Answers
ANSWER
The mass of the solute is 8.2 grams
EXPLANATION
Given that;
The volume of the solute is 125mL
The molarity of the solution is 0.25M
Follow the steps below to find the mass of the solute
Note; the solute is Ba(NO3)2
Step 1; Convert the volume of the solution to L
Recall, that 1 mL is equivalent to 0.001L
Let v represent the volume of the solution in L
\(\begin{gathered} \text{ 1mL }\rightarrow\text{ 0.001L} \\ \text{ 125mL }\rightarrow\text{ vL} \\ \text{ cross multiply} \\ \text{ 1mL}\times vL\text{ }=\text{ 125mL }\times\text{ 0.001L} \\ \text{ Isolate vL} \\ \text{ vL}=\text{ }\frac{125\cancel{mL}\times0.001L}{1\cancel{mL}} \\ \text{ v }=\text{ 0.125L} \end{gathered}\)Hence, the volume in Liters is 0.125L
Step 2; Find the number of moles of the solute using the below formula
\(\text{ n }=\text{ cv}\)\(\begin{gathered} \text{ n }=\text{ 0.25 }\times\text{ 0.125} \\ \text{ n }=\text{ 0.03125 mole} \end{gathered}\)STEP 3: Find the mass of the solute using the formula below
\(\text{ Mole }=\text{ }\frac{\text{ mass}}{\text{ molar mass}}\)Recall, that the molar mass of Ba(NO3)2 is 261.337 g/mol
\(\begin{gathered} \text{ 0.03125 }=\frac{mass}{261.337} \\ \text{ Cross multiply} \\ \text{ mass }=\text{ 0.03125 }\times\text{ 261.337} \\ \text{ mass }=\text{ 8.2 grams} \end{gathered}\)Hence, the mass of the solute is 8.2 grams
Related Questions
Write balanced equation for:
the incomplete combustion of pentane
The complete combustion of heptane
The incomplete combustion of heptane
Answers
Answer:
hmmm good luck
Explanation:
1.write the balanced equation for
CuCl2+HNO3+AgNO3=
What is the general molecular formula for phenol?
Answers
1
1
The general molecular formula for phenol is C6H5OH. It is an aromatic organic compound with a hydroxyl group bonded to a carbon atom of a cyclic structure. The ring structure has alternate double and single bonds. The formula of phenol is C6H5OH. Benzene has the formula C6H6. It is a hexagonal ring of six carbon atoms bonded with alternate single and double bonds. Each carbon atom in benzene has a bond with a hydrogen atom. In phenol, a hydroxyl group replaces one of the hydrogen atoms.
The reaction of CuCl2, HNO3, and AgNO3 is a redox reaction. In a redox reaction, one substance loses electrons and is oxidized, while another substance gains electrons and is reduced. In this reaction, CuCl2 is oxidized to Cu2+, HNO3 is reduced to NO, and AgNO3 is reduced to Ag.
The balanced equation for the reaction is:
CuCl2 + 2HNO3 + 2AgNO3 → Cu2+ + 2NO + 2AgCl
The products of the reaction are copper(II) ions, nitrogen monoxide gas, and silver chloride precipitate.
Here are some of the properties of phenol:
Phenol is a white crystalline solid that is soluble in water.
Phenol has a strong, characteristic odor.
Phenol is a mild acid.
Phenol is toxic and can cause burns to the skin and eyes.
Phenol is used in a variety of products, including disinfectants, antiseptics, and plastics.
This type of matter does not have a definite shape but does have volume and takes the shape of whatever container it is in? Shape liquid or gas
Answers
Answer:
liquid takes the shape of whatever container it is in and have a volume but gas doesn't have a volume
Answer:
liquid because you can take the volume of water and the shape depends on the container
Upon reaction of 1.274 g of copper(II) sulfate with excess zinc metal, 0.392 g copper metal was obtained according to the equation: CuSO4 (aq) + Zn (s) Cu (s) + ZnSO (aq) What is the percent yield? (77.3%)
Answers
The Percent Yield=(Actual YieldTheoretical Yield)×100% = 77.3%
What does the term percent yield mean?A real yield divided by hypothetical yield multiplied by 100 is known as percent yield.Yield Percentage Equals Actual YieldA theoretical yield of 100%There are various reasons why a chemical reaction's theoretical yield might not match its actual yield, and these will be covered in more detail in later chapters of the course.
What formula is used to determine percentage yield?Percent yield = Actual Yield/Theoretical Yield x 100 is the formula we use to calculate the yield percentage.
To know more about yield percentage visit:
https://brainly.com/question/29714892
#SPJ4
X-rays are often used in medical settings to create images of the body's internal structures such as bones. This is made possible by the
fact that X-rays are able to pass through the body's softer tissues without being absorbed.
Radio waves are also able to pass through the body's softer tissues without being absorbed. Why are radio waves not used to generate
medical images?
OA. The electrons in most atoms are not in high enough energy states to absorb the photons of radio waves.
OB. Radio waves tend to bend too much when they encounter solid materials to be used for generating accurate images,
OC
The frequency of most radio waves is too low to allow them to pass through bones or other solid materials.
OD.
Radio waves carry so little energy that they tend to pass through most atoms without an interaction taking place.
Answers
Answer:D
Explanation:I did it on study island.
Radio waves are not used to generate medical images because as per the electromagnetic spectrum ,the radio waves carry little energy that they pass through most atoms without interaction.
What is an electromagnetic spectrum?The electromagnetic spectrum consists of radiation which consists of waves made up of electromagnetic field which are capable of propogating through space and carry the radiant electromagnetic energy.
The radiation are composed of electromagnetic waves which are synchronized oscillations of electric and magnetic fields . They are created due to change which is periodic in electric as well as magnetic fields.
In vacuum ,all the electromagnetic waves travel at the same speed that is with the speed of air.The position of an electromagnetic wave in an electromagnetic spectrum is characterized by it's frequency or wavelength.They are emitted by electrically charged particles which undergo acceleration and subsequently interact with other charged particles.
Learn more about electromagnetic spectrum,here:
https://brainly.com/question/15576247
#SPJ2
why is coal and fossil fuels a nonrenewable resource
Answers
Answer:
It is a finite resource. Fossil fuels such as oil, natural gas, and coal are examples of nonrenewable resources. Humans constantly draw on the reserves of these substances while the formation of new supplies takes eons. Renewable resources are the opposite: Their supply replenishes naturally or can be sustained.
Explanation:
What is the mass number of an atom with 17 protons, 17 electrons,and 18 nuetrons?
Answers
Answer:
35
Explanation:
the formula is: mass number = protons + neutrons
so 17+18=35
what molecule is not a compound
Answers
Easy | Grade 8 practice sheet "Vocabulary of Chemical Equations"

Answers
Based on how chemical equations are written, the given chemical reaction: Four atoms of aluminum metal react quickly with 3 molecules of oxygen in the air to produce 2 molecules of aluminum oxide can be written as follows:
Word equation: Aluminum metal + oxygen gas ---> Aluminum oxide
Reactants: Aluminum metal and oxygen gas
Products: Aluminum oxide
Known states of matter: solid + gas ---> solid
Equation: 4 Al (s) + 3 O₂ (g) ---> 2 Al₂O₃ (s)
What is a chemical reaction?A chemical reaction is a type of change that results in changes in the chemical properties of substances.
Chemical reactions involve the rearrangement of atoms of substances and result in the formation of new substances.
Chemical reactions can be represented by a chemical equation.
Chemical equations use symbols to represent the reactants and the products of a reaction.
Learn more about chemical equations at: https://brainly.com/question/26694427
#SPJ1
Diethylene glycol is an organic compound used as a fuel in heating cans. Based on its structure and your data recorded in Data Table 3, how do you hypothesize its energy efficiency (cal/g) compared to isopropanol and paraffin wax?
Answers
Due to its higher oxygen content and based on its structure dietheylene glycol has a higher energy efficiency (cal/g) compared to isopropanol and paraffin wax.
What is the calorific value of a fuel?The calorific value of a fuel is the amount of heat energy released from the combustion of the fuel under standard conditions.
Dietheylene glycol, isopropanol and paraffin wax are all used as fuels.
However, due to it higher oxygen content and based on its structure, diethethylene glycol has a higher calorific value than either isopropanol or paraffin wax.
Therefore, dietheylene glycol has a higher energy efficiency (cal/g) compared to isopropanol and paraffin wax.
Learn more about calorific value at: https://brainly.com/question/12975147
#SPJ1
what is a formula car? (This is for my chemistry project)
Answers
Answer:
A formula car is a single seat, open cockpit, open wheel racing car with substantial front and rear wings and an engine positioned behind the driver intended to be used in competition.
Does MgSO4 react with ZnSO4?
Answers
There is no reaction between zinc II sulfate and magnesium sulfate.
Is there a reaction?We know that a reaction is said to occur when two species are combined and there are new products that appear in the system. This implies that if there are no new substances that appear in the system we can not say that a chemical reaction has taken place.
In this case, we can see that we can not be able to observe any change when wee mix a solution of magnesium sulfate and zinc II sulfate since the both of them have the same anion.
Learn more about reaction:https://brainly.com/question/28984750
#SPJ1
O
Question 2 of 10
Which of the following values best classifies a bond between 2 atoms as
being covalent?
A. An electronegativity difference of greater than 2.7 between the
atoms
B. An electronegativity difference of greater than 1.7 between the
atoms
C. An electronegativity difference of less than 1.7 between the atoms
D. An electronegativity difference of less than 2.7 between the atoms
← PREVIOUS
SUBMIT
Answers
A bond between 2 atoms as being covalent an electronegativity difference of greater than 1.7 between the atoms.
What is bond?Bond is a debt instrument, which is issued by governments and corporations. These bonds are used to raise capital for various projects and investments. Bonds are a form of loan and investors are usually paid a fixed interest rate for lending their money. Bonds are typically issued for a fixed period of time, usually for a period of 5-30 years, and have a fixed maturity date. Investors receive interest payments at regular intervals and receive the principal amount of the bond at the end of the period.
To learn more about bond
https://brainly.com/question/29794367
#SPJ1
How will you describe the pattern as electrons are distributed
Answers
How will you describe the pattern as electrons are distributed?
In a simple way, you can say, electrons are arranged around an atom's nucleus.
When electrons are near the nucleus, have the lowest energy. Electrons further aways will have higher energy.
We can say electrons are in different shells with different energy. Inside those shells, electrons are in subshells too.
Then you have some numbers that describe the completly characteristics of an electron in an atom.
They are called Quantum Numbers. They are four
1) The principal Quantum number (n): describes the energy of an electron and the most probable distance of the electron from the nucleus. In other words, it refers to the size of the orbital and the energy level an electron is placed in.
2) The number of subshells or (l): describes the shape of the orbital. It can also be used to determine the number of angular nodes.
3) The magnetic Quantum number (ml): describes the energy levels in a subshell. (Remember we have shells and inside of them we have subshells)
4) The Spin of the Electron (ms): refers to the spin of the electron which can either be up or down.
\(_{m_{s\text{ }}}\)Net ionic equation for potassium sulfide and magnesium iodide
Answers
The net ionic equation for the reaction between potassium sulfide and magnesium iodide is S2- + Mg2+ -> MgS, as the potassium and iodide ions are spectator ions and do not participate in the reaction.
To determine the net ionic equation for the reaction between potassium sulfide (K2S) and magnesium iodide (MgI2), we first need to identify the ions present in each compound and then determine the products formed when they react.
Potassium sulfide (K2S) dissociates into two potassium ions (K+) and one sulfide ion (S2-):
K2S -> 2K+ + S2-
Magnesium iodide (MgI2) dissociates into one magnesium ion (Mg2+) and two iodide ions (I-):
MgI2 -> Mg2+ + 2I-
Now, we need to determine the possible products when these ions combine. Since potassium (K+) has a +1 charge and iodide (I-) has a -1 charge, they can combine to form potassium iodide (KI):
K+ + I- -> KI
Similarly, magnesium (Mg2+) and sulfide (S2-) can combine to form magnesium sulfide (MgS):
Mg2+ + S2- -> MgS
Now, we can write the complete ionic equation by representing all the ions present before and after the reaction:
2K+ + S2- + Mg2+ + 2I- -> 2KI + MgS
To obtain the net ionic equation, we remove the spectator ions, which are the ions that appear on both sides of the equation and do not participate in the actual reaction. In this case, the spectator ions are the potassium ions (K+) and the iodide ions (I-).
Thus, the net ionic equation for the reaction between potassium sulfide and magnesium iodide is:
S2- + Mg2+ -> MgS
For more such questions on ionic equation visit:
https://brainly.com/question/25604204
#SPJ8
the presumptive definition of driving under the influence in florida is having a blood alcohol content (bac) of .08.
Answers
True. The presumptive definition of Driving Under the Influence in Florida is having a Blood Alcohol Content (BAC) of .08.
True. In Florida, it is illegal to operate a motor vehicle with a blood alcohol content (BAC) of .08 or higher. This is known as driving under the influence (DUI). The legal limit for DUI in Florida is .08.
If a driver is found to have a BAC of .08 or higher, they can be arrested for DUI. Florida also has a Zero Tolerance Law for drivers under 21, meaning that any BAC .02 or higher can lead to an arrest for DUI.
For more questions like DUI click the link below:
https://brainly.com/question/29771200
#SPJ4
Complete question:
The presumptive definition of Driving Under the Influence in Florida is having a Blood Alcohol Content (BAC) of .08. true or false
draw the major thermodynamic and kinetic products of the reaction. draw the kinetic product. select draw rings more erase select draw rings more erase select draw rings more erase c h br draw the thermodynamic product.
Answers
The major thermodynamic product of the reaction between C₅H₈ and HBr is the most stable product, which is usually the one with the lowest free energy. On the other hand, the major kinetic product is the product that is formed through a faster and less controlled reaction pathway.
This thermodynamic product product is formed through a slower and more controlled process, which allows for the formation of stronger chemical bonds and results in a more stable final product.
This kinetic product is formed due to its lower activation energy, which makes it easier to form, but it may not necessarily be the most stable product.
In the case of C₅H₈ and HBr, the reaction can result in both thermodynamically and kinetically stable products, meaning that there can be multiple products formed, each with different stability properties. The thermodynamically stable product is the one with the lowest free energy, while the kinetically stable product is the one with the lower activation energy.
In summary, the major thermodynamic product of the reaction is the most stable product, while the major kinetic product is the product that is formed through a faster and less controlled reaction pathway. However, it is important to note that the exact products of the reaction can vary based on the reaction conditions and reactant concentrations.
To know more about kinetic products click on below link:
https://brainly.com/question/22616533#
#SPJ11
Complete question:
What are themajor thermodynamic and kinetic products of the reaction.
C₅H₈ + HBr (1 equivalent) ------> thermodynamically stable + Kinetically stable
One mole of atoms of any element has a mass equal to the atomic mass of that element. This means that 1 mole of oxygen atoms
A) has a mass of 8 grams
B) has a mass of 6.02 x 1023 grams
C) has a mass of 16 grams
D) has a mass of 6 grams
Answers
This means that 1 mole of oxygen atoms has a mass of 16 grams.
option C.
What is mole?A mole is defined as the amount of a substance that contains the same number of entities (such as atoms, molecules, or ions) as there are in 12 grams of carbon-12, which is an isotope of carbon.
This number is known as Avogadro's number, which is approximately 6.02 x 10²³ entities per mole.
So, when we say "1 mole of oxygen atoms," we are referring to Avogadro's number of oxygen atoms, which is approximately 6.02 x 10²³ oxygen atoms.
Since the atomic mass of oxygen is 16 grams per mole, 1 mole of oxygen atoms would have a mass of 16 grams, which is option C) in the given choices.
Learn more about number of moles here: https://brainly.com/question/14357742
#SPJ1
Acetic acid (mm = 60.05 g/mol) is a monoprotic acid; an amount of acetic acid was dissolved in enough water to make a 10.00 mL solution. Titration of this acid required 18.53 mL of 0.300 M sodium hydroxide. How many grams of acetic acid were used in this titration?
Answers
Since most reactions take place in solutions, it's critical to comprehend how the substance's concentration is expressed in a solution. The number of chemicals in a solution can be stated in a variety of ways. The grams of acetic acid is 611.90 g.
The number of moles of dissolved solute per liter of solution is the definition of molarity, a unit of concentration. Molarity is defined as the number of millimoles per milliliter of the solution by dividing the number of moles and the volume by 1000.
The equation which is used to calculate the molarity of two different solutions is:
M₁V₁ = M₂V₂
M₁ = M₂V₂ / V₁
0.300 × 18.53 / 10.00 = 0.55 M
Number of moles = Molarity × volume
n = 0.55 × 18.53 = 10.19
Mass = n × Molar mass = 10.19 × 60.05 = 611.90 g
To know more about molarity, visit;
https://brainly.com/question/16587536
#SPJ1
A balloon contains 7.36 g of oxygen gas (02). How many oxygen molecules
are in the balloon?
The Periodic Table
O A. 236 molecules
O B. 1.38 x 1023 molecules
O C. 4.43 x 1024 molecules
O D. 15 molecules
Answers
Answer:
15 molecules
Explanation:
A balloon contains 7.36 g of oxygen gas (02). How many oxygen molecules
are in the balloon?
Arteries do not contain muscle of any type.
True or False?
Answers
False.
Arteries are said to be more muscular.
That means Arteries ARE* MUSCLES
A sheet of aluminum foil has a volume of 0.555 cm3. If the foil measures 10.0 cm by 10.0 cm, what is the thickness of the foil?
Answers
Answer: B) 0.005 55 cm
Hope you have a blessed day!
calculate the number of moles for the quanity 8.06 x 1021 atoms of Pt
Answers
The number of moles for the quanity 8.06 x\(10_{21\) atoms of Pt is approximately 2.61 grams.
To calculate the number of moles for a given quantity of atoms, we can use Avogadro's number and the molar mass of the element. Avogadro's number is 6.022 x 10²³ atoms/mol.
In this case, you have 8.06 x 10²¹ atoms of Pt. To find the number of moles, divide this quantity by Avogadro's number:
8.06 x 10²¹ atoms Pt / 6.022 x 10²³ atoms/mol = 0.0134 mol Pt
So, there are approximately 0.0134 moles of Pt in 8.06 x 10²¹ atoms of Pt.
The molar mass of Pt (platinum) is 195.08 g/mol. To convert the number of moles to grams, multiply the number of moles by the molar mass:
0.0134 mol Pt x 195.08 g/mol = 2.61 g Pt
Therefore, there are approximately 2.61 grams of Pt in 8.06 x10²¹ atoms of Pt.
In summary, the number of moles for the quantity 8.06 x 10²¹ atoms of Pt is approximately 0.0134 moles. This is equivalent to approximately 2.61 grams of Pt. Remember to use Avogadro's number and the molar mass to perform these calculations accurately.
Know more about moles here:
https://brainly.com/question/29367909
#SPJ8
vbvjkkkp[kpojsdbfoijaefoibhfeboqi
Answers
Answer:
sdfhioupsdfiuhikdfjsdfhsdksdflk";d089sdfojskdfk
pls give brainlyest
Explanation:
200 IQ intellectual right here ^^
Swag? Swag? Swag? Swag? Swag?
Answers
how many atoms of nitrogen is in Boron nitride
Answers
Answer:
Boron nitride
Magnified sample of crystalline hexagonal boron nitride
Names
IUPAC name
Boron nitride
Identifiers
CAS Number
10043-11-5 check
3D model (JSmol)
hexagonal (graphite) structure: Interactive image
sphalerite structure: Interactive image
wurtzite structure: Interactive image
ChEBI
CHEBI:50883 check
ChemSpider
59612 check
ECHA InfoCard 100.030.111 Edit this at Wikidata
EC Number
233-136-6
Gmelin Reference 216
MeSH Elbor
PubChem CID
66227
RTECS number
ED7800000
UNII
2U4T60A6YD ☒
CompTox Dashboard (EPA)
DTXSID5051498 Edit this at Wikidata
InChI
SMILES
Properties
Chemical formula BN
Molar mass 24.82 g·mol−1
Appearance Colorless crystals
Density 2.1 (h-BN); 3.45 (c-BN) g/cm3
Melting point 2,973 °C (5,383 °F; 3,246 K) sublimates (cBN)
Solubility in water insoluble
Electron mobility 200 cm2/(V·s) (cBN)
Refractive index (nD) 1.8 (h-BN); 2.1 (c-BN)
Structure
Crystal structure hexagonal, sphalerite, wurtzite
Thermochemistry
Heat capacity (C) 19.7 J/(K·mol)[1]
Std molar
entropy (So298) 14.8 J/K mol[1]
Std enthalpy of
formation (ΔfH⦵298) -254.4 kJ/mol[1]
Gibbs free energy (ΔfG˚) -228.4 kJ/mol[1]
Hazards
GHS labelling:
Pictograms GHS07: Exclamation mark
Signal word Warning
Hazard statements H319, H335, H413
Precautionary statements P261, P264, P271, P273, P280, P304+P340, P305+P351+P338, P312, P337+P313, P403+P233, P405, P501
NFPA 704 (fire diamond)
NFPA 704 four-colored diamond
000
Related compounds
Related compounds Boron arsenide
Boron carbide
Boron phosphide
Boron trioxide
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
☒ verify (what is check☒ ?)
Infobox references
Explanation:
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic (zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form.[2]
Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment. Boron nitride has potential use in nanotechnology.
Boron nitride exists in multiple forms that differ in the arrangement of the boron and nitrogen atoms, giving rise to varying bulk properties of the material.
Amorphous form (a-BN)
The amorphous form of boron nitride (a-BN) is non-crystalline, lacking any long-distance regularity in the arrangement of its atoms. It is analogous to amorphous carbon.
All other forms of boron nitride are crystalline.
Hexagonal form (h-BN)
The most stable crystalline form is the hexagonal one, also called h-BN, α-BN, g-BN, and graphitic boron nitride. Hexagonal boron nitride (point group = D6h; space group = P63/mmc) has a layered structure similar to graphite. Within each layer, boron and nitrogen atoms are bound by strong covalent bonds, whereas the layers are held together by weak van der Waals forces. The interlayer "registry" of these sheets differs, however, from the pattern seen for graphite, because the atoms are eclipsed, with boron atoms lying over and above nitrogen atoms. This registry reflects the local polarity of the B–N bonds, as well as interlayer N-donor/B-acceptor characteristics. Likewise, many metastable forms consisting of differently stacked polytypes exist. Therefore, h-BN and graphite are very close neighbors, and the material can accommodate carbon as a substituent element to form BNCs. BC6N hybrids have been synthesized, where carbon substitutes for some B and N atoms.
HELP ME PLS- 8th grade science
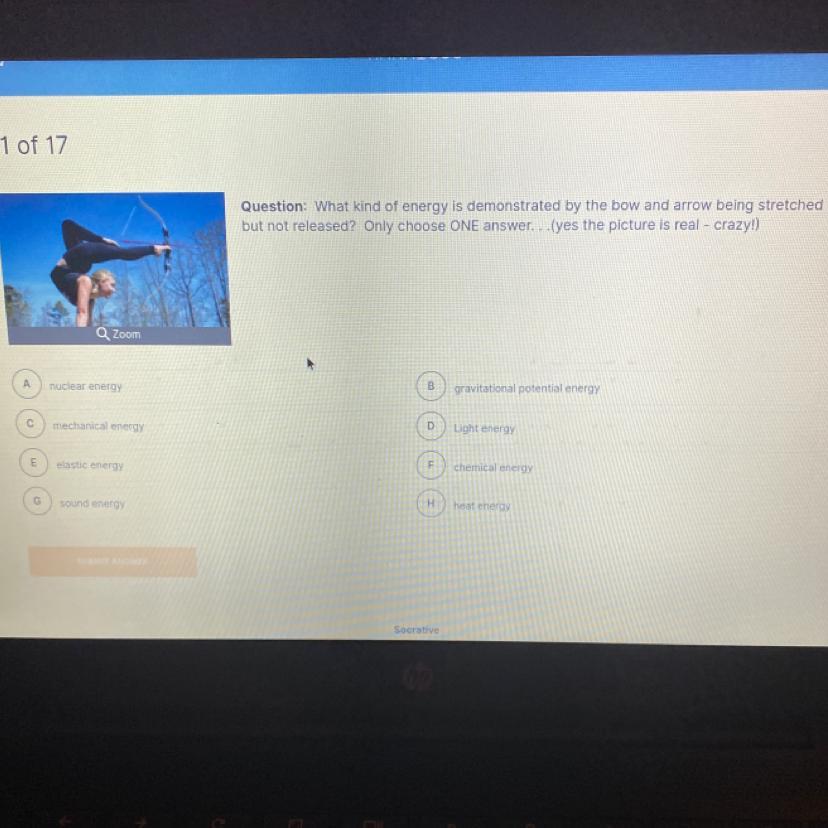
Answers
Answer:
Elastic Energy
Explanation:
Elastic energy is the mechanical potential energy stored in the configuration of a material or system as it is subjected to elastic deformation by work performed upon it. Elastic energy occurs when objects are impermanently compressed, stretched or generally deformed in any manner.
five differences between true solution and false solution
Answers
Answer:
k im silly frrr lol have a great day
What has more kinetic energy? 120 180, 105
Answers
Answer:
If those values are in terms of mass, weight or velocity
Kinetic energy will be in the order 180 » 120 » 105
Answer:
105, if you're dealing with the weight of object
Explanation:
It's the amount of speed/movement an object can move. The lighter the weight the more amount of speed it has.
Hope this helps!
Scenario: You place a spoonful of frozen ice cream on your tongue a) Type of energy transfer b) Where will each form of transfer occur c) What will happen and why?
Answers
The type of energy transfer between the frozen ice cream and the tongue is convection. The frozen ice cream will gradually melt because of the temperature difference.
What is convection?Convection is the method of heat transfer that involves the transmission of heat in a fluid (liquid or gas) by the circulation of currents.
According to this question, a spoonful of frozen ice cream is placed on the tongue. The heat transfer will occur between the tongue with warmer temperature and the ice cream with colder temperature.
The heat energy will flow from the tongue to the ice cream, hence, making it defreeze or melt with time. This type of heat transfer is convection because it involves a fluid.
Learn more about convection at: https://brainly.com/question/4138428
#SPJ1