A 0.0367 M solution of a weak base has a pH of 11.59. What is the identity of the weak base?
Weak Base Kb
Ethylamine (CH3CH2NH2) 4.7 × 10-4
Hydrazine (N2H4) 1.7 × 10-6
Hydroxylamine (NH2OH) 1.1 × 10-8
Pyridine (C5H5N) 1.4 × 10-9
Aniline (C6H5NH2) 4.2 × 10-10
a. hydrazine
b. pyridine
c. aniline
d. ethylamine
e. hydroxylamine
Answers
The correct answer to the given question is option (a) hydrazine.
To determine the identity of the weak base in the solution, we need to use the pH and Kb values of each candidate weak base to calculate which one would result in a pH of 11.59 for a 0.0367 M solution. First, we can use the pH to find the pOH of the solution using the equation pH + pOH = 14. So, pOH = 2.41.
Next, we can use the Kb values of each weak base to calculate their corresponding pKb values, which is equal to -log(Kb).
The pKb values for the given weak bases are:
Ethylamine (CH3CH2NH2) 3.33
Hydrazine (N2H4) 5.77
Hydroxylamine (NH2OH) 8.96
Pyridine (C5H5N) 8.85
Aniline (C6H5NH2) 9.38
We can then use the pKb values and the pOH of the solution to calculate the degree of ionization (α) of each weak base using the formula:
α = sqrt(Kb/[H3O+]) = sqrt(Kb/10^-pH)
The degree of ionization for each weak base is:
Ethylamine (CH3CH2NH2) 0.50%
Hydrazine (N2H4) 61.8%
Hydroxylamine (NH2OH) 1.15%
Pyridine (C5H5N) 1.04%
Aniline (C6H5NH2) 0.65%
From the calculations, we can see that hydrazine has the highest degree of ionization and is therefore the most likely candidate for the weak base in the solution. So the answer is (a) hydrazine.
To know more about pH please visit:
https://brainly.com/question/15289741
#SPJ11
Related Questions
If a mixture is 4.6 g of water and .5 g of copper chloride ,what is the percent composition of water?
Answers
The percent composition of water : 90.2%
Further explanationThe concentration of a substance can be expressed in several quantities such as moles, percent (%) weight / volume,), molarity, molality, parts per million (ppm) or mole fraction. The concentration shows the amount of solute in a unit of the amount of solvent.
mass of water = 4.6 g
mass of Copper chloride = 0.5 g
mass of solution :
\(\tt mass~solution=mass~water+mass~Copper~chloride\\\\mass~solution=4.6+0.5\\\\mass~solution=5.1~g\)
So the percent composition of water (%mass) :
\(\tt \%water=\dfrac{mass~water}{mass~solution}\times 100\%\\\\\%water=\dfrac{4.6~g}{5.1~g}\times 100\%\\\\\%water=90.2\%\)
after ten years, 75 grams remain of a sample that was
originally 100 grams of some unknown radio isotope. find the half
life for this radio isotope
Answers
The half-life of the radioisotope, calculated based on the given information that after ten years only 75 grams remain from an initial 100 grams, is approximately 28.97 years.
To find the half-life of the radioisotope, we can use the formula for exponential decay:
N(t) = N₀ × (1/2)^(t / T₁/₂)
T₁/₂ is the half-life of the substance.
In this case, we know that the initial amount N₀ is 100 grams, and after ten years (t = 10), 75 grams remain (N(t) = 75 grams).
We can plug these values into the equation and solve for T₁/₂:
75 = 100 × (1/2)^(10 / T₁/₂)
Dividing both sides of the equation by 100:
0.75 = (1/2)^(10 / T₁/₂)
Taking the logarithm (base 2) of both sides to isolate the exponent:
log₂(0.75) = (10 / T₁/₂) × log₂(1/2)
Using the property log₂(a^b) = b × log₂(a):
log₂(0.75) = -10 / T₁/₂
Rearranging the equation:
T₁/₂ = -10 / log₂(0.75)
Using a calculator to evaluate the logarithm and perform the division:
T₁/₂ ≈ 29.13 years
Therefore, the half-life of the radioisotope is approximately 28.97 years.
Read more on half-life period here: https://brainly.com/question/12341489
#SPJ11
Based on this equation, which of the following statements best describes what would be observed as the reaction takes place?
\(AgNO3(aq) + KCl(aq) ~~---\ \textgreater \ ~~ AgCl(s) + KNO3(aq)\)
a) silver chloride forming a solid precipitate (CORRECT)
b) solid silver dissolving
c) two clear solutions mixing to form one clear solution
d) bubbles forming from aqueous solution production
Answers
Answer:
a
Explanation:
i took the quiz
how many kilograms are 129.569grams?
Answers
0.129569. You have to divide the mass value by 1000.
how to determine which direction the equilibrium will shift with the addition of a new ionic compound
Answers
The direction of equilibrium shift upon addition of a new ionic compound depends on the nature of the compound being added and the equilibrium constant (K) of the reaction. Le Chatelier's principle is a useful tool to predict the direction of equilibrium shift.
If an ionic compound is added to a reaction mixture, it will dissociate into its respective ions. If the added ions are the same as the ones present in the reaction, they will not affect the equilibrium position. However, if the added ions are different, they can affect the equilibrium position by increasing or decreasing the concentration of one or more reactants or products.
If a new compound is added to a reaction, and it contains an ion that is common to one side of the equilibrium, then the equilibrium will shift towards the opposite side. If the added compound contains an ion that is unique to one side of the equilibrium, then the equilibrium will shift towards that side.
Additionally, if the added compound reacts with one of the reactants or products to form a new compound, the equilibrium will shift to restore the original concentrations of the reactants and products. The extent of the shift will depend on the relative magnitudes of the equilibrium constants of the original and new reactions.
Overall, the direction of equilibrium shift upon addition of a new ionic compound is dependent on the specific conditions of the reaction, and can be predicted using Le Chatelier's principle.
To learn more about ionic compound refer to:
brainly.com/question/3222171
#SPJ4
how to make a buffer solution using henderson- hasselbalch
Answers
A buffer solution is a solution that opposes a sudden change in pH value when a small amount of acid or base is added to it. Buffer solutions are made up of a weak acid and its conjugate base, or a weak base and its conjugate acid. A buffer solution is formed by mixing a weak acid or base with its salt, or by mixing two salts with slightly different anions and cations.
The Henderson-Hasselbalch equation is utilized to compute the pH of a buffer solution. It is as follows:
Henderson-Hasselbalch equation: pH = pKa + log([A-]/[HA])where pH is the negative logarithm of the hydrogen ion concentration in moles per liter, pKa is the negative logarithm of the acid dissociation constant, [A-] is the concentration of the base, and [HA] is the concentration of the acid.
To make a buffer solution using Henderson-Hasselbalch, the following steps should be followed: Firstly, choose a weak acid or base as the buffer component. A buffer system has the most buffering capability when the pH is near the pKa value of the weak acid or base. As a result, choose a weak acid or base whose pKa is close to the desired pH of the buffer solution.
Secondly, determine the concentration of the weak acid or base. The concentration of the weak acid or base used in the buffer solution is usually in the range of 0.1 to 1 Molar.
Thirdly, calculate the concentration of the conjugate base or acid. The Henderson-Hasselbalch equation can be used to determine the concentration of the conjugate base or acid needed to achieve the desired pH value. The concentration of the conjugate base or acid is usually between 10 and 100 times that of the weak acid or base.
Finally, mix the weak acid or base with its salt, or mix two salts with slightly different anions and cations to form a buffer solution. The pH of the buffer solution can be measured with a pH meter, and any necessary adjustments can be made by adding acid or base to the solution.
To know more about Buffer visit-
https://brainly.com/question/31847096
#SPJ11
Draw the Lewis structure for BrF5. The hybridization on the Br is (sp, sp2, sp3, sp3d, sp3d2). The molecule is (choose one: polar or nonpolar)
Answers
The hybridization on the Br in BrF₅ is sp³d². the molecule is polar molecule .
The hybridization of bromin pentafluoride , BrF₅ sp³d² hybridize. in the structure of BrF₅ the boromine contains one lone pair of electron. the lewis structure is shown as follows :
F
|
F - Br : - F
| |
F F
the valence electron the Br atom are 7 valence electron out of which 5 are making bonds with the fluorine atom. two of them are present as lone of electrons. one s , three p and two d orbitals are taking part in hybridization . BrF₅ is the polar molecule as the difference in electro negativity in Br and the F atom contributes to the unequal charge distribution.
To learn more about hybridization here
https://brainly.com/question/28207543
#SPJ4
The table above shows the structural formulas and molar masses for three different compounds. Which of the following is a list of the compounds in order of increasing boiling points?
A. Butane < 1-propanol < acetone
B. Butane < acetone < 1-propanol
C. 1-propanol < acetone < butane
D. Acetone = butane < 1-propanol

Answers
Butane < acetone < 1-propanol is a list of the compounds in order of increasing boiling points.
Which substance has a greater boiling point?The intermolecular interactions between molecules in a compound play a major role in boiling point. Higher boiling temperatures are a result of greater intermolecular interactions, bigger masses, and less branching.
The highest boiling point is for HF. As we go from HF to HI, the van der Waals forces of attraction between all hydrogen halides get stronger.
Boiling point rises as the difference in electronegativity grows. Additionally, the bp grows as the molecule's size does. So the sequence is CO2, CS2, CCl4, and then H2O.
Van der Waals attraction and dipole-dipole attraction draw acetone molecules together.
To learn more about compounds refer to:
https://brainly.com/question/26487468
#SPJ1
please answer quick please, im have a test

Answers
PLEASEEE HELP!!!! How many grams of copper are required to replace 10.4 g of gold (III)
nitrate, which is dissolved in water?
3 Cu + 2 Au(NO3)3(aq) → 3 Cu(NO3)2(a) + 2 Au(s)
Answers
Answer:
5.3584 g
Explanation:
What element is the catalyst in the reaction above? H N O Pt
Answers
Answer:
The element that is the reaction is Pt
Explanation:
Answer:
The answer is pt on edge
Explanation:
20 cm³ of carbon monoxide are reacted with 10 cm³ of oxygen. The equation for the reaction is
shown
2CO (g) + O₂(g) → 2CO₂ (g)
What volume of carbon dioxide is produced?
Answers
Answer: 20.16cm³ of CO₂ produced
Explanation: the equation for the reaction between carbon monoxide (CO) and oxygen (O2) is:
2CO (g) + O₂ (g) → 2CO₂ (g)
Given that 20 cm³ of carbon monoxide is reacted with 10 cm³ of oxygen. From the equation,
we can see that two molecules of CO react with one molecule of O₂to produce two molecules of CO₂
Since we have 20 cm³ of CO and 10 cm³ of O₂, we can calculate the number of moles of CO and O₂ as follows:
CO: 20 cm³ x (1 mole / 22.4 cm³) = 0.89 moles
O₂: 10 cm³ x (1 mole / 22.4 cm³) = 0.45 moles
Since two molecules of CO react with one molecule of O₂, the number of moles of O₂ is half the number of moles of CO. Since we have 0.45 moles of O₂, we can calculate the number of moles of CO₂ produced as follows:
0.45 moles x 2 = 0.9 moles
Finally, to find the volume of CO₂ produced, we can multiply the number of moles of CO₂ by the volume occupied by one mole of CO₂:
0.9 moles x 22.4 cm³/mole = 20.16 cm³
Read more about stoichiometry and mole concept:
https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Beginning_Chemistry_(Ball)/05%3A_Stoichiometry_and_the_Mole#:~:text=5.2%3A%20Stoichiometry,mass%20in%20atomic%20mass%20units.
Most scientific investigations follow a specific approach called the __________________ __________________________.
A.general method
B. system of studies
C. scientific method
D.none of the above
Answers
The following are halogens except
a. fluorine
b. iodine
c. chlorine
d. phenol
Answers
Answer:
i think this is [d. phenol]
The correct answer:
Phenol
\( \large{ \boxed{ \bf{ \color{green}{Option \: D}}}}\)
Phenol is a compound, While other are elments found under the 17th group, and popularly known as Halogens.
Explore more:-The name halogen comes from the Greek words "hals", meaning "salt", and "gen", meaning "to make."The halogens are a group of elements in the periodic table. They are located to the right of the other nonmetals and to the left of the noble gases.The halogens include the five elements fluorine, chlorine, bromine, iodine, and astatine. They make up column 17 of the periodic table.They have seven valence electrons in their outer shell.They all exist as diatomic molecules (two atoms) when in their pure form.Simple compounds that contain halogens are called halides.━━━━━━━━━━━━━━━━━━━━
what is the ratio of the concentrations of acetate ion and undissociated acetic acid at ph 5.22? (the pka of acetic acid is 4.76.)
Answers
To determine the ratio of the concentrations of acetate ion and undissociated acetic acid at pH 5.22, we can use the Henderson-Hasselbalch equation, which relates the pH, pKa, and the concentrations of the acid and its conjugate base.
Given to us is
pH = 5.22
pKa = 4.76
\(pH = pKa + log\frac{[A-]}{[HA]}\)
In this equation, [A-] represents the concentration of acetate ion, and [HA] represents the concentration of undissociated acetic acid.
We can rearrange the Henderson-Hasselbalch equation to solve for the ratio \(\frac{A-}{HA}\):
\(\frac{A-}{HA} = 10^(pH - pKa)\)
Substituting the given values:
\(\frac{A-}{HA} = 10^(5.22 - 4.76)\)
\(\frac{A-}{HA} = 10^{0.46}\)
Using logarithmic properties, we can calculate:
\(\frac{A-}{HA} = 2.82\)
Therefore, at pH 5.22, the ratio of the concentrations of acetate ion to undissociated acetic acid is approximately 2.82.
Learn more about Henderson-Hasselbalch equation here:
https://brainly.com/question/31732200
#SPJ 4
Giving BRAINLIEST, please HELP!

Answers
Answer:
-65 kJ
Explanation:
To use Hess's Law, you need to combine the equations in a way that results in the desired equation. To add reactants/products, just add the equations and their subsequent heat of reactions. Subtracting reactants/products involves flipping the signs of enthalpies, duplicating reactants/products involves multiplying the coefficients and enthalpies by a desired number.
A + 2B --> C + D
+ B --> 2C + D
------------------------------------
A + 3B --> 3C + 3D
In this case, you only need to add the equations together to get the desired equation. Because you just add the equations, combine each equations' heat of reaction.
(-20 kJ) + (-45 kJ) = -65 kJ
The overall heat of reaction of the desired reaction is -65 kJ.
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
Ruth rode home at a constant speed for the first 10 minutes of the trip. What was her constant speed?
______ m/min
What was Ruth's average speed for the entire trip?
______ m/min
Ruth stopped to talk with another friend during the trip.
How far was she from home when she stopped?
______ m
How long was she stopped?
_____ min
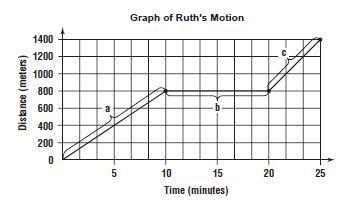
Answers
From the graph, the distance for the first 10 minutes is 600 meters.
10 minutes = 10 x 60 = 600 seconds
Ruth's speed for the first 10 minutes = distance/time
= 600/600 = 1 m/s
Average speed = total distance traveled/total time taken
Total distance = 1400 meters
Total time = 25 minutes = 25 x 60 = 1500 seconds
Average speed = 1400/1500 = 0.93 m/s
Ruth had traveled 800 meters before stopping to talk to her friend:
1400 - 800 = 600 meters.
Thus, she was 600 meters away from home when she stopped.
She stopped between 10 and 20 minutes:
20 - 10 = 10 minutes
Thus, she stopped to talk to her friend for 10 minutes.
More on velocity-time graphs can be found here: https://brainly.com/question/11290682
#SPJ1
PLEASE I NEED HELP ASAP

Answers
Answer:
Because the density of water is one
Which term describes “one or more cells that carry out all of the processes needed to sustain life”?
A. DNA
B. organism
C.organelles
D. cytoplasm
Answers
Answer:
B. Organism
Explanation:
Answer: The answer would be organism/organelle
If im wrong im srry!
Hope this helps!
Explanation:
it is often possible to determine mass of products without knowing the actual chemical equation for the reaction by using the concept of percent composition. for example, ammonia (nh3) can be used to synthesize hydrazine (n2h4). assuming that all the nitrogen in ammonia is converted to the nitrogen in hydrazine, how many grams of hydrazine can be produced from 19.2 g of ammonia?
Answers
11.4 g of hydrazine can be produced from 19.2 g of ammonia.
What is hydrazine?Hydrazine is a colorless, flammable chemical compound with a strong odor.
To solve this problem, you first need to calculate the percent composition of nitrogen in both ammonia and hydrazine. The percent composition of nitrogen in ammonia is 17.03%. The percent composition of nitrogen in hydrazine is 28.57%.
Next you need to calculate the amount of nitrogen in 19.2 g of ammonia. This is done by multiplying 19.2 g by the percent composition of nitrogen in ammonia, which is 17.03%. This gives you 3.26 g of nitrogen.
Now you need to calculate how much hydrazine can be produced from 3.26 g of nitrogen. This is done by dividing 3.26 g of nitrogen by the percent composition of nitrogen in hydrazine, which is 28.57%. This gives you 11.4 g of hydrazine.
Therefore, 11.4 g of hydrazine can be produced from 19.2 g of ammonia.
To learn more about hydrazine
https://brainly.com/question/14253477
#SPJ4
If one metric ton = 1000 kg, then how many metric tons are in 5.3 × 10^3 lb?
Answers
The metric tons of \(5.3 * 10^3\) lb is 11.74 metric tons
A metric ton is a unit of measurement for mass or weight, and it is equal to 1000 kilograms. It is commonly used to measure the weight of large objects such as ships, steel beams, or large quantities of material. It is also used to measure the capacity of a cargo container, or the weight of a truckload of goods. Metric tons are often abbreviated as "t" or "MT".
One metric ton is equal to 1000 kg, and one pound is equal to 0.45359237 kg.
To convert\(5.3 * 10^3\) lb to metric tons, we can use the following equation:
\(Metric tons =\frac{ (5.3 *10^3 lb) }{ (1000 kg/metric ton)} \\ = \frac{(5.3 * 10^3 lb) }{ (0.45359237 kg/lb)} \\ \\= 11.74 metric tons\)
Therefore, The metric tons of \(5.3 * 10^3\) lb is 11.74 metric tons
learn more about metric tons Refer:brainly.com/question/4062096
#SPJ4
how does the periodic table tell me if a element is solid, liquid, gas, or man made?
Answers
Answer:
if you search up the periodic table and go to any website it will let you press on the element and it will say if it is a gas,solid or liquid and it might say if it is man made!
Explanation:
i really hope this helps:)
How many moles are in 55g of NH3(g)?
Answers
Answer:
1 grams NH3 is equal to 0.058718113128665 mole. Note that rounding errors may occur, so always check the results. Use this page to learn how to convert between grams NH3 and mole.
Explanation:
Water is added to liquid isopropanol (a polar liquid) to form a solution of rubbing alcohol. Describe what happens on the molecular level as the water dissolves in the isopropanol. Select the correct step order from the drop-down menu for each step listed.
Answers
1. Water molecules approach isopropanol molecules. 2. Intermolecular forces between water and isopropanol molecules weaken and break. 3. Water molecules insert themselves between isopropanol molecules. 4. Water molecules surround and solvate the isopropanol molecules. 5. Isopropanol molecules fully dissolve in the water solution.
Here's a step-by-step explanation of what happens on the molecular level when water is added to liquid isopropanol to form a solution of rubbing alcohol:
1. The water and isopropanol molecules are both polar, meaning they have regions of positive and negative charges.
2. As water is added to the isopropanol, the polar water molecules begin to interact with the polar isopropanol molecules.
3. The positive region of a water molecule (hydrogen) is attracted to the negative region of an isopropanol molecule (oxygen).
4. Similarly, the negative region of a water molecule (oxygen) is attracted to the positive region of an isopropanol molecule (hydrogen).
5. These attractions, known as hydrogen bonds, cause the water and isopropanol molecules to mix and form a homogeneous solution.
6. The water effectively dissolves in the isopropanol as their molecular interactions create a uniform mixture.
Learn more about hydrogen bonding here: brainly.com/question/30885458
#SPJ11
What is an atom according to Modern understanding? Select the most accurate response.
O A solid, divisible particle that is the building block of matter.
O A solid, indivisible particle that is the building block of matter.
O The smallest particle of matter that retains the properties of that element.
O The smallest particle of matter that retains the properties of that element and is solid and indivisible
Answers
The answer is A.
The question is a bit tricky
What are the three technologies used to convert biomass energy into heat and electricity? Describe each one of them.
Answers
The three technologies used to convert biomass energy into heat and electricity are Combustion, Gasification, Anaerobic Digestion.These technologies enable the efficient utilization of biomass resources, reducing reliance on fossil fuels and contributing to renewable energy generation.
Combustion: Biomass combustion is a widely used technology that involves burning biomass materials, such as wood, agricultural residues, or dedicated energy crops, to produce heat and electricity. In this process, biomass is burned in a controlled manner, and the heat generated is used to produce steam, which drives a turbine connected to a generator. The combustion process releases carbon dioxide (CO2), but since biomass is considered a renewable energy source, the CO2 emitted is part of the natural carbon cycle and does not contribute to net greenhouse gas emissions.
Gasification: Biomass gasification is a thermochemical process that converts biomass into a combustible gas known as syngas. The biomass is subjected to high temperatures in a low-oxygen environment, resulting in the production of syngas, which mainly consists of carbon monoxide (CO), hydrogen (H2), and traces of other gases. The syngas can be used directly for heating purposes or for the production of electricity through internal combustion engines, gas turbines, or fuel cells.
Anaerobic Digestion: Anaerobic digestion is a biological process that breaks down biomass, such as animal manure, crop residues, or organic waste, in the absence of oxygen. During the anaerobic digestion process, microorganisms break down the biomass, producing biogas, which is primarily composed of methane (CH4) and carbon dioxide (CO2). The biogas can be combusted to produce heat and electricity, or it can be upgraded to biomethane and injected into the natural gas grid or used as a transportation fuel.
To know more about biomass energy, click here, https://brainly.com/question/32175810
#SPJ11
11) What is the relationship between temperature and the speed of sound? Include the concept of molecule
vibration in your explanation.
Answers
Answer:
Temperature is another condition that affects the speed of sound. Heat, like sound, is a form of kinetic energy. Molecules at higher temperatures have more energy and can vibrate faster and allow sound waves to travel more quickly. The speed of sound at room temperature air is 346 meters per second.
Which of the following explains how marie and pierre Curie tried to influence the use of their discovery?
Answers
Answer:
Option 3 is the correct approach.
Explanation:
In the given questions, options are not given. Please find the attachment of the complete question.
Marie Curie (1867 to 1934) as well as Pierre Curie (1859 to 1906) have been scientists but most leaders of nuclear science. Marie Curie becomes credited with the Radioactivity Hypothesis as well as the identification or observation of the components-Polonium and perhaps Radium.Pierre, Marie as well as Henri's pioneering work regarding radiation earned together the year 1903 Prize throughout Physics.Some other options in question are not relevant to something like the given situation. So that the option above will be the right one.

what will happen to the atoms or molecules of a substance when it boils
Answers
Answer: It will change to gas
Explanation: It will change to gas because of evaporation. When the heat intensifies it will happen.