a calorimeter has a heat capacity of 1265 j/o c. a reaction causes the temperature of the calorimeter to change from 22.34o c to 25.12o c. how many joules of heat were released in this process?
Answers
The amount of heat energy (q) released in the reaction can be calculated using the following formula:q = CΔTWhere C is the heat capacity of the calorimeter and ΔT is the change in temperature. Substituting the values into the formula, we get:q = 1265 J/°C × 2.78°Cq = 3520.7 Therefore, 3520.7 joules of heat were released in the reaction.
A calorimeter is an instrument used to measure the amount of heat energy transferred to or from a substance in a chemical reaction. It is designed to prevent heat exchange between the system and the surroundings.The heat capacity of the calorimeter is the amount of heat energy required to raise its temperature by one degree Celsius. In this case, the heat capacity of the calorimeter is 1265 J/°C.The temperature of the calorimeter was initially 22.34°C and it changed to 25.12°C due to a chemical reaction. The change in temperature (ΔT) can be calculated by subtracting the initial temperature from the final temperature.ΔT = T₂ - T₁ = 25.12°C - 22.34°C = 2.78°CSince the calorimeter is designed to prevent heat exchange with the surroundings, the heat energy released in the reaction is absorbed by the calorimeter. The amount of heat energy (q) released in the reaction can be calculated using the following formula:q = CΔTWhere C is the heat capacity of the calorimeter and ΔT is the change in temperature.Substituting the values into the formula, we get:q = 1265 J/°C × 2.78°Cq = 3520.7 JTherefore, 3520.7 joules of heat were released in the reaction.
To Know more about heat energy visit:
brainly.com/question/29210982
#SPJ11
Related Questions
HELP HELP HELPP! Based on the formula of kinetic energy, how will the temperature change if you increase the average velocity of the molecules in a gas?

Answers
Answer:
Explanation:
In a hot gas, the molecules move faster than in a cold gas; the mass remains the same, but the kinetic energy, and hence the temperature, is greater because of the increased velocity of the molecules. ... We can sense that one gas is hotter than another gas and therefore has a higher temperature.
what is the opposite of a heterotroph
Answers
The opposite of heterotroph is autotroph.
What is heterotroph?
An organism is referred to as a heterotroph if it consumes other plants or animals for food and energy. Its origins are in the Greek words hetero, which means "other," and trophe, which means "nutrition."
Examples - Human, birds, dogs, etc.
What is autotroph?
A primary producer, also known as an autotroph, is an organism that uses energy from light or inorganic chemical reactions to create complex organic chemicals from simple ones, such as carbon dioxide.
Example - plants, algae etc.
The opposite of heterotroph is autotroph.
To know more about autotroph, check out:
https://brainly.com/question/10253663
#SPJ1
which statement is true about alkali metals?
A. Some of them explode when exposed to water.
B. They aren't conductors of heat or electricity.
C. They're in group 18 of the periodic table.
D. They aren't very reactive
Answers
Answer:
answer is a . because they have enough heat is given off during exothermic reaction
Answer: answer is a . because they have enough heat is given off during exothermic reaction
Explanation:
What is magma called once
leaves a volcano?
Answers
Answer:
lava
Explanation:
an insulated tank is divided by a thin partition. (a) on the left is 0.79 mole of n2 at 1 bar and 298 k; on the right is 0.21 mole of o2 at 1 bar and 298 k. the partition ruptures. what is dsuniv for the process? (b) on the left is 0.79 mole of n2 at 2 bar and 298 k; on the righ
Answers
The entropy is -3.65 and -0.049 J per kelvin per mole respectively are the entropy of an insulated tank.
Entropy is a measure of randomness in a system. It is a state function. It can be denoted by heat supplied for a reversible system per temperature.
The final pressure will be the partial pressure of each gas in the mixture.
a) Partial pressure of nitrogen = mole fraction of nitrogen x total pressure = (0.79 mol) × (1 + 1 bar)/(0.79 + 0.29 mol) = 1.46 bar
Partial pressure of oxygen = mole fraction of oxygen x total pressure = (0.29 mol) ×(1 + 1 bar)/(0.79 + 0.29 mol) = 0.54 bar
Therefore,
ΔS of nitrogen= -R ln1.46 = -0. 38R
∆S of oxygen= -R ln 0.54= -0. 61R
ΔSuniv=−(0.79mol/0.79+0.29mol)×0.38 R−(0.29mol/0.79+0.29mol)×0.61
R=−0.27 R−0.16
R=−0.44
R=−3.65JK−1mol−1
(b) Partial pressure of nitrogen = mole fraction of nitrogen x total pressure = (0.79 mol) × (2 + 1 bar)/(0.79 + 0.29 mol) = 2.19 bar
Partial pressure of oxygen = mole fraction of oxygen x total pressure = (0.29 mol) × (2 + 1 bar)/(0.79 + 0.29 mol) = 0.80 bar
ΔS of nitrogen gas=−Rln1.46=−0.09R
ΔSof sulphur oxide =−Rln0.54=0.22R
ΔSuniv=−(0.79mol/0.79+0.29mol)×0.09R+(0.29mol/0.79+0.29mol)×0.22
R=−0.066 R+0.060
R=−0.049JK−1mol−1
To know more about entropy visit
https://brainly.com/question/13146879
#SPJ4
The highest pressure measured in Jupiter's upper atmosphere by the Galileo probe is ×1.67104torr. Calculate the highest pressure measured in mmHg and atm. Round each of your answers to 3 significant digits.
Answers
Answer: 16700 mmHg , 22,0 atm
Explanation: 1 torr = 1 mmHg, so p = 16700 torr = 16700 mmHg
1 atm = 101325 Pa = 760 torr
16700 torr / 760 = 21,97 atm
if you mixed 20 mL of a 12 M acid into 500 mL, what's the concentration of the final solution (rounded to one sig fig)?
Answers
The concentration of the final solution, rounded to one significant figure, is 0.5 M.
What is the concentration of the final solution?To find the concentration of the final solution, we need to use the formula:
C1V1 = C2V2
where;
C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.In this problem, we can plug in the given values and solve for C2:
C1 = 12 M
V1 = 20 mL = 0.02 L
V2 = 500 mL = 0.5 L
C1V1 = C2V2
(12 M)(0.02 L) = C2(0.5 L)
0.24 = 0.5C2
C2 = 0.48 M
Learn more about concentration of solution here: https://brainly.com/question/26255204
#SPJ1
If these teams are pulling with the same amount of force what will happen?
The team with the green and orange shirts will win.
bo
The team with the yellow and red shirts will win.
They will not move and neither team will win.
Answers
Ascorbic acid has a molar mass of 176. 14 g/mol. What is the molecular formula of ascorbic acid?
Answers
The molecular formula of Ascorbic acid is C6H8O6 having the molar mass of 176.14 g/ mole.
Ascorbic acid is known as the chemical name for Vitamin C. Ascorbic acid is commonly found in high concentrations in citrus fruit. This acid is also found in tomatoes, broccoli, and many other fruits and vegetables. Vitamin C is a nutrient of the body needs to form blood vessels, cartilage, muscle and collagen in bones. This vitamin is also vital to the body's healing process.
A molecular formula is defined as a chemical formula of a molecular compound that shows the kinds and numbers of atoms present in a molecule of the compound. This is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule using chemical element symbols and numbers.
A molecule of the ascorbic acid will have a mass of 176.124 atomic mass units.
This is determined by adding 6 X 12.011 for carbon + 8 X 1.008 for hydrogen + 6 X 15.999 for oxygen.
This is equals to the 72.066 for carbon + 8.064 for hydrogen + 95.994 for oxygen. Added together, these equal 176.124 molecular mass.
To learn more about Ascorbic acid
https://brainly.com/question/16996726
#SPJ4
PESTLE and SWOT analysis for Harvard case CLEAN EDGE RAZOR: SPLITTING HAIRS IN PRODUCT POSITIONING.
Answers
The PESTLE and SWOT analyses provide a comprehensive assessment of the external and internal factors influencing the Harvard case "Clean Edge Razor: Splitting Hairs in Product Positioning."
The PESTLE analysis examines the external factors that may impact the case. Politically, regulations on product safety and environmental sustainability could affect the razor industry. Economically, consumer purchasing power and economic trends may impact the demand for high-end razors. Sociocultural factors, such as grooming habits and preferences, influence consumer behavior. Technological advancements, such as electric razors and online shopping, pose opportunities and challenges. Legal factors include intellectual property rights and advertising regulations. Environmental considerations involve sustainability and eco-friendly practices.
The SWOT analysis evaluates the internal strengths and weaknesses of the case. Strengths may include the Clean Edge brand reputation and innovative product features. Weaknesses could involve high manufacturing costs or limited market presence. Opportunities may arise from market growth and expanding into new segments. Threats may come from intense competition and changing consumer preferences.
By conducting both analyses, the case can gain insights into the broader industry landscape, identify potential risks and opportunities, and assess its own internal capabilities. This holistic understanding aids in making informed decisions and formulating effective strategies for positioning the Clean Edge Razor product.
Learn more about environmental sustainability here:
https://brainly.com/question/28602390
#SPJ11
nitrous oxide/oxygen analgesia use in dentistry dates back to 1844 and is also known as:
Answers
Nitrous oxide/oxygen analgesia use in dentistry dates back to 1844 and is also known as laughing gas.
What is nitrous oxide known as ?It is a colorless, odorless, and tasteless gas that has been used for sedation in dentistry for over 170 years. It is a safe and effective way to manage anxiety and pain during dental procedures.
Laughing gas works by depressing the central nervous system, which results in a feeling of relaxation and reduced sensitivity to pain. It is also a mild anesthetic, which can help to numb the area being treated.
It is administered through a mask that the patient wears over their nose and mouth. The dentist can control the amount of gas that the patient receives.
Find out more on nitrous oxide at https://brainly.com/question/30570662
#SPJ4
Calculate the decrease in temperature when 6.00 L at 20.0 °C is compressed to 4.00 L.
Answers
To calculate the decrease in temperature when 6.00 L at 20.0 °C is compressed to 4.00 L, we need to use the ideal gas law, PV = nRT.
How do you calculate temperature drop due to pressure drop?You subtract the final temperature from the starting temperature to find the difference. So if something starts at 50 degrees Celsius and finishes at 75 degrees C, then the change in temperature is 75 degrees C – 50 degrees C = 25 degrees C. For decreases in temperature, the result is negative.Use the formula: k = T1/P1. For example, if a gas at an initial temperature of 300 K and an initial pressure of 100 Pa, drops by 50 Pa, the proportionality constant k = 3 K/Pa = 300/100 = T1/P1. Multiply the drop in pressure by the proportionality constant k to obtain the drop in temperature.First, we need to convert the temperature from Celsius to Kelvin: 20.0 °C + 273.15 = 293.15 KNext, we need to find the initial pressure, using the ideal gas law: P1V1 = nRT, where P1 = initial pressure, V1 = initial volume, n = number of moles, R = ideal gas constant, T = temperature in Kelvin. We know that V1 = 6.00 L, T = 293.15 K, and R = 8.314 J/mol·K. We do not know the value of n, so we can assume it is 1 mole. Therefore, P1 = (nRT) / V1 = (1 x 8.314 x 293.15) / 6.00 = 476.89 J/LFinally, we can calculate the final pressure, using the same equation: P2V2 = nRT, where P2 = final pressure, V2 = final volume. We know that V2 = 4.00 L, T = 293.15 K, and R = 8.314 J/mol·K. Substituting these values into the equation, we get P2 = (nRT) / V2 = (1 x 8.314 x 293.15) / 4.00 = 715.34 J/LTo find the decrease in temperature, we subtract the final pressure from the initial pressure: P1 - P2 = 476.89 J/L - 715.34 J/L = -238.45 J/LSo the decrease in temperature when 6.00 L at 20.0 °C is compressed to 4.00 L is -238.45 J/L.To learn more about temperature refer to:
https://brainly.com/question/14671786
#SPJ1
Which of these is a physical property?
A)ability to burn a piece pf paper
B) pure sodium reacts violently with water
C)the boiling point of the water is 100⁰C
D)helium does not tend to react with anything
Answers
Answer:
the answer is B because it is a chemical reaction
A mixture of two amino acids, glycine and alanine, what is the best separating technique to separate them
Answers
One of the best techniques to separate a mixture of two amino acids, glycine and alanine, is chromatography. Chromatography is a versatile separation method commonly used in chemistry and biochemistry to separate and analyze mixtures.
Chromatography is a versatile separation technique used in various scientific fields to separate and analyze complex mixtures of substances. It is based on the principle of differential migration of components in a sample mixture through a stationary phase and a mobile phase. The stationary phase can be a solid or a liquid, while the mobile phase is typically a liquid or a gas.
During chromatographic analysis, the sample mixture is introduced into the system, and the different components interact differently with the stationary and mobile phases. This leads to their separation as they travel at different rates through the system. The separated components are then detected and analyzed. Chromatography finds applications in a wide range of disciplines such as chemistry, biochemistry, pharmaceuticals, forensics, environmental science, and more.
To know more about Chromatography refer to-
brainly.com/question/28731153
#SPJ4
Please help I need it asap

Answers
Note that the experiment described is the Rutherford Scattering Experiment invented by Ernest Rutherford for simulating alpha particle dispersion. See the procedure below.
How do you carry out the Rutherford Scattering Experiment?Students might use the above contraption to simulate alpha particle dispersion by rolling the marble down the moveable slope towards the circular weight placed on the huge sheet of lined paper.
The scattering of alpha particles can be studied by measuring the angle of deflection using a protractor.
The significance of this experiment is that it contributes to our understanding of subatomic particle behavior, notably how alpha particles disperse when they contact with other particles. See a clearer image attached.
Learn more about Alpha Particles:
https://brainly.com/question/2288334
#SPJ1

Stretching a rubber band is an example of________.
A. frictional
B. tension
C. gravitation
D. compression
Answers
Answer:
B. tension
Explanation:
a straight stretched rubber band experiences tension which is a stress that tries to pull matter apart.
Which layer of the atmosphere is chemically stratified, with the lightest molecules riding above heavier molecules?a. the thermosphereb. the tropospherec. the mesosphered. the stratosphere
Answers
The stratosphere is the most chemically stratified layer in the atmosphere.
What is the stratosphere?
The stratosphere is the layer of the atmosphere that is chemically stratified, with the lightest molecules riding above heavier molecules. The ozone layer, which contains a high concentration of ozone (O3) molecules, is located in the stratosphere.
The stratosphere is located above the troposphere and extends from about 15 km to about 50 km above the Earth's surface. This layer is characterized by a relatively constant temperature and is mostly composed of nitrogen and oxygen molecules.
The ozone layer protects the Earth from harmful ultraviolet radiation from the sun. Because ozone is lighter than the air below it, it remains in the stratosphere, while the heavier gases in the air below do not reach that high. This chemical stratification is maintained by the balance of ozone production and destruction processes in the stratosphere.
Hence, the stratosphere is the most chemically stratified layer in the atmosphere.
To learn more about the stratosphere from the given link:
https://brainly.com/question/28097222
#SPJ4
Indicate the charge the following elements as they achieve the noble gas configuration.
Ga O Br P Rb As
S Mg Al Se Li I
Answers
Answer:
See explanation
Explanation:
Ga is in group 13 hence it must loose three electrons to form Ga^3+ in order to achieve the noble gas configuration because it has three electrons on its outermost shell.
O is in group 16 hence it must accept two electrons in order to attain the noble gas configuration to form O^2- since oxygen has six electrons on its outermost shell.
Br in group 17 has seven electrons in its outermost shell hence it must form Br^- (gain one electron) in order to attain the noble gas configuration.
P in group 15 must accept three electrons and form P^3- in order to attain the noble gas configuration since it has five electrons on its outermost shell.
S is in group 16 hence it must accept two electrons in order to attain the noble gas configuration to form S^2- since sulphur has six electrons on its outermost shell.
Mg in group 2 has two electrons on its outermost shell and must loose both to attain the noble gas configuration forming Mg^2+.
Al is in group 13 hence it must loose three electrons to form Al^3+ in order to achieve the noble gas configuration because it has three electrons on its outermost shell.
Se is in group 16 hence it must accept two electrons in order to attain the noble gas configuration to form Se^2- since selenium has six electrons on its outermost shell.
Lithium is in group 1 and must loose its only outermost electron in order to attain the noble gas configuration to form Li^+.
Rb is in group 1 and must loose its only outermost electron in order to attain the noble gas configuration to form Rb^+.
As in group 15 must accept three electrons and form As^3- in order to attain the noble gas configuration since it has five electrons on its outermost shell.
I in group 17 has seven electrons in its outermost shell hence it must form I^- (gain one electron) in order to attain the noble gas configuration.
5. how can a diene be differentiated from an alkene in this experiment?
Answers
In an experiment, a diene can be differentiated from an alkene by performing a reaction that specifically involves dienes. One such reaction is the Diels-Alder reaction, which is a cycloaddition reaction between a diene and a dienophile (an alkene or alkyne). This reaction is highly specific for dienes and does not occur with simple alkenes.
The Diels-Alder reaction involves the formation of a cyclic compound by the reaction of a diene with a dienophile, resulting in the formation of a new ring system. The reaction proceeds through a concerted mechanism and is typically thermally activated. The reaction is highly regioselective and stereospecific.
To differentiate a diene from an alkene using the Diels-Alder reaction, you can perform the following steps:
1. Choose a dienophile that is known to undergo the Diels-Alder reaction. Common dienophiles include maleic anhydride, acrylates, and fumarates.
2. Prepare a reaction mixture containing the suspected diene and the dienophile. This can be done by dissolving both compounds in an appropriate solvent.
3. Heat the reaction mixture to a suitable temperature. The reaction is typically performed at elevated temperatures, ranging from 80-150°C.
4. Monitor the reaction progress by various techniques such as thin-layer chromatography (TLC) or spectroscopic methods like infrared (IR) or nuclear magnetic resonance (NMR) spectroscopy.
5. If the suspected compound is a diene, it will react with the dienophile via the Diels-Alder reaction, resulting in the formation of a new cyclic compound. This can be confirmed by analyzing the reaction mixture using the techniques mentioned above.
6. If the suspected compound is an alkene, it will not undergo the Diels-Alder reaction with the chosen dienophile, and there will be no formation of a cyclic compound.
By performing the Diels-Alder reaction and observing the formation or lack of formation of a cyclic compound, you can differentiate a diene from an alkene in the experiment.
To know more about the Diels-Alder reaction visit:
https://brainly.com/question/30751490
#SPJ11
please answer
Classifying Unfamiliar Substances (Chemical names): Questions to be asking
1. Is it on the Periodic table?
a.Yes – Then its an element
b. No – Go to Number 2.
2. Is it an “Ide”?
a.Yes – Then it is a compound
b. No – Go to Number 3
3. Is it an “Ate”?
a.Yes – Then it is a compound
b. No – Go to Number 4
4. Is it an “Ane”?
a.Yes – Then it is a compound
b. No – Go to Number 5
5. Does it have “and” in it?
a.Yes – then it is a Mixture
b.No – then the substance could still be a Mixture
10
Substance
Classification
(eg Element)
Reason
Oxygen
Element
On periodic table
Petroleum
Hydrogen and
Helium
Iron Sulphide
Carbon Dioxide
Salt Water
Milk and Sugar
Silver Nitrate
Sodium Carbonate
Carbon Electrode
Astatine
Argon
Oxidane
Iron and Sulphur
Methane
Answers
Answer:
1) petroleum, mixture, not found on periodic table
2) hydrogen and helium, mixture, contains "and"
3) iron sulphide, compound, ends with "ide"
4) carbon dioxide, compound, ends with "ide"
5) salt water, mixture, not found on periodic table
6) milk and sugar, mixture, contains "and"
7) silver nitrate, compound, ends with "ate"
8) sodium carbonate, compound, ends with "ate"
9) carbon electrode, mixture, not found on periodic table
10) astatine, element, found on periodic table
11) argon, element, found on periodic table
12) oxidane, compound, contains "ane"
13) iron and sulphur, mixture, contains "and"
14) methane, compound, contains "ane"
Explanation:
HELP MEEEEE (100 points)
In these chemical compounds, C is for carbon, O is for oxygen, N is for nitrogen, and K is for potassium.
Which chemical compound is most likely inorganic?
a.H2O
b.C12H22O11
c.C6H12O6
d.CH4
Answers
Answer: b: C12H22O11
Explanation: have a good day!
Answer:
b.C12H22O11
Explanation:
I love chemistry.
The elements on the Periodic Table of the Elements are arranged in order of increasing
Answers
Answer: The Atomic Number increases. Hope this helps!!!
Explanation:
Elements are arranged from left to right and top to bottom in order of increasing atomic number. Order generally coincides with increasing atomic mass.
Answer:
The Atomic number increases....
Explanation:
Duh
Question 1 of 10
Match the term in Column 1 to the corresponding statement in Column 2.
BRUH CAN SOMEONE HELP!?!?!?
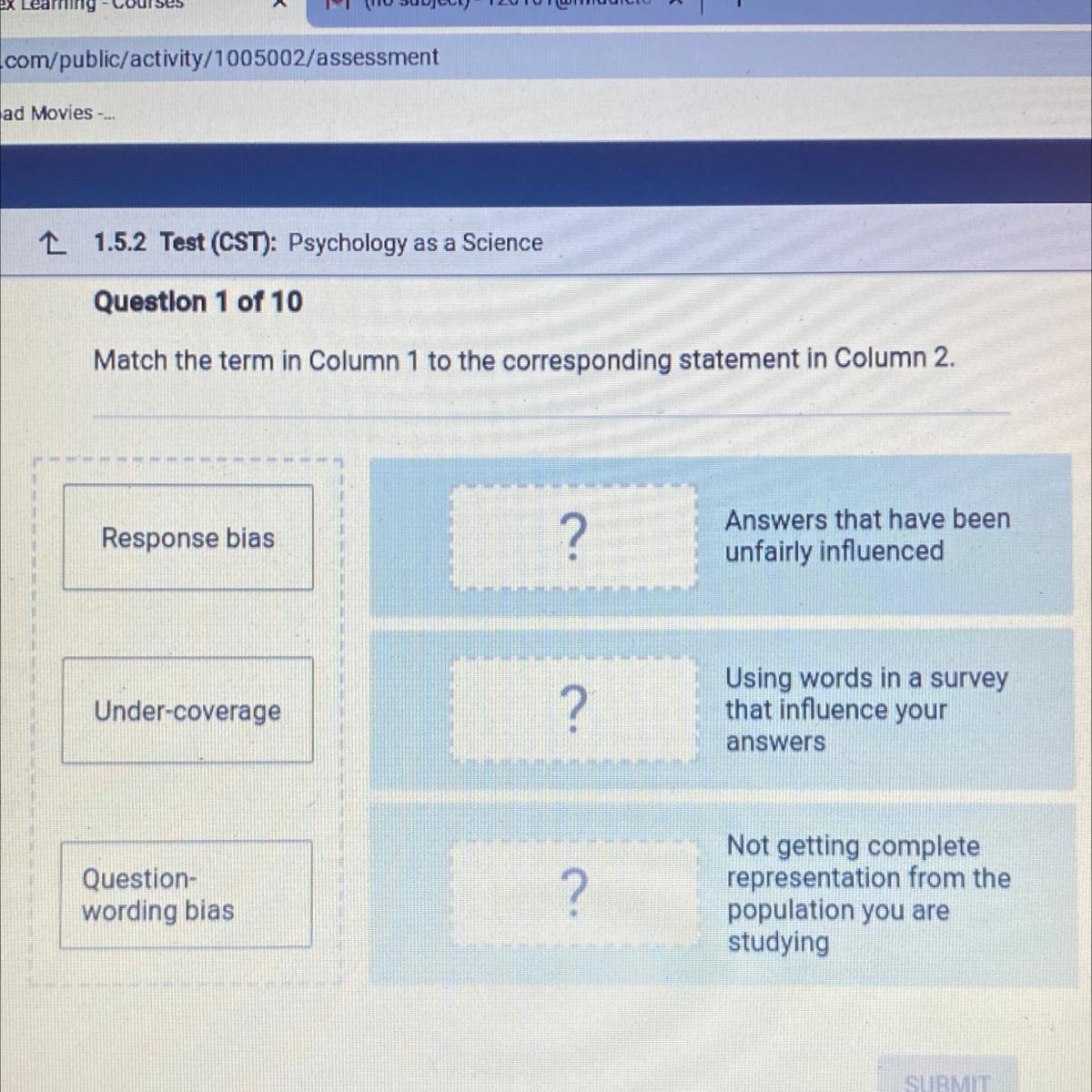
Answers
the 6^14 text(c) activity of an ancient artifact is found to be 0.170 bq per gram of carbon. what is its age? the half-life of 6^14 text(c) is 5730 yr.
Answers
The 6¹⁴ text(c) activity of an ancient artifact is found to be 0.170 bq per gram of carbon. 49,305 is its age of ancient artifact.
To find the age of the artifact, we can use the formula for exponential decay:
N(t) = N₀ e(-λt)
Where N0 is the initial amount of 6¹⁴ text(c), N(t) is the current amount, λ is the decay constant (which is related to the half-life), and t is the time elapsed.
We know that the half-life of 6¹⁴ text(c) is 5730 years. This means that after 5730 years, half of the initial amount of 6¹⁴ text(c) will have decayed. So, we can find λ as follows:
λ = ln(2) / t1/2
λ = ln(2) / 5730
λ = 0.000120968
Now, we can use the activity of the artifact to find the current amount of 6¹⁴ text(c):
A = λN
N = A / λ
N = 0.170 / 0.000120968
N = 1403.94
So, the artifact currently has 1403.94 grams of 6¹⁴ text(c). We can use this value and the known initial amount of 6¹⁴ text(c) (which would have been present in the organism when it died) to find the age of the artifact:
N(t) = N₀ e(-λt)
1403.94 = N₀e(-0.000120968t)
We can solve for t:
ln(1403.94/N0) = -0.000120968t
t = -ln(1403.94/N0) / 0.000120968
We don't know the exact initial amount of 6¹⁴ text(c) in the organism, but we can assume it was the same as the current amount in the atmosphere (which is about 1.2 × 10¹² grams). So:
t = -ln(1403.94/1.2e12) / 0.000120968
t = 49,305 years
Therefore, the artifact is approximately 49,305 years old.
Learn more about Half life here
https://brainly.com/question/31666695
#SPJ11
Explain why the product at the negative electrode is not always a metal.
Answers
The product at the negative electrode is not always a metal because it will only be produced if it is less reactive than hydrogen.
What is a Metal?These are materials which donate electrons in a chemical reaction and have high heat and electrical conductivity.
Metals are more reactive than hydrogen which is why it is not always produced at the cathode.
Read more about Metals here https://brainly.com/question/4701542
#SPJ2
Which of these is the basis for some traditional calendars?
A-cycles of the moon
B-constellations in the heavens
C-solar and lunar eclipses
D-the appearance of seasonal rainstorms
Answers
Answer:
A-cycles of the moon
Explanation:
The people of Ancient Greece, Rome and China counted their year based on a 12 moon cycle ( that is, about 354 days). A 13th cycle of the moon is included so that the lunar year remains in step with the seasons of the year.
Today, the Gregorian calendar is mostly used around the world. This calendar was
actually adapted from an earlier lunar version. Ancient civilizations used the phases of the moon to help identify the seasons.
The major difference between the lunar calendar and the solar calendar is the kind of celestial body that is used to determine time.
How do carbon atoms form many organic compounds
Answers
Carbon has four valence electrons, so it can achieve a full outer energy level by forming four covalent bonds. When it bonds only with hydrogen, it forms compounds called hydrocarbons. Carbon can form single, double, or triple covalent bonds with other carbon atoms.
a wrong
b wrong
c right
d wrong
mL? PLEASEEE HELP
What is the answer

Answers
The most outer solid portion of
the planet Earth
Answers
Answer:
The lithosphere is the most outer solid portion of
he most outer solid portion ofthe planet Earth.
crust is the outermost layer of earth.
Researchers are studying a fish population in a small pond that has algae as the only producer in the ecosystem. The scientists are concerned about what might happen to the fish, if the algae suddenly disappear. Why is this a concern? What might be done to prevent this from occurring?
Answers
Explanation:
Algae is a group of a photosynthetic eukaryotic organisms that plays a very important role on the ecosystem. It forms the lower base of the food chain and supports all the life forms of the ecosystem. It takes up water and carbon dioxide from the atmosphere to prepare food during the process of photosynthesis and maintains a balance in the ecosystem.
In the context, if the algae disappears, the fish might die as the small fishes feeds on this algae. This is a concern as the algae is main component of food source and forms the base of the food chain. If there will be no algae there will be no life form on earth, as all species depend on each other to survive.
In order to prevent this from occurring, we must protect our algae. We must plant more trees, keep our water bodies healthy, we should not litter our ponds and lakes. We should also help these algae to grow and survive so that other life forms can feed them and thus maintain the ecosystem.