represent constitutional isomers?
Answers
Constitutional isomerism is a type of isomerism in which molecules have the same atoms, but the order in which the atoms are bonded is different. They can have the same molecular formula but different functional groups
The members of the following set of compounds are all alcohols:
2-Butanol
3-Methyl-1-pentanol
2-Methyl-2-butanol
Pentan-1-ol
2-Methyl-1-butanol
1-Pentanol
Therefore, we must recognize the structural formula that represents the same compound and the one that represents constitutional isomers of each other.The constitutional isomers are
2-Methyl-1-butanol, 3-Methyl-1-pentanol, and 2-Methyl-2-butanol.
The following two pairs of alcohols represent the same compound:
2-Butanol and Pentan-1-ol.
Their structural formulas contain five carbon atoms.
1-Pentanol and 3-Methyl-1-pentanol. They contain five carbon atoms and are primary alcohols as well.Each alcohol has its own unique structural formula that separates it from other compounds. Isomers are compounds that have the same chemical formula but differ in structure, and this includes constitutional isomers.Therefore, the structural formulas that represent the same compound are Pentan-1-ol and 2-Butanol. The structural formulas that represent constitutional isomers are 2-Methyl-1-butanol, 3-Methyl-1-pentanol, and 2-Methyl-2-butanol.
Constitutional isomers are compounds that have the same number and kind of atoms, but the atoms are connected differently.
To know more about constitutional isomers visit:
https://brainly.com/question/30556576
#SPJ11
Which of the following molecules or ions will exhibit delocalized bonding?
O32-
903
SO2
SO32- only
SO2, SO3, and SO32-
SO2 and SO3
none of these will exhibit delocalized bonding.
SO3 and SO32-
Answers
Among the given options, SO2, SO3, and SO32- can exhibit delocalized bonding.
Delocalized bonding is a type of bonding where the electrons are not localized between two atoms but rather are shared among multiple atoms in a molecule. This type of bonding is characterized by the presence of resonance structures and is commonly observed in molecules that contain pi bonds.
SO2 has a bent structure with a lone pair of electrons on the sulfur atom. The double bond between sulfur and oxygen involves the overlap of a p orbital on sulfur and a p orbital on one of the oxygen atoms. This results in a pi bond and a sigma bond. The pi electrons are delocalized between the sulfur and oxygen atoms, and resonance structures can be drawn to show the delocalization.
SO3 has a trigonal planar structure with all three sulfur-oxygen bonds being identical. The sulfur atom in SO3 uses its three 3p orbitals to form three sigma bonds with the three oxygen atoms. The remaining 3p orbital on sulfur overlaps with the p orbitals on the three oxygen atoms to form three pi bonds. The pi electrons are delocalized over all three sulfur-oxygen bonds.
SO32- has a resonance structure in which the negative charge is delocalized over the sulfur and two oxygen atoms. The two sulfur-oxygen double bonds can be described as a combination of one strong sigma bond and one weaker pi bond, resulting in the delocalization of electrons over the sulfur-oxygen bonds.
In summary, SO2, SO3, and SO32- can exhibit delocalized bonding due to the presence of pi bonds and the ability to form resonance structures.
For more such questions on delocalized bonding
https://brainly.com/question/30218039
#SPJ11
2 NaN3 ---> 2 Na + 3 N2
1) Given 8.38 moles of N2, how many moles of NaN3 are needed?
a. 3.45 moles NaN3
b. 5.59 moles NaN3
c. 8.06 moles NaN3
d. 11.98 moles NaN3
Answers
in a compressional wave, when the particles are far apart, what is the part known as
Answers
Answer:
The answer is: "Rarefactions"
Explanation:
In a longitudinal wave, particles of the medium vibrate in a direction that is parallel to the direction that the wave travels. Places where particles of a medium crowd closer together are called compressions, and places where the particles spread farther apart are called rarefactions.
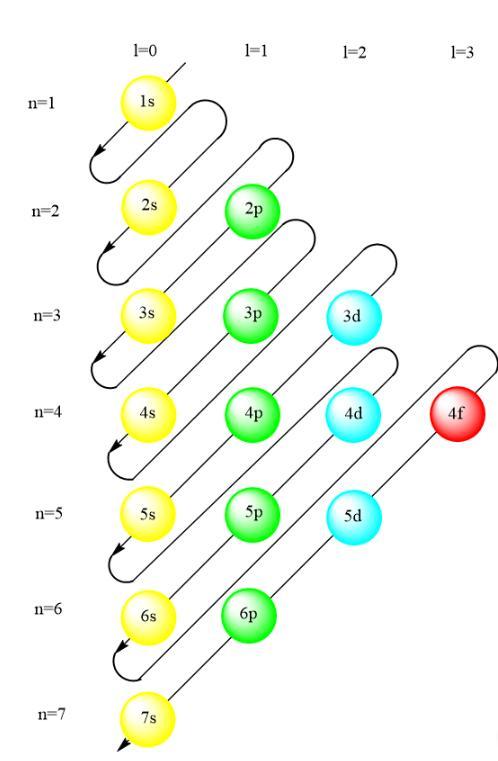
what is wrong with the notation 1s22s22p63s23p63d104s24p2 for germanium (atomic number 32)? why should the 4s subshell be filled before the 3d ?
Answers
The 4s orbital in the electronic configuration of a germanium should be filled first before 3d orbital because 4s orbital has a less energy than 3d orbital.
The electronic configuration of element is written in the order which is given in the attached image. The order of filling electron is given by Madelung rule in which electrons tries to achieve the order which fills the energy sublevels of atoms. According to the Aufbau principle, electrons occupy the lowest energy sublevel.
In other words, the orbital with a smaller value of n+l will be filled first. The n and l value for 4s orbital is 4 and 0 respectively. The n and l value for 3d orbital is 3 and 2. Hence, their n+l value will be
4s=(4+0)=4
3d=(3+2)=5
Hence, the electronic configuration of germanium should be written as 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰.
Therefore, 4s orbital in the electronic configuration of a germanium should be filled first before 3d orbital because 4s orbital has a less energy than 3d orbital.
To know more about electronic configuration
https://brainly.com/question/7466098
#SPJ1
what is the difference between hydrogen and oxygen? give at least 3 or more answers
Answers
Answer:
Hydrogen v.s. Oxygen
Explanation:
Hydrogen is a Light gas and Oxygen is a heavy gasAn Oxygen atom is bigger than a Hydrogen atom Hydrogen is Explosive Oxygen is necessary for combustionHope this helps <3
SCIENCE
why is it important to distinguish the two types of crust?
Answers
Answer:
It is important to identify it because the two types of crust are made up of two different types of rock
Explanation:
Have a nice day
An elephant and a parakeet standing on ledge will have a different amount of potential energy because of their . this is for science actually
Answers
Answer:
Mass
Explanation:
Based on their number of valence electrons, which group of elements will gain two electrons by bonding with other atoms?
Question 1 options:
F, Cl, Br, I, At
N, P, As, Sb, Bi
O, S, Se, Te, Po
Be, Mg, Ca, Sr, Ba
Answers
Answer:
O, S, Se, Te, Po
Explanation:
O, S, Se, Te, Po are all elements found in group sixteen of the periodic table. Remember that the commonality between all the elements in the same group of the periodic table is that they all possess the same number of outermost electrons. All elements in the same group must have the same number of outermost electrons in their outermost shell.
Now, having six electrons in their outermost shell implies that they readily bond with two other atoms to complete their octet. In accordance with the octet rule. This explains why oxygen bonds with calcium by gaining two electrons in calcium oxide.
An atom has 8 protons, 9 Neutrons, and 10 electrons. What is the mass number?
Answers
Answer:
17
Explanation:
Mass number = # of protons + # of neutrons
Mass number is calculated using ONLY protons and neutrons. Electrons are not in the count.
Thus, we simply add them together: 8 + 9 = 17
This element is Oxygen - 17.
a chemist must prepare of aqueous silver(ii) oxide working solution. she'll do this by pouring out some aqueous silver(ii) oxide stock solution into a graduated cylinder and diluting it with distilled water. calculate the volume in of the silver(ii) oxide stock solution that the chemist should pour out. round your answer to significant digits.
Answers
The chemist should pour out 10 mL of the silver(ii) oxide stock solution into a graduated cylinder and dilute it with distilled water to prepare a 100 mL aqueous silver(ii) oxide working solution with a concentration of 0.01 M.
To calculate the volume of the silver(ii) oxide stock solution that the chemist should pour out, we need to use the dilution equation:
C1V1 = C2V2
where C1 is the concentration of the stock solution, V1 is the volume of the stock solution to be poured out, C2 is the desired concentration of the working solution, and V2 is the final volume of the working solution.
Let's assume that the concentration of the silver(ii) oxide stock solution is 0.1 M and the desired concentration of the working solution is 0.01 M. We also need to know the final volume of the working solution, which is not given in the question. Let's assume that the chemist wants to prepare 100 mL of the working solution.
Substituting the values in the dilution equation, we get:
0.1 M x V1 = 0.01 M x 100 mL
Solving for V1, we get:
V1 = (0.01 M x 100 mL) / 0.1 M
V1 = 10 mL
Therefore, the chemist should pour out 10 mL of the silver(ii) oxide stock solution into a graduated cylinder and dilute it with distilled water to prepare a 100 mL aqueous silver(ii) oxide working solution with a concentration of 0.01 M. This calculation assumes that the chemist has a silver(ii) oxide stock solution with a known concentration and that she wants to prepare a working solution with a lower concentration.
for more such question on stock solution
https://brainly.com/question/24697661
#SPJ11
Match each term below with its definition or description.
1 The point in a titration when the added amount of standard reagent is equal to the amount of analyte being titrated.
2 The analyte is titrated with the standard reagent and the volume of standard solution required to complete the reaction is measured.
3 A reagent that is pure and stable, which can be used directly after weighing.
4 The analyte that is being analyzed in the titration.
5 Standard reagent is added in excess to ensure complete reaction with the analyte. The excess reagent is then titrated with a second standard reagent.
6 A solution, whose concentration is known, often made from a reagent of known purity.
7 The standard reagent of known concentration that is added from a buret to the analyte solution.
8 The analyte does not react directly with the titrant so it is converted to another form which will react with the titrant.
9 The point in a titration when a change in the analyte solution is observed, indicating equivalency.
10 It is added to the analyte solution and aids in the observation of the completion of the reaction.
a) End Point
b) Indicator
c) Direct Titration
d) Back Titration
e) Indirect Titration
f) Primary Standard
g) Standard Solution
h) Titrand
i) Equivalence Point
j) Titrant
Answers
Answer:
1. Equivalence point
2. Direct titration
3. Primary standard
4. Titrand
5. Back titration
6. Standard solution
7. Titrant
8. Indirect titration
9. End point
10. Indicator
Explanation:
1. The equivalence point is the tiration point at which the quantity or moles of the added titrant is sufficient or equal to the quantity or moles of the analyte for the neutralization of the solution of the analyte.
2. Direct titration is a method of quantitatively determining the contents of a substance
3. A primary standard is an easily weigh-able representative of the mount of moles contained in a substance
4. A titrand is the substance of unknown concentration which is to be determined
5. The titration method that uses a given amount of an excess reagent to determine the concentration of an analyte is known as back titration
6. A standard solution is a solution of accurately known concentration
7. A titrant is a solution that has a known concentration and which is titrated unto another solution to determine the concentration of the second solution
8. Indirect titration is the process of performing a titration in athe reverse order
9. The end point is the point at which the indicator indicates that the equivalent quantities of the reagents required for a complete reaction has been added
10 An indicator is a compound used to visually determine the pH of a solution.
The titration has been the neutralization reaction in which the titrand and the titrant react to form the salt and the water and help in the determination of the qualitative and quantitative properties.
What is an Endpoint?In a titration reaction, the endpoint has been the point at which the equivalent amount of reagent has been completely neutralized.The Indicator has been the chemical that changes to indicate the endpoint of the reaction.Direct titration involves the reaction for the quantitative determination of the substances.The back titration can be given as the reaction in which the excess reagent is used to titrate the second standard reagent in the reaction.Indirect titration can be given as the reaction of the analyte to convert to another form and then the analysis with the titrant.The primary standard has been the known concentration of the pure and stable weighing reagent.The standard solution has been the solution of the known concentration in the reaction.Titrand has been the unknown sample that has to be analyzed.The equivalence point is the concentration point at which the quantity of titrant added to the titrand has been equal.Titrant has been the known concentration of sample that has been added to equivalent the unknown sample.Learn more about titration here:
https://brainly.com/question/24704707
why do water molecules have a stronger attraction than helium?
answer needed before 3:00 June 2nd 2023
Answers
Water molecules have a stronger attraction than helium due to the presence of dipole-dipole interactions resulting from the polarity of the water molecule.
Water molecules have a stronger attraction than helium due to the difference in their intermolecular forces. Intermolecular forces are the attractive forces that exist between molecules and play a crucial role in determining the physical properties of substances.
Water molecules have a polar nature, meaning they have a partial positive charge on the hydrogen atoms and a partial negative charge on the oxygen atom.
This polarity arises from the unequal sharing of electrons in the O-H bonds due to oxygen's higher electronegativity compared to hydrogen. The presence of polar bonds within the water molecule gives rise to a dipole-dipole interaction.
In contrast, helium is a noble gas and exists as individual atoms. Helium atoms are electrically neutral and do not possess a permanent dipole moment.
As a result, helium exhibits weak intermolecular forces known as London dispersion forces or Van der Waals forces. These forces arise due to temporary fluctuations in electron distribution, causing temporary dipoles that induce dipoles in neighboring atoms or molecules.
The dipole-dipole interaction in water is stronger than the London dispersion forces in helium. This is because dipole-dipole forces are more significant when there are permanent dipoles in the molecules.
The stronger attraction between water molecules leads to higher boiling and melting points compared to helium.
For more such question on molecules. visit :
https://brainly.com/question/24191825
#SPJ8
I do believe i need help.....


Answers
Answer:
C,D, and A I believe
Explanation:
Developing Scientific knowledge
Fill in each blank with the word that best completes the statement
Scientists develop knowledge by making
about the natural world that may lead to a
scientific question
A scientific question may lead to an)
which can be tested
The results of
can lead to changes in scientific knowledge.

Answers
Answer:
Scientists develop knowledge by making observation about the natural world that may lead to a scientific question.
A scientific question may lead to a(n) hypothesis which can be tested.
The results of experimentation can lead to changes in scientific knowledge.
Explanation:
In the process of developing scientific knowledge, scientists carry out series of observations on what happens in the natural world. This helps them to develop scientific questions. These scientific questions drawn can then lead to hypotheses which are testable.
The testing process leads to experimentation. It is in this process that much scientific questions receive answers. Testing actually lies at the core of scientific inquiry for all scientists. Whatever hypothesis made must always be tested.
The result of the testing or experimentation brings changes in scientific knowledge.
When rocks break down or decompose, they can form
A.
soil.
B.
magma.
C.
bigger rocks.
D.
lava.
Answers
Answer:
C
Explanation:
because when rocks break down they can form and once they form they can make more and even bigger rocks hopes this helps.
Having problems figuring out molar mass in general. steps would be most appreciated.
Answers
Answer:
The periodic table
Explanation:
As you can see in the screenshot. Each element on the periodic table has an atomic number and a mass number. The atomic numbers are arranged in numerical order from right to left in the periodic table. The second number there is the atomic mass.
When doing stoichiometric ratios, you need to use the atomic mass of each element to determine the molecular/molar mass.
example. Find the molar mass of
H₂SO₄
Hydrogen (2) + sulphur (1) +oxygen (4)
= atomic mass of H(2)+ atomic mass S(1)+ atomic mass oxygen(4)
=1(2) + 32(1)+16(4)
=2+32+64
=98
N.B We do not use the co-efficient when looking for molar mass, we only use the atom and subscript
Review the poster.
https://www.cdc.gov/handwashing/pdf/wash-your-hands-fact-sheet-508.pdf
What is the greatest advantage of using a poster to present the information in “Stop Germs! Wash Your Hands”?
A poster that features brief sentences conveys urgency and simplifies content.
The extensive detail in posters captures viewers and causes them to stop and read.
The colors and images of a poster often convey a stronger message than words.
A poster appeals to several senses at once, which makes it hard to ignore.
Answers
A. A poster that features brief sentences conveys urgency and simplifies content.
Calculate the mole fraction of ki in a solution made by dissolving 3. 4 g of ki in 5. 8 g of water.
Answers
The mole fraction of ki in a solution made by dissolving 3. 4 g of ki in 5. 8 g of water: 0.060.
Mole fraction = Moles of componentMoles of all species in solution. And thus, Mole fraction, KI = Moles of KIMoles of KI + moles of water.
The mole fraction may be calculated by using dividing the wide variety of moles of one issue of an answer by means of the overall variety of moles of all the additives of an answer. it is mentioned that the sum of the mole fraction of all the additives inside the answer needs to be identical to at least one. Mole percentage ( ) is the same as a mole fraction in chemistry, however in a special guise. Multiplying the mole fraction through one hundred yields the mole percent of the given component. Mole Fraction = the variety of moles of 1 aspect in the given combination and the overall quantity of moles in the combination.
A mole fraction is a unit of attention, defined to be the same as the number of moles of an issue divided by means of the whole number of moles of a solution. because it's far a ratio, the mole fraction is a unitless expression.
Learn more about mole fraction here:
https://brainly.com/question/14783710
#SPJ4
Geothermal energy is produced when water touches _______________.
A oil
B hot rocks
C biomass
Answers
Answer: Hot Rocks
Explanation:
which example is nonpolar? a. a negative ion b. a neutral ion c. a positive ion d. a molecule with no partial charges
Answers
The example that is nonpolar is d. a molecule with no partial charges.
When the charges of the molecule are symmetrical and there are no partial charges, it indicates that the molecule is nonpolar.
Polar molecules have partial positive and negative charges on either end of the molecule.
This occurs as a result of the polarity of the molecule, which is created by the difference in electronegativity between the two atoms forming the bond.
The charge distribution on the molecule is unbalanced due to this polarity, with the electron density more concentrated around the more electronegative atom.
The measurement of the polarity of a molecule is based on the difference in electronegativity between the two atoms forming the bond.
The polarity of a molecule can be determined using various methods, including the dipole moment method, which measures the magnitude of the dipole moment of the molecule.
The dipole moment measures the charge distribution in the molecule and is measured in Debye (D) units, where 1 D = 3.336 × 10-30 Cm.
In conclusion, a molecule with no partial charges is nonpolar.
The other options such as a negative ion, a neutral ion, and a positive ion are polar molecules as they have partial charges on either end of the molecule.
To know more about Polar molecules, visit:
https://brainly.com/question/31023968
#SPJ11
A balloon at sea level on earth (1 atm pressure, 19°C) takes up 14.5 L of space. The balloon travels to Mars where atmospheric pressure is 4.55 torr and the temperature is -55°C What is the volume of the balloon on Mars?
Answers
Answer:
1807.24L
Explanation:
Using combined gas law equation:
P1V1/T1 = P2V2/T2
Where;
P1 = pressure on Earth
P2 = Pressure on Mars
V1 = volume on Earth
V2 = volume on Mars
T1 = temperature on Earth
T2 = temperature on Mars
According to the information provided of the balloon in this question;
P1 = 1 atm
P2 = 4.55 torr = 4.55/760 = 0.00599atm
V1 = 14.5L
V2 = ?
T1 = 19°C = 19 + 273 = 292K
T2 = -55°C = -55 + 273 = 218K
Using P1V1/T1 = P2V2/T2
1 × 14.5/292 = 0.00599 × V2/218
14.5/292 = 0.00599V2/218
Cross multiply
14.5 × 218 = 292 × 0.00599V2
3161 = 1.74908V2
V2 = 3161 ÷ 1.74908
V2 = 1807.24L
a student obtained a wet burette from the cart but failed to rinse it with a small amount of the base before starting a titration. will more or less titrant (base) be required to neutralize the acid?
Answers
The student failed to rinse a wet burette with a small amount of base before starting a titration. The student would need more titrant (base) to neutralize the acid than if they had rinsed the burette before starting the titration.
The reason for this is that when a wet burette is not rinsed with the titrant, the remaining water in the burette can dilute the titrant, thereby decreasing its concentration. If the titrant is diluted, more of it would be required to neutralize the same amount of acid. This would result in a titration that requires more titrant (base) to neutralize the acid than if the burette had been properly rinsed with the base.
In other words, not rinsing the burette with a small amount of base can affect the accuracy of the titration results. It is, therefore, important to properly rinse the burette with the titrant before starting a titration to avoid diluting the titrant and ensure accurate titration results.
In conclusion, more titrant (base) would be required to neutralize the acid if a wet burette is not rinsed with a small amount of base before starting a titration. It is essential to rinse the burette before starting a titration to avoid dilution of the titrant and ensure accurate titration results.
for more such question on titration
https://brainly.com/question/186765
#SPJ11
List two ways in which enzymatic browning can be prevented?
Answers
Adding citric and irradiation
Hope it helps.