Answers
Related Questions
Please Help! (Chemistry Molecules to Grams)

Answers
Answer:
20.95 g of caffeine, C₈H₁₀N₄O₂
Explanation:
From the question given above, the following data were obtained:
Number of molecules of C₈H₁₀N₄O₂ = 6.5×10²² molecules
Mass of C₈H₁₀N₄O₂ =?
From Avogadro's hypothesis,
1 mole of C₈H₁₀N₄O₂ = 6.02×10²³ molecules
Next, we shall determine the mass of 1 mole of C₈H₁₀N₄O₂. This can be obtained as follow:
1 mole of C₈H₁₀N₄O₂ = (8×12) + (10×1) + (4×14) + (2×16)
= 96 + 10 + 56 + 32
1 mole of C₈H₁₀N₄O₂ = 194 g
Thus,
194 g of C₈H₁₀N₄O₂ = 6.02×10²³ molecules
Finally, we shall determine the mass of caffeine, C₈H₁₀N₄O₂ that contains 6.5×10²² molecules. This can be obtained as follow:
6.02×10²³ molecules = 194 g of C₈H₁₀N₄O₂
Therefore,
6.5×10²² molecules = (6.5×10²² × 194) / 6.02×10²³
6.5×10²² molecules = 20.95 g of C₈H₁₀N₄O₂.
Therefore, 20.95 g of caffeine, C₈H₁₀N₄O₂ contains 6.5×10²² molecules
what are thetypes of luminous flame
Answers
Types of luminous flames:
1. Yellow Luminous Flame
2. Smoky Luminous Flame
3. Orange Luminous Flame
4. Blue Luminous Flame
Luminous flames are characterized by their visible glow, which is caused by the incomplete combustion of fuel. The presence of soot particles in the flame causes the emission of light. There are different types of luminous flames, which can be classified based on their fuel composition and burning conditions. Here are some common types of luminous flames:
1. Yellow Luminous Flame: This is the most common type of luminous flame, often seen in open fires, candles, and gas stoves. It appears yellow due to the presence of soot particles in the flame. Yellow flames indicate incomplete combustion of hydrocarbon fuels, such as methane, propane, or natural gas. The high carbon content in these fuels leads to the formation of soot, which emits visible light.
2. Smoky Luminous Flame: This type of flame is characterized by a significant amount of black smoke and soot production. It is commonly observed in poorly adjusted or malfunctioning burners or engines. The excessive presence of unburned fuel in the flame results in incomplete combustion and the emission of dark smoke particles.
3. Orange Luminous Flame: An orange flame indicates a higher combustion temperature compared to a yellow flame. It is often seen in more efficient burners or when burning fuels with a higher carbon content, such as oil or diesel. The higher temperature helps in burning more of the carbon particles, reducing the amount of soot and making the flame appear less yellow.
4. Blue Luminous Flame: A blue flame is typically associated with complete combustion. It indicates efficient burning of fuel, resulting in minimal soot formation. Blue flames are commonly observed in gas burners or Bunsen burners. The blue color is a result of the combustion of gases, such as methane, in the presence of sufficient oxygen.
It's important to note that the luminosity of a flame can vary depending on factors such as fuel-air mixture, combustion temperature, and the presence of impurities. Achieving complete combustion and minimizing the production of soot is desirable for efficient and cleaner burning processes.
for more questions on luminous
https://brainly.com/question/27163038
#SPJ8
Can someone help me on this?

Answers
Molecules are in constant motion due to their thermal energy, which is related to their temperature.
Why do molecules move faster and spread apart when heated?As the molecules move faster, they are more likely to overcome the intermolecular forces that hold them together, causing them to break apart and become less cohesive. This can cause a solid to melt into a liquid, or a liquid to evaporate into a gas.
In summary, heating a substance increases the kinetic energy of its molecules, causing them to move faster, collide with one another with greater force, and spread apart from each other, resulting in an increase in volume and thermal expansion.
Learn more about temperature:https://brainly.com/question/11464844
#SPJ1
volume reading
final: 28.5 mL
start: 7.5 mL
Total Volume: 21 mL
What is the Molarity of vinegar?
Based off the work information provided
Answers
The molarity of vinegar is 0.47368421 moles per liter.
To calculate this, we can use the following formula:
molarity = (initial_volume - total_volume_change) / final_volume
In this case, the initial volume is 7.5 mL, the total volume change is 21 mL, and the final volume is 28.5 mL. Plugging these values into the formula, we get:
molarity = (7.5 - 21) / 28.5 = -0.47368421
The negative value for molarity indicates that the solution is diluted. This is because the total volume of the solution increased by 21 mL, while the amount of solute (acetic acid) remained the same.
It is important to note that the molarity of a solution can change depending on the temperature. This is because the volume of a solution expands as it gets warmer. Therefore, it is important to measure the volume and temperature of a solution at the same time to get an accurate measurement of its molarity.
For such more questions on molarity
https://brainly.com/question/30704561
#SPJ8
Scientists are studying ways to improve the efficiency of solar cells by studying a process called photon upconversion . In this process , the energy of two photons can become combined to form a new photon with an energy equal to the sum of the two combined photons . With this process , abundant infrared radiation can be converted into visible light that can be used by solar celis to produce electricity . Suppose an infrared photon with a wavelength of 853 nm were combined with another infrared photon with a wavelength of 935 nm , what would be the wavelength of the new 'combined photon , in nm ?
Answers
Answer:
λ = 4.46 x 10⁻⁷ m = 446 nm
Explanation:
Applying Law of Conservation of Energy in this condition, we will get the following equation:
Total Energy of Combined Photons = Energy of 1st Photon + Energy of 2nd Photon
hc/λ = hc/λ₁ + hc/λ₂
hc/λ = hc(1/λ₁ + 1/λ₂)
1/λ = 1/λ₁ + 1/λ₂
where,
λ = wavelength of combined photon = ?
λ₁ = wavelength of 1st photon = 853 nm = 8.53 x 10⁻⁷ m
λ₂ = wavelength of 2nd photon = 935 nm = 9.35 x 10⁻⁷ m
Therefore,
1/λ = 1/(8.53 x 10⁻⁷ m) + (9.35 x 10⁻⁷ m)
1/λ = (0.1172 x 10⁷ m⁻¹) + (0.1069 x 10⁷ m)
1/λ = 0.2241 x 10⁷ m⁻¹
λ = 1/(0.2241 x 10⁷ m⁻¹)
λ = 4.46 x 10⁻⁷ m = 446 nm
Answer:
446
Explanation:
What volume, in mL, of 0.100 M NaOH is required to neutralize 35.0 mL of 0.102 M hydroiodic acid HI?
Answers
Answer:
35.7 mL NaOH
Explanation:
M1V1 = M2V2
M1 = 0.100 M NaOH
V1 = ?
M2 = 0.102 M HI
V2 = 35.0 mL
Solve for V1 --> V1 = M2V2/M1
V1 = (0.102 M)(35.0 mL) / (0.100 M) = 35.7 mL NaOH
The equation below represents the dissociation of vinegar which is a weak acid. How can you tell that it is an acid and it is weak? Just from looking at the equation.

Answers
Because it is not very effective at transferring \(H^{+}\) ions to water, vinegar is a weak acid. Less than 0.4% of the \(CH_{3}CO_{2}H\)molecules in a 1 M solution interact with water to create \(H_{3}O^{+}\)and \(CH_{3}CO_{2}^{-}\) ions. More than 99.6% of the acetic acid molecules are still whole.
Weak acidsAcids that partially dissociate in solution are referred to as weak acids. To put it another way, a weak acid is any acid that is not a strong acid. A weak acid's strength is influenced by how much it dissociates; the more it dissociates, the stronger the acid.In comparison to weak acids, strong acids have a lower pH. 2) Strong acids dissociate more, resulting in a lower pH (greater concentration of \(H^{+}\) ions in solution). 3) This can be verified by usingFor more information on weak acid kindly visit to
https://brainly.com/question/22104949
#SPJ1
If a jar of saltwater sits in the sun, the liquid level steadily decreases, and finally, crystals appear. Explain what is happening.
Answers
When a jar containing saline solution is exposed to sunlight, its liquid content gradually diminishes and eventually forms crystals as a result of the evaporation process.
What is the saltwater about?The energy from the sun's heat accelerates the movement of molecules in saltwater, which leads to their subsequent release from the liquid surface as water vapor. As a result of this process, the moisture transforms into gas and ascends, scattering throughout the atmosphere.
The concentration of salt in the remaining liquid increases as water evaporates from saltwater, as salt is unable to evaporate like water. As time passes, the salt concentration within the water triples to a point where it becomes incapable of remaining dissolved and commences the process of crystal formation.
Learn more about saltwater from
https://brainly.com/question/18761619
#SPJ1
A characteristic used to describe something is called a
Answers
adjectives that can be used to describe characteristics of people are called.
738.90 m has ____ significant figures
Answers
Answer: 4
Explanation: because the zero doesn't count
Given this slope equation, calculate the x-intercept (the point at which y = 0).
y = 0.523x + 16.8
Answers
Answer:
The answer is (-31.58, 0) when rounded to 2 decimal places.
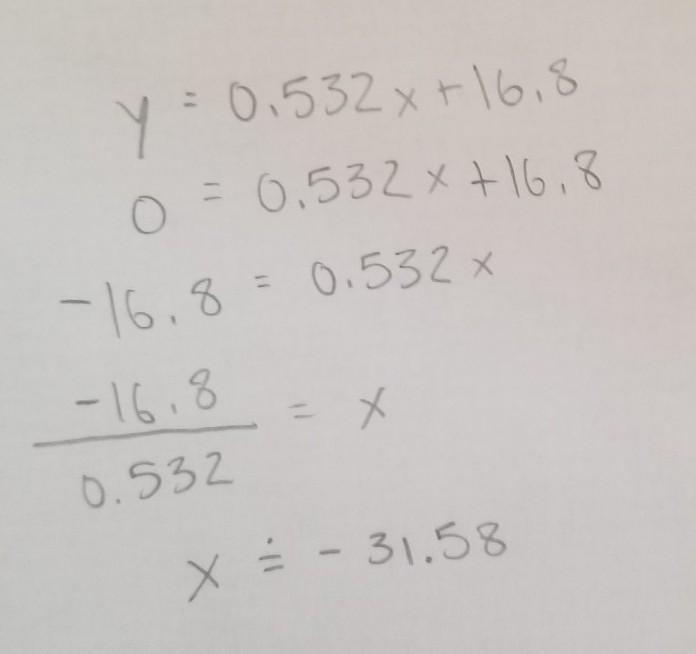
HELP!⚠️ I’ll mark you as brainlist.
Caffeine is a bitter stimulate drug and is found in vary quantities. It’s formula is C8H10N4O2. Match the number of moles of each element found
• numbers are 1.2, 2.0, 4.0, 5.0, 8.0, 10, 16, 27
• There are ___ mol of nitrogen in 0.30 mol
• There are ___ mol of hydrogen in 2.7 mol caffeine
• There are ___ mol of carbon in 2.0 mol caffeine
• There are __ mol of oxygen in 2.5 mol of caffeine
Answers
Answer:
• There are 1.8 mol of nitrogen in 0.30 mol of caffeine.
• There are 21.6 mol of hydrogen in 2.7 mol of caffeine.
• There are 16.0 mol of carbon in 2.0 mol of caffeine.
• There are 5.0 mol of oxygen in 2.5 mol of caffeine.
Explanation:
Using this information, we can calculate:
There are 4.8 x 10^23 (1.2 x 4) nitrogen atoms in 0.30 mol of caffeine.
There are 1.62 x 10^24 (2.7 x 6) hydrogen atoms in 2.7 mol of caffeine.
There are 1.6 x 10^24 (2 x 8) carbon atoms in 2.0 mol of caffeine.
There are 2.7 x 10^24 (2.5 x 2 + 8 x 2) oxygen atoms in 2.5 mol of caffeine.
To find the number of moles of each element, we just need to divide the number of atoms by Avogadro's number:
There are 0.8 mol of nitrogen in 0.30 mol of caffeine.
There are 2.7 mol of hydrogen in 2.7 mol of caffeine.
There are 0.2 mol of carbon in 2.0 mol of caffeine.
There are 1.5 mol of oxygen in 2.5 mol of caffeine.
The dimensions of a box are measured to be 18.4 inches by 17.92 inches by 26 inches. The volume of the box can be found by multiplying these three dimensions. What is the volume of the box expressed to the correct number of significant figures?
A.
8,600 in3
B.
8,573 in3
C.
8,572.9 in3
D.
8,570 in3
Answers
Answer:
Explanation:The volume of the box is 8.6 × 10³ in².
V = lwh = 26 in × 18.4 in × 17.92 in = 8.6 × 10³ in²
Note: The answer can have only two significant figures because that is all you gave for the length of the box.
bezglasnaaz and 23 more users found this answer helpful
How many full orbitals are in phosphorus
Answers
Answer:
three half-filled orbitals
Answer:
6p
Explanation:
It can hold a total of 6
a mixture of 121.5 g of p and 125.5 g of o2 reacts completely to form p4o6 and p4o10. find the masses of p4o6 and p4o10 that are formed by the reaction.
Answers
The masses of P4O6 and P4O10 that are formed by the reaction are 215.5 g and 278.3 g, respectively.
The reaction can be represented by the balanced chemical equation:
4P + 3O2 → P4O6
4P + 5O2 → P4O10
To find the masses of P4O6 and P4O10 that are formed by the reaction, we can use the mole ratio from the balanced chemical equation and the molar masses of the compounds.
First, we need to find the moles of P and O2 that are reacting:
moles of P = 121.5 g / 30.97 g/mol = 3.922 mol
moles of O2 = 125.5 g / 32.00 g/mol = 3.922 mol
Next, we can use the mole ratio from the balanced chemical equation to find the moles of P4O6 and P4O10 that are formed:
moles of P4O6 = 3.922 mol P / 4 mol P * 1 mol P4O6 = 0.9805 mol
moles of P4O10 = 3.922 mol P / 4 mol P * 1 mol P4O10 = 0.9805 mol
Finally, we can use the molar masses of P4O6 and P4O10 to find the masses of the compounds that are formed:
mass of P4O6 = 0.9805 mol * 219.89 g/mol = 215.5 g
mass of P4O10 = 0.9805 mol * 283.89 g/mol = 278.3 g
Therefore, the masses of P4O6 and P4O10 that are formed by the reaction are 215.5 g and 278.3 g, respectively.
To know more about mass of compounds, refer here:
https://brainly.com/question/2998100#
#SPJ11
2C2H6 + 7O2 —> 4CO2 + 6H2O how many grams of oxygen react in order to produce 7.2 moles of carbon dioxide
Answers
Answer:
403.2 grams of oxygen
Explanation:
According to the balanced chemical equation, 2C2H6 + 7O2 —> 4CO2 + 6H2O, 4 moles of carbon dioxide (CO2) are produced for every 7 moles of oxygen (O2) that react. Therefore, if 7.2 moles of carbon dioxide are produced, the number of moles of oxygen that react is (7.2 moles CO2) * (7 moles O2 / 4 moles CO2) = 12.6 moles O2.
Since the molar mass of oxygen is approximately 32 g/mol, the mass of oxygen that reacts is (12.6 moles O2) * (32 g/mol) = 403.2 g. Therefore, 403.2 grams of oxygen react in order to produce 7.2 moles of carbon dioxide.
Fe(ClO_4)_2
please help me with this question

Answers
Answer:
ferrous perchlorate
Explanation:
Which type of cell is shown in the following image?

Answers
Answer:
A prokaryotic cell is shown in the image
Explanation:
Prokaryotic cells are the most basic types. Since they are basic, they don't have a nucleus. All eukaryotic cells have it but prokaryotic cells don't have it. A nucleus contains organelles such as a mitochondria which the diagram doesn't show as well. Thus a prokaryotic cell is shown in the image.
Am I right????????????????Btw question was “Name four abiotic factors shown in the above prairie ecosystem?

Answers
Hope this helps
Have a great day/night
Feel free to ask any questions
plssss i need this immediately
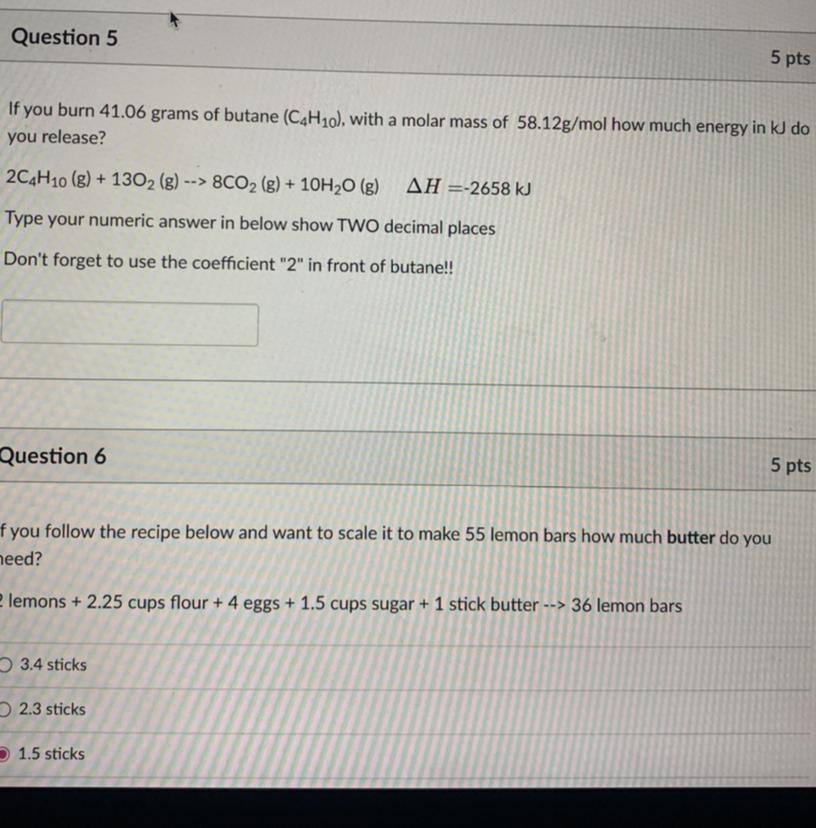
Answers
The heat that is going to be released in the reaction can be obtained as 930.3 kJ.
What is the heat of reaction?The heat of reaction, also known as the enthalpy change of a reaction, is the amount of heat energy that is released or absorbed during a chemical reaction. It is denoted by the symbol ΔH and has units of joules per mole (J/mol) or kilojoules per mole (kJ/mol).
Number of moles of butane = 41.06g/58 g/mol = 0.7 moles
If 2 mole release 2658 kJ
0.7 moles will release 0.7 * 2658/2
= 930.3 kJ
Learn more about heat of reaction:https://brainly.com/question/30464598
#SPJ1
How many grams of NaOH is needed to neutralize 90 mL of 1.5 N HCl?
Answers
mol = conc × v
= 1.5 × 0.09
= 0.135 moles of HCl
HCl + NaOH > NaCl + H2O
1 mole HCl = 1 mole NaOH
0.135 mol HCl = x
x = 0.135 mol NaOH
mass = mol × molar mass
= 0.135 × 40
= 5.4 g
NaOH = 23 + 16 + 1 = 40 g/mol
I'm not a 100% sure if it's correct
i need a little assistance with understanding this

Answers
Q_w = 307.3 J
C_m = .233 J/g•K
%error = 39.95%
For simplicity's sake, I'm relabeling
Q_w as Q1
m_w as m1
C_w as c1
T_eq as T2
T_w as T1
Q_m as Q2
m_m as m2
C_m as c2
experimental value as exp
actual value as actual
Question 1
Q1 = m1•c1•(T2-T1)
Identify what you know
m1 = 124g
c1 = 4.13 J/g • K
T2 = Final temperature = 22.3°C
T1 = Initial temperature = 21.7°C
Convert Celsius to Kelvin (C+273.15=K)
T2 = 295.45 K
T1 = 294.85 K
Plug in
Q1 = 124g•(4.13 J/g•K)•(295.45K - 294.85K)
Solve
Q1 ≈ 307.3 J
Question 2
-Q1 = Q2 = m2•(c2)•(T2-100)
Ignore Q2 for a second, and you're left with
-Q1 = m2•(c2)•(T2-100)
which is the same thing.
Identify what you know
Q1 = 307.3 J
m2 = 17g
T2 = 22.3°C
Plug in
-(307.3J) = 17g • c2 • (22.3°C-100°C)
Solve
-307.3 J = (-1320.9 g•°C) • c2
c2 = .233 J/g•°C or J/g•K (I'll explain later)
Question 3
%err = ((exp - actual)/actual) • 100%
Identify what you know
exp = .233 J/g•K
actual = .388 J/g•K
Plug in
%err = ((.233 J/g•K - .388 J/g•K)/ .388 J/g•K) • 100%
Solve
%err = -39.95 %
Take the absolute value
%err = 39.95%
Referring to earlier change in units:
The reason we can not use the K value of T2 (295.45K) is because the formula provided (T2-100) does not account for T2 being in K. It only accounts for T2 being in °C.
Which statement best explains why magnesium and chlorine combine in a 1:2 ratio?
Chlorine can accept twice as many electrons as magnesium has in its outer shell.
Magnesium has two valence electrons, and chlorine can accept one electron in its outer shell.
Magnesium has one electron shell, and chlorine has two electron shells.
Chlorine’s atomic number is twice magnesium’s atomic number.
Answers
The statement that best explains why magnesium and chlorine combine in a 1:2 ratio is; Magnesium has two valence electrons, and chlorine can accept one electron in its outer shell.
The number of electrons that an atom of an element has in its outermost shell determines the chemical formula of the compounds formed by atoms such elements.
Magnesium is in group 2, as such it has two electrons in its outermost shell while chlorine in group 17 only accepts one electron in its outermost shell. This one electron will give chlorine an inert gas configuration while the loss of two electrons give magnesium an inert gas configuration.
Therefore; The compound MgCl2 is formed in the ratio of 1:2 because Magnesium has two valence electrons, and chlorine can accept one electron in its outer shell.
Learn more: https://brainly.com/question/11527546
Answer:
Magnesium has two valence electrons, and chlorine can accept one electron in its outer shell.
Explanation:
Conx's 2023
Find the density of an object that has a mass of 5 kg and a
volume of 50 cm3.
Answers
\(\\ \rm\longmapsto Density=\dfrac{Mass}{Volume}\)
\(\\ \rm\longmapsto Density=\dfrac{5000}{50}\)
\(\\ \rm\longmapsto Density=100g/cm^3\)
18.00 g hydrated magnesium
sulfate is heated to drive off the
water, leaving 9.211 g MgSO4 solid.
What is the formula of the hydrate?
Answers
The formula of the hydrate would be \(MgSO_4.6H_2O\).
Deducing the formula of a hydrate saltIn order to get the formula of the hydrated salt, the empirical formula method can be used to know the mole of the salt and that of the water.
The formula of magnesium sulfate = \(MgSO_4\)
The formula of water = \(H_2O\)
Mass of hydrated magnesium sulfate = 18.00 g
Mass of anhydrous magnesium salt = 9.211g
Mass of water = 18 - 9.211
= 8.789 g
Now, let's find the mole equivalent of each component:
Molar mass of magnesium sulfate = 120.366 a/mol
Molar mass of water = 18.01 g/mol
Mole of magnesium sulfate anhydrous = 9.211/120.366
= 0.0765 mole
Mole of water = 8.789/18.01
= 0.4880 mole
Now, let's divide by the lowest mole:
Magnesium sulfate = 0.0765/0.0765
= 1
Water = 0.4880/0.0765
= 6.37
Thus, the mole ratio of magnesium sulfate and water of hydrate is 6 to 1. Therefore, the formula of the hydrated salt would be \(MgSO_4.6H_2O\).
More on hydrated salts can be found here: https://brainly.com/question/5586505
#SPJ1
Determine the molar mass of an unknown monoprotic acid to two decimal places if 16.98 mL of a 0.086 M NaOH solution were used to titrate 0.236 g of the unknown acid
Answers
1 ) Chemical equation
\(\text{NaOH + HX}\rightarrow H_2O+Na^++X^-\)HX represents the unknown acid.
2) Moles of NaOH in the reaction
\(M=\frac{\text{moles of solute}}{\text{liters of solution}}\)Convert mL into L
\(L=16.98mL\cdot\frac{1L}{1000mL}=0.01698L\)Plug in known values in the equation and solve for moles.
\(0.086M=\frac{\text{moles of NaOH}}{0.01698L}\)\(\text{mol NaOH= 0.086M}\cdot0.01698L=0.00146028\text{ mol NaOH}\)3) Moles of the unknown acid that reacted with 0.00146028 mol NaOH
Molar ratio
1 mol NaOH: 1 mol HX
\(\text{mol HX=0.00146028 mol NaOH}\cdot\frac{1\text{ mol HX}}{1\text{ mol NaOH}}=0.00146028\text{ mol HX}\)4) Molar mass of the unknown monoprotic acid
\(\text{Molar Mass=}\frac{\text{mass of solute (g)}}{moles\text{ of solute}}\)Plug in known values and solve
\(\text{Molar Mass}=\frac{0.236\text{ g HX}}{0.00146028\text{ mol HX}}=161.61\text{ g/mol}\)The molar mass of the unknown monoprotic acid is 161.61 g/mol
.
6NaCl + Ba3P2 - 3BaCl₂ + 2Na3P
If 17 moles sodium phosphide is produced, how many moles of sodium chloride is needed?
Round to the nearest hundredths.
Answers
6NaCl + Ba3P2 -> 3BaCl₂ + 2Na3P
We can see that for every 2 moles of Na3P, we need 6 moles of NaCl. This means there is a 3:1 molar ratio between NaCl and Na3P.
If 17 moles of Na3P is produced, we can calculate the moles of NaCl needed as follows:
17 moles Na3P * (6 moles NaCl / 2 moles Na3P) = 51 moles NaCl
Therefore, approximately 51 moles of sodium chloride are needed.
Consider the reaction of 1-butanol with K2Cr2O7, H2SO4, heat. Draw only the organic product derived from 1-butanol.
Answers
Answer:
Butanoic acid.
Explanation:
Hello,
In this case, when a primary alcohol such as 1-butanol (OH is bonded to a primary carbon) is oxidized in the presence of a strong oxidizing media such as potassium dichromate (K2Cr2O7) and sulfuric acid, the stepwise oxidation goes to the corresponding aldehyde with a further oxidation to the corresponding carboxylic acid:
\(R-CH_2-OH\longrightarrow R-COH\longrightarrow R-COOH\)
Therefore, on the attached picture you can find that the formed aldehyde is butanal and the inly organic product, due to the strong oxidizing media is finally butanoic acid.
Best regards.

Which of the following statements is FALSE in regards to herpes?
A. A symptom of herpes is flu-like symptoms.
B. Herpes simplex symptoms will occur in about a week of contraction.
C. Only herpes simplex I affects the face as a cold sore and herpes simplex Il affects the genitals.
D. There is no cure for HSV.
Answers
Answer:
B. is false
Explanation:
-there is no cure for hsv,
-herpes simplex I does affect the face as a cold sore, and herpes simplex II does affect the genitals
- a symptom of herpes can be flu like symptoms, everyone is different, some people don't even have any symptoms at all
If the mass of a stick of butter________ then no mass was created when it melted
Answers
Answer:
decreases
Explanation:
Melting is the process of turning solid materials into liquids by introducing heat. If the mass of a stick of butter decreases then no mass was created when it melted.
Melting is the process of a solid changing from a solid to a liquid when heated to a specific temperature. The set temperature at which a solid begins to transform into a liquid is known as the melting point.
A substance will change from a solid to a liquid when the temperature is raised to its melting point. since of this, the substance's volume will grow since its molecules will be spaced more apart.
To know more about melting, visit;
https://brainly.com/question/34580322
#SPJ7