5
volume (cm³)
1. Based on this graph, how does metal B differ from metal A?
2. What is the density of metal B? Show all your work and include appropriate units.
3. What is the mass of 9.0 cm³ of metal B? Find this in two different ways.
a. Mark on the above graph how you might determine this.
b. Show how you could calculate this mathematically.

Answers
Density is an intensive property as it does not depend on the quantity of the substances Whereas mass and volume are extensive property. Therefore, Metal A is lesser denser than metal B.
What is density?Density tells about the compactness of the substances, how much dense is the substances in other words. Object that is more denser than water they just sink in the water.
Mathematically,
Density = Mass of the metal ball ÷volume marked on the graduated cylinder
Since mass of metal a and metal B is same, so now density depend on volume. Volume of metal A is higher than metal B. From formula we can see density is inversely proportional to volume. So, Metal A is lesser denser than metal B.
Therefore, Metal A is lesser denser than metal B.
To know more about density, here:
https://brainly.com/question/16894337
#SPJ1
Related Questions
Why are sodium (Na) and potassium (K) in the same group on the periodic table?
A They are both hard and brittle.
B They have similar reactivity.
C They are both colorless.
D They have similar atomic weights.
Answers
Sodium (Na) and potassium (K) in the same group on the periodic table because of option B They have similar reactivity.
The periodic table, also called the periodic table of the factors, is a tabular show of the chemical elements. it is broadly used in chemistry, physics, and other sciences, and is typically visible as an icon of chemistry.
Lithium, sodium, and potassium have the same wide variety of valence electrons i.e. 1, for this reason, they belong to the same organization. The institution is referred to as the alkali metals group because these kinds of elements shape oxides that dissolve in water to form alkali.
Potassium is observed clearly in lots of meals and as a complement. Its major position within the body is to assist maintain ordinary levels of fluid inside our cells. Sodium, its counterpart, keeps regular fluid ranges outdoors of cells. Potassium also helps muscles to settle and supports normal blood pressure.
Learn more about the periodic table here:-https://brainly.com/question/15987580
#SPJ1
PLEASE HELP THIS IS SCIENCE
ILL MARK U BRAINLIEST
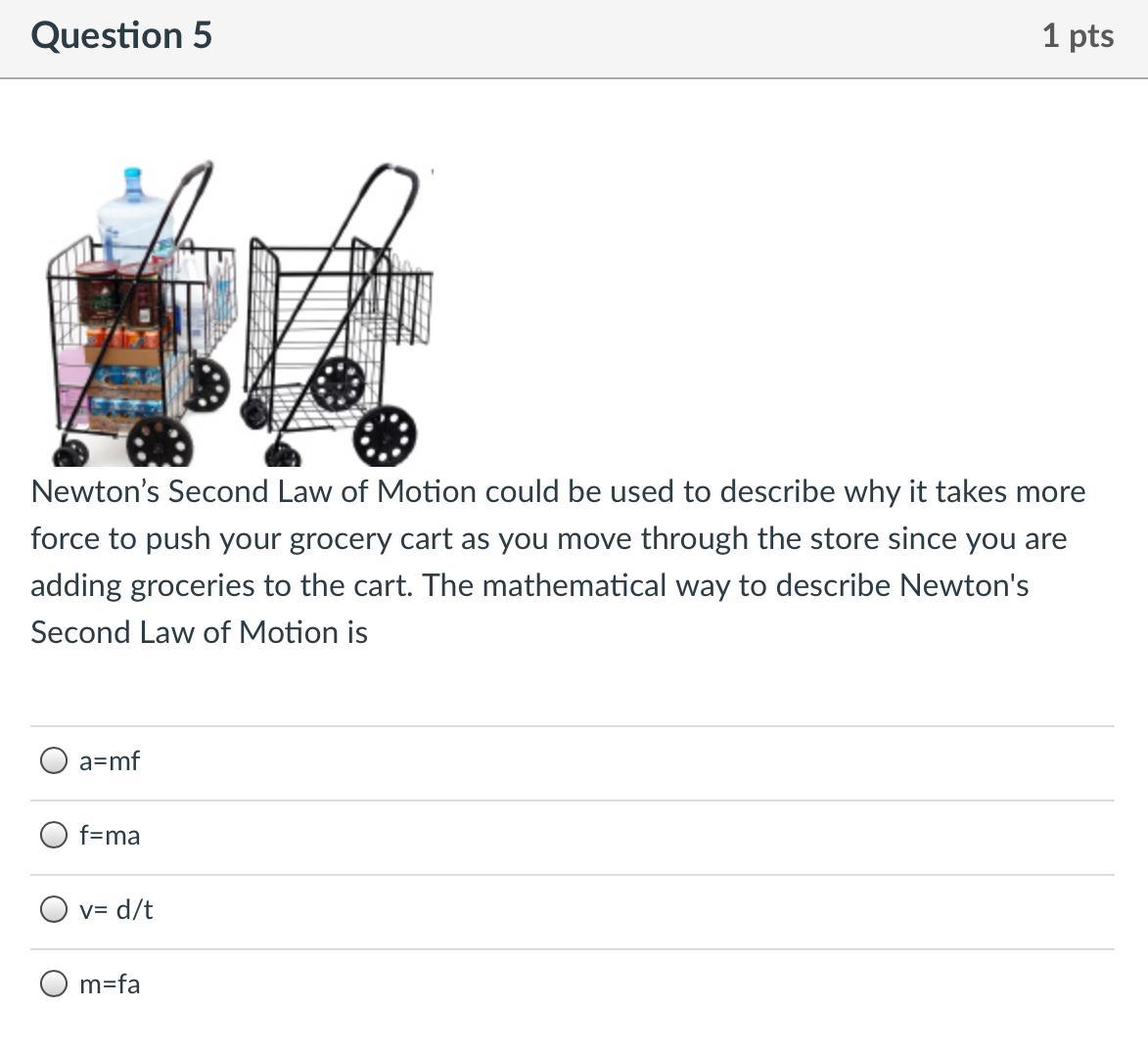
Answers
Answer: f=ma
Explanation:
What is inertia?
the motion of an object
a force that acts on an object at rest
an object’s resistance to a change its motion
the speed and direction of an object in motion
Answers
Inertia of an object is its tendency to stay in its current state. Hence it is the resistance to change its motion. Thus, option c is correct.
What is Newton's law of motion ?According to Newtons; first law of motion every object tend to continue in the state of rest or motion until an external force act on it. This tendency is called inertia.
The impacts of inertia can be well explained based on on real life examples. If we might had the experience of moving to front in a vehicle which suddenly breakes. It is the tendency of the vehicle to stay in motion.
Similarly the car when started from rest initially tends to stay at rest and that's why we feel it moves back ward. Similarly the car will move forward when it is suddenly stops. Hence, option c is correct.
Find more on more inertia:
brainly.com/question/3268780
#SPJ1
On the left a Circuit A, which is a square box. The top, left, and right sides have circles with X's in them. The bottom side has a stack of vertical lines, which are from left to right very short, short, very short, short. On the right a Circuit B, which is a square box. 2 extra lines cross the box near its top, parallel with the top side. The top side and the 2 extra lines have circles with X's in them. The bottom side has a stack of vertical lines, which are from left to right very short, short, very short, short.
Use the diagram and drop-down menus to answer each question.
Which circuit is a parallel circuit?
Which circuit is a series circuit?
In which circuit do the light bulbs all shine at their maximum brightness?
Answers
Answer:
B A B sorry if its wrong
Explanation:
Circuits can be connected in series or parallel but the maximum voltage is obtained in series connection, thus, light bulbs shine brightest in series connection.
What is parallel and series connection in a circuit?A parallel circuit connection is one in which the terminals of all the components in th circuit meet at two points.
The components are parallel to each other and current flows in multiple directions.
A series connection in a circuit is one in which the terminals of the component parts in a circuit are joined end to end. Current flows in one direction.
The voltage in a series connection is the sum of all the individual cells, therefore, the light bulb in series connection shines brightest.
Learn more about parallel and series circuits at: https://brainly.com/question/1122566
#SPJ2
Two identical blocks are heated to different temperatures. The blocks are placed so that they touch, and heat begins to flow between the blocks. The pair of blocks is insulated, so no energy escapes. Later, the temperature of each block is measured again. Which pair of temperatures is possible.
PLEASE HURRY.

Answers
When two items of different temperatures come into touch with one other, energy is transferred from the hotter (higher temperature) object to the colder (lower temperature) object until both objects reach the same temperature.
What is the term for when two materials that are in contact transfer heat until they achieve the same temperature?Conduction occurs when two materials or objects come into direct touch with one another. The warmer object's molecules vibrate quicker than the cooler object's. The molecules that vibrate faster clash with the molecules that vibrate slower. This causes the colder molecules to vibrate faster, warming the item.
This signifies that the substance's constituent material is a third component.
learn more about Conduction
https://brainly.com/question/893656
#SPJ1
g an enzyme is operating at its optimum ph. if the ph were increased, how would the rate of the enzyme-catalyzed reaction change? a. decrease b. would not change c. increase d. could increase or decrease
Answers
If an enzyme is operating at its optimum pH and the pH was increased, the rate of the enzyme-catalyzed reaction would likely decrease (option A).
This is because enzymes have an optimal pH range for their activity, and deviations from this range can cause denaturation or reduced efficiency of the enzyme.
Enzymes are sensitive to pH and their activity can be affected by changes in the pH of the environment. Each enzyme has an optimal pH range in which it functions most efficiently, and any deviation from this range can lead to a decrease in its activity. The optimal pH range for enzymes is usually between pH 6 and 8, which is close to the pH range found in most living organisms.
If the pH is too low or too high, the enzyme may become denatured, which means that its three-dimensional structure is altered and it loses its ability to bind to its substrate. This can result in a decrease in enzymatic activity and ultimately lead to a loss of function.
Learn more about enzyme : https://brainly.com/question/1596855
#SPJ11
What is the molarity of 1. 5 liters of an aqueous solution that contains 52 grams of lithium fluoride.
Answers
Answer:
1.3 M
Explanation:
The formula mass of lithium fluoride is 7+19=26 g/mol. This means that in 52 grams of LiF, there is 2 moles.
Molarity = (moles of solute)/(liters of solution), so the answer is 2/1.5 = 1.3 M
To calculate the Ksp value in the presence of ion activity, it is necessary to measure the ion product at the point of saturation for multiple . The ion product nears the Ksp value at Choose... Choose... concentrations is finally used to determine the Ksp Value. due to lower ionic strer temperatures compounds
Answers
To calculate the Ksp value in the presence of ion activity, it is necessary to measure the ion product at the point of saturation for multiple concentrations.
The ion product nears the Ksp value at higher concentrations, and this value is finally used to determine the Ksp value. This is because at higher concentrations, there is a lower ionic strength and lower temperatures which promote the formation of solid compounds and increase the solubility of the ions.
To know more about Ksp value click this link-
brainly.com/question/3162744
#SPJ11
How does density affect the water cycle
Answers
Have a nice day :)
Explanation:
Biological in the ocean is affected by the water cycle via the Mixed Layer Depth and Run off from land. Salinity and temperature determine the density of ocean water, and density influences the circulation. E-P determines surface salinity of the ocean, which helps determine the stability of the water column.
A silver ring reacts with compounds containing sulfur in the air to form silver sulfide, a black substance that makes up the tarnish on the surface of silver objects. To remove the tarnish from the ring, students placed it in a pan lined with aluminum foil and added hot water. Baking soda was added to the hot water and stirred. Students made observations about the process. Which observation of this process provides evidence of a chemical reaction?
Question 4 options:
A Hot water heated the aluminum foil.
B The liquid solution changed color.
C The pan was lined with aluminum foil.
D The hot water cooled.
Answers
Answer:
c lined with aluminum foil
9. Describe two ways in which you can conserve and recycle lab resources.
I
Answers
Answer:
Using solar energy, hydroelectric energy and wind are major ways of conserving natural resources. Trees and other organic energy sources are traditional sources of renewable energy. Forests can be replanted and hemp and other organic materials such as ethanol can be used to preserve natural resources.
explain why is energy input required to add an electron to zinc
Answers
Answer: When you add an electron to zinc, it needs some extra energy. This is because zinc atoms naturally don't like having an extra electron. The extra electron and the electrons already present in zinc repel each other due to their negative charges. So, you have to give some energy to the zinc atom to overcome this repulsion and make it accept the additional electron. Basically, energy input is required to make zinc accept an extra electron because the electron doesn't fit easily and needs some force to be added.
Explanation: hope this helps
How does the mass of each atom compare to the average atomic mass of the
element given in the periodic table? *
Answers
The mass given in the periodic table is a weighted average of all isotopes of an element. For example Hydrogen is normally just one proton so one would expect its atomic mass to be exactly 1, but there are other naturally occurring isotopes of H (deuterium with a mass of 2 and tritium with a mass of 3) that have more neutrons resulting in a weighted average slightly greater than 1.
The mass of the atoms is given by the number of neutrons and protons. The mass of the atom and the average mass of the element looks a little different due to mass defect.
What is a mass defect?The mass defect has been the difference in the mass and the average mass of the elemental atom. It has been the energy that binds the neutrons and the protons together in the nucleus. The mass and the average atomic mass differ slightly.
The mass of an individual atom differs from the average atomic mass as average atomic mass is the sum of the masses of the isotope of the elements. Isotopes have little difference in masses due to the number of neutrons. For example mass and average atomic mass of hydrogen differs.
Therefore, the mass and the average atomic mass differ.
Learn more about the mass defects, here:
https://brainly.com/question/16485729
#SPJ2
What is the volume of 0.150 M sodium hydroxide, required to react completely with 250.0 mL of 0.545 M acetic acid, CH3COOH solution?
Answers
Answer:
68.81ml
Explanation:
Using the formula:
CaVa = CbVb
Where;
Ca = concentration of acid (M)
Cb = concentration of base (M)
Va = volume of acid (ml)
Vb = volume of base (ml)
Based on the information given in this question;
Ca = 0.545M
Cb = 0.150M
Va = 250ml
Vb = ?
Using CaVa = CbVb
0.545/250 = 0.150/Vb
Cross multiply
250 × 0.150 = 0.545Vb
37.5 = 0.545Vb
Vb = 37.5/0.545
Vb = 68.81ml
On the fictional planet Castiana, a somewhat fictional element with an atomic mass of 121.764 a.m.u. has two naturally occuring isotopes, with atomic masses of 120.905 and 122.921. What is the percent abundance of the isotope whose atomic mass is 120.905?
Answers
The percent abundance of the isotope with an atomic mass of 120.905 is approximately XX%.
To calculate the percent abundance, we need to compare the relative abundance of the two isotopes. Given that there are two isotopes with atomic masses of 120.905 and 122.921, we can assume that the sum of their percent abundances is 100%.
Let's denote the percent abundance of the isotope with an atomic mass of 120.905 as "x". The percent abundance of the other isotope (with atomic mass 122.921) can then be expressed as (100 - x).
Since the atomic mass is directly proportional to the percent abundance, we can set up the following equation:
(120.905 * x) + (122.921 * (100 - x)) = 121.764
Solving this equation will give us the value of x, which represents the percent abundance of the isotope with an atomic mass of 120.905. Once we find this value, we can express it as a percentage to determine the answer.
Learn more about Percent abundance
brainly.com/question/1594226?
#SPJ11
Again I am here with privious question
I have post this question last time also but no one answers. So can you solve this. I need it.
I will support your account

Answers
Answer:
The answer is below
Explanation:
1. Fractional Distillation
2. Simple Distillation
3. Filtration
4. Steam distillation or lemon grass oil extraction
What name is given to a metal when it forms an ion, and what type of charge does it have?
Answers
Answer:
Cation metal. Positive charge.
Answer:
DHS has been working hard for years and it is still unclear whether this would happen if it is to end the market with the engine is a little less than the next 5years to be 8years in the first quarter and the same year in a quarter or less than a quarter or more than that
what is a simple explanation of electrolysis??? :)
Answers
Answer:
electrolysis is the process by which electric current is passed through a substance to effect a chemical change.
Answer:
The production of a chemical reaction by passing an electric current through an electrolyte is called electrolysis. We know that an electrolyte contains ions, which are charged. The positively charged ions are called cations, because they are attracted to the cathode, and the negatively charged ones are called anions because they are attracted to the anode. We know that unlike charges attract and like charges repel. Cations, being positively charged, get attracted to the negatively charged cathode and move toward it. Anions, being negatively charged, get attracted to the positively charged anode and move toward it. This explains how ions move in an electrolytic cell, and thus ‘conduct’ an electric current. A chemical reaction takes place at the anode and the cathode. This can be observed as the formation of bubbles (due to the production of gases) or deposition of metal on the electrodes or a change in the color of the electrolyte. The reaction varies depending on the metals used for the electrodes and the electrolyte chosen. Electrolysis of a solution of sodium chloride (NaCl) produces hydrogen gas (H2), chlorine gas (Cl2), and sodium hydroxide (NaOFI).
The pictures of electrolysis examples are shown below:


A flexible container has 5.00 L of nitrogen gas at 298 K. If the temperature is increased to 333 K, what will be the new volume of that sample of nitrogen?
0.179 L
0.223 L
4.47 L
5.59 L
Answers
Answer:
5.59 L
Points earned on this question: 4
Explanation:
took the test
The new volume of the sample of gas when the temperature is increased from 298 K to 333 K is 5.59 L
Charles' lawCharles' law states as follow:
V₁ / T₁ = V₂ / T₂
Where
V₁ is the initial volume T₁ is the initial temperature V₂ is the new volume T₂ is the new temperature How to determine the new volumeFrom the question given above, the following data were obtained
Initial volume (V₁) = 5 LInitial temperature (T₁) = 298 KNew temperature (T₂) = 333 KNew Volume (V₂) =?V₁ / T₁ = V₂ / T₂
5 / 298 = V₂ / 333
Cross multiply
298 × V₂ = 5 × 333
Divide both side by 298
V₂ = (5 × 333) / 298
V₂ = 5.59 L
Learn more about gas laws:
https://brainly.com/question/6844441
What does this picture represent?
А. 0
Hot Plate
B.
Graduated cylinder
С.
Goggles
D.
Electronic Balance

Answers
the answer is d electronic balance
What is the charge of Cl2?
Answers
Answer:
Cl2 does not have any charge
Explanation:
But when it is present in its ionic form then chlorine have -1 charge on it. But charge on chlorine varies from -1 to +7
What molecule is butane
Answers
Butane is a gaseous molecule which is part of the organic compounds called hydrocarbon,
What kind of molecule is butane?Butane is a gaseous molecule which is generally called a hydrocarbon.
Hydrocarbons are composed of only carbon and hydrogen. They are of organic origin.
Butane is a low molecular weight hydrocarbon which is used as a fuel.
In conclusion, butane is a gaseous hydrocarbon,
Learn more about hydrocarbons at: https://brainly.com/question/1489747
#SPJ1
Suppose you are running a trial of the reaction between acetic acid and calcium carbonate, caco3. starting with 2.47 g of caco3, you determine that 1.11 g of carbon dioxide are produced.
Answers
Based on the given information, we can calculate the percent yield of the reaction using the formula: Percent yield = (Actual yield / Theoretical yield) * 100
First, we need to determine the theoretical yield of carbon dioxide (CO2) based on the stoichiometry of the reaction. The balanced chemical equation for the reaction between acetic acid (CH3COOH) and calcium carbonate (CaCO3) is:
2 CH3COOH + CaCO3 -> Ca(CH3COO)2 + H2O + CO2
The actual yield of CO2 is given as 1.11 g. Now we can calculate the percent yield:
Percent yield = (1.11 g CO2 / 1.086 g CO2) * 100
= 102.2%
The percent yield of the reaction is 102.2%. The percent yield is a measure of the efficiency of a chemical reaction. It compares the actual yield to the theoretical yield and expresses it as a percentage. In this case, the actual yield of CO2 is 1.11 g, while the theoretical yield is calculated to be 1.086 g. By plugging these values into the percent yield formula, we find that the percent yield is 102.2%.
To know more about reaction visit:
https://brainly.com/question/16737295
#SPJ11
When 2.47 g of calcium carbonate (CaCO3) is reacted, approximately 0.025 mol of CaCO3 is consumed and 0.025 mol of carbon dioxide (CO2) is produced. The calculated mass of CO2 is approximately 1.1 g, which closely matches the measured mass of 1.11 g.
Based on the given information, we can calculate the molar mass of calcium carbonate (CaCO3) and the number of moles of CO2 produced.
1. To find the molar mass of CaCO3, add the atomic masses of calcium (Ca), carbon (C), and three oxygen (O) atoms:
- Ca: 40.08 g/mol
- C: 12.01 g/mol
- O: 16.00 g/mol
- Molar mass of CaCO3 = 40.08 g/mol + 12.01 g/mol + (16.00 g/mol x 3)
- Molar mass of CaCO3 = 100.09 g/mol
2. Use the molar mass to convert the mass of CaCO3 to moles:
- Moles of CaCO3 = 2.47 g / 100.09 g/mol
- Moles of CaCO3 ≈ 0.025 mol
3. According to the balanced equation, one mole of CaCO3 produces one mole of CO2.
Therefore, the number of moles of CO2 produced is also approximately 0.025 mol.
4. To find the mass of CO2 produced, use the molar mass of CO2:
- C: 12.01 g/mol
- O: 16.00 g/mol
- Molar mass of CO2 = 12.01 g/mol + (16.00 g/mol x 2)
- Molar mass of CO2 = 44.01 g/mol
5. Mass of CO2 produced = moles of CO2 x molar mass of CO2
- Mass of CO2 = 0.025 mol x 44.01 g/mol
- Mass of CO2 = 1.1 g
Comparing the calculated mass of CO2 (1.1 g) with the measured mass of CO2 (1.11 g), we can see that they are very close. This suggests that the reaction between acetic acid and calcium carbonate is stoichiometric, meaning the reaction proceeds according to the balanced equation.
In conclusion, when 2.47 g of calcium carbonate (CaCO3) is reacted, approximately 1.11 g of carbon dioxide (CO2) is produced. The stoichiometry of the reaction is confirmed by the close agreement between the calculated and measured masses of CO2.
Learn more about molar mass:
https://brainly.com/question/31545539
#SPJ11
explain why zn reacts more slowly with dilute hydrochloric acid then with concentrated hydrochloric acid
Answers
Answer:
When the concentration is higher, more hydrogen ions are near the Zn atoms at any given time. This allows for more Zn atoms to be ionized and dissolved into the solution per second.
Explanation:
_____ Ni(OH)2 + _____ H2(SO4) → _____ Ni(SO4) + _____ H2O this is balancing chemical equations I need help fast
Answers
Double Replacement Reaction
When 1-chlorobutane is treated with sodium hydroxide, two products are formed. what are these products?
Answers
The two products formed when 1-chlorobutane is treated with sodium hydroxide are 1-butene and 1-butanol.
The common name of 1-chlorobutane is Butyl chloride. The reaction of 1 -chlorobutane with sodium hydroxide is catalyzed by sodium iodide to give 1 -butanol.
1-Butene is the compound with the formula CH₃CH₂CH=CH₂. This is a colorless gas which easily condensed to give a colorless liquid. 1-butene is classified as a linear alpha-olefin. It is the organic compound with the formula CH3CH2CH=CH2.
1-Butanol is known as butan-1-ol or n-butanol. It is a primary alcohol with the chemical formula C₄H₉OH. It is of linear structure. The Isomers of 1-butanol are butan-2-ol and tert-butanol. It refers to the straight chain isomer.
To learn more about 1-Chlorobutane please visit:
https://brainly.com/question/26615309
#SPJ4
The chemical formula of a compound can tell you - A. The proportions of elements in the compound. B. The three-dimensional structure of the compound. C. The type and arrangement of bonds in the compound. D. The properties of the elements in the compound
Answers
Answer:
A.
Explanation:
A chemical formula tells us the number of atoms of each element in a compound.
It contains the symbols of the atoms of the elements present in the compound as well as how many there are for each element in the form of subscripts.
Which example best fits
in with the rest?
Sodium, Aluminum, Gold,
Helium
a. potassium
b. salt
c. vinegar
d. vitamin C
Answers
Answer: a. Potassium
Explanation:
Sodium, Aluminum, Gold and Helium are all elements on the periodic table. The only example given that is also an element is Potassium.
This means the answer is;
➜ a. potassium
As a pure element the oxidation number of magnesium (Mg)is _____, but in compounds such as MgCl2 its oxidation number is _____.O 0,+1O +2,0O 0.+2O +1.0
Answers
As a pure element, the oxidation number of magnesium (Mg) is 0. However, in compounds such as MgCl2, its oxidation number is +2. Therefore, the correct answer is +2, 0.
How does oxidation number work?The total amount of electrons that an atom acquires or loses in order to create a chemical connection with another atom is known as the oxidation number, also known as the oxidation state. Magnesium and oxygen therefore undergo an oxidation-reduction process.
Do stable complex ions of magnesium form?The metal ion cofactor that is by far the most common in enzyme systems is magnesium. The capacity of the metal ion magnesium to form stable complexes with phosphate-containing species, such as ATP, which is often paired with the metal ion when functioning in a physiological setting, is most likely responsible for this.
To know more about oxidation number visit:-
https://brainly.com/question/29263066
#SPJ1
Calculate the volume in mL of a 1.420 M NaOH solution required to titrate the following solutions:
(a) 25.00 mL of a 2.430 M HCI solution
(b) 25.00 mL of a 4.500 M H2SO4 solution
(c) 25.00 mL of a 1.500 M H3PO4 solution
Answers
=158.4ml
Explanation:
Solution:
Chemical\; reaction:Chemicalreaction:
H_2SO_4(aq)\;+\;2NaOH(aq)\implies Na_2SO_4(aq)\;+\;H_2O(l)H
2
SO
4
(aq)+2NaOH(aq)⟹Na
2
SO
4
(aq)+H
2
O(l)
1) By using the acid-base equation:
M_1V_1=M_2V_2M
1
V
1
=M
2
V
2
4.5M*25ml=1.42M*V_24.5M∗25ml=1.42M∗V
2
V_2=\frac{(4.5M*25ml)}{1.42M}V
2
=
1.42M
(4.5M∗25ml)
Remember 1 mole H2SO4 is equivalent to 2 moles NaOH, that is Normality of H2SO4 = (Molarity x 2)
79.2*2ml=158ml\;of\;1.42M\;NaOH\;will\;be\;required.79.2∗2ml=158mlof1.42MNaOHwillberequired.
Answer:
V_2=158.4mlV
2
=158.4ml