Answers
Answer:
Copper
Explanation:
Copper has excellent electrical conductivity, as well as malleability and ductility, making it easy to form into thin wires. Hope this helps!
Related Questions
where is the answers?>???!!!!
Answers
Answer:
Explanation:
For what?
PLEASE HELP ME NO ONE WILL THIS IS IMPORTANT ALOT OF POINTS! A student's favorite drink is sweet tea. Every morning he makes it by adding exactly thirty grams of sugar and one tea bag to one liter of hot water. Some days his tea does not taste as sweet as other days. Those same days he notices that there is sugar sitting at the bottom of the cup that will not dissolve no matter how long he stirs. He decided to filter out the remaining sugar and keep track of the data in the graph below.
Explain why different amounts of sugar might dissolve at different times.
Consider:
1. which day the least sugar dissolved and which day the most sugar dissolved.
2. what could have caused less sugar to dissolve on some days
3. what the student could do to his drink to make more sugar dissolve.
Be sure to consider the completeness of your response, supporting details, and accurate use of terms. Your response should be 6-8 complete sentences.

Answers
The solubility of a substance, such as sugar, depends on several factors, including temperature, pressure, and the presence of other solutes. In this case, the student adds the same amount of sugar and tea bag to the same amount of hot water every day, but the temperature of the water could vary from day to day, affecting how much sugar dissolves.
According to the graph, the least amount of sugar dissolved on day 3, while the most sugar dissolved on day 5. The difference in temperature on these days could explain this variation. On day 3, the water may have been cooler, making it more difficult for the sugar to dissolve. On the other hand, on day 5, the water may have been hotter, which could have increased the solubility of the sugar.
To increase the amount of sugar that dissolves in the tea, the student could try using hotter water or stirring the sugar more vigorously to distribute it evenly throughout the water. Alternatively, the student could try adding the sugar gradually while stirring to give it more time to dissolve before adding more.
Answer:
I'm not 100% sure on this because mine hasn't been graded yet, but here are the answers I submitted.
Explanation:
Which day the least sugar dissolved and which day the most sugar dissolved:
Day 4 is when the least sugar dissolved, and Day 2 is when the most sugar dissolved.
What could have caused less sugar to dissolve on some days:
The temperature of the tea could have caused the sugar not to dissolve on some days.
What the student could do to his drink to make more sugar dissolve.
The student would need to add the sugar to the tea as soon as it's done boiling. The Sugar will dissolve faster in a warmer tea due to more energy of movement.
Be sure to stir the tea as you add the sugar. Stirring the sugar into the tea speeds up the rate of dissolving by helping distribute the sugar particles throughout the tea.
If the student were to use granulated sugar, those are smaller particles and have greater surface area. Greater surface area allows for more contact between the tea and the sugar.
Use standard enthalpies of formation to calculate ΔH∘rxn for the following reaction. 3NO2(g)+H2O(l)→2HNO3(aq)+NO(g)
Answers
The standard enthalpy change (∆H°rxn) for the given reaction is -38.0 kJ/mol. This negative value indicates that the reaction is exothermic, meaning that it releases heat to the surroundings.
The standard enthalpies of formation (∆Hf°) for the reactants and products involved in the reaction are: ∆Hf°[NO2(g)] = +33.2 kJ/mol. ∆Hf°[H2O(l)] = -285.8 kJ/mol. ∆Hf°[HNO3(aq)] = -207.2 kJ/mol. ∆Hf°[NO(g)] = +90.4 kJ/mol. We can use these values to calculate the standard enthalpy change (∆H°) for the reaction using the following equation:
∆H°rxn = Σn∆Hf°(products) - Σm∆Hf°(reactants) where n and m are the stoichiometric coefficients of the products and reactants, respectively. Substituting the values, we get:∆H°rxn = [2 × ∆Hf°(HNO3(aq))] + [∆Hf°(NO(g))] - [3 × ∆Hf°(NO2(g))] - [1 × ∆Hf°(H2O(l))]. ∆H°rxn = [2 × (-207.2 kJ/mol)] + [90.4 kJ/mol] - [3 × (+33.2 kJ/mol)] - [1 × (-285.8 kJ/mol)]. ∆H°rxn = -414.4 kJ/mol + 90.4 kJ/mol - 99.6 kJ/mol + 285.8 kJ/mol. ∆H°rxn = -38.0 kJ/mol
Therefore, the standard enthalpy change (∆H°rxn) for the given reaction is -38.0 kJ/mol. This negative value indicates that the reaction is exothermic, meaning that it releases heat to the surroundings.
For more such question on enthalpy visit:
https://brainly.com/question/16985375
#SPJ11
How do the valence electrons of an atom affect chemical reactions?
Answers
Valence electrons are the electrons in the outermost shell of an atom and are responsible for chemical reactions. In a chemical reaction, atoms gain or lose electrons to achieve a stable electron configuration, which is known as the octet rule. The number of valence electrons an atom has determines its chemical reactivity and how it will bond with other atoms. For example, atoms with only a few valence electrons, such as hydrogen, are highly reactive and will readily form chemical bonds, while atoms with many valence electrons, such as noble gases, are relatively unreactive and do not easily form chemical bonds.
How would you monitor the progress of a neutralization reaction? Question 2 options: We will use a funnel to separate the solid as it forms We will use a balance to see the changes in mass We will use a thermometer to check the changes in temperature We will use an acid-base indicator to see changes in color depending on the pH
Answers
Answer:
We will use an acid-base indicator to see changes in colour depending on the pH
Explanation:
The pH changes during a titration, so you could use an acid-base indicator to follow the changes in pH.
A is wrong. An acid-base titration does not usually form a solid, and it would be impractical to isolate a solid with a funnel.
B is wrong. There are no changes in mass.
C is wrong. Any changes in temperature would be too small to measure precisely with an ordinary thermometer.
The best way to monitor the progress of a neutralization reaction such as acid-base titration: D. Use an acid-base indicator to observe the changes in color depending on the pH.
The chemical reaction that occurs when you mix an acid and a base together is referred to as neutralization reaction.
In a neutralization reaction, what is formed is salt and water.
Acid-base titration is a neutralization method.
During acid-base titration, the neutralization reaction that occurs is usually monitored by observing the pH changes that occurs.
Change in pH is an indicator that there is progress in the neutralization reaction.
An acid-base indicator, can be used to detect the changes that occur via the pH changes in relation to the color change.
Therefore, the best way to monitor the progress of a neutralization reaction such as acid-base titration: D. Use an acid-base indicator to observe the changes in color depending on the pH.
Learn more about neutralization reaction here:
https://brainly.com/question/12442828
If you begin with 5.000 grams of KClO3(s) how many moles and KClO3(s)
will be used, and b) how many grams, moles, and molecules of the product species will
be formed?

Answers
Based on the given data, the amount of products from 5.00 g of KClO₃ is:
0.04 moles of KCl2.98 g KCl2.41 * 10²² molecules of KCl 0.06 moles of O₂.1.92 g of O₂3.61 * 10²² molecules of O₂What amount of products is formed from the decomposition of KClO₃?The decomposition of KClO₃ is given by the equation below:
2 KClO₃ -----> 2 KCl + 3 O₂
2 moles of KClO₃ produces 2 moles of KCl and 3 moles of O₂
Molar mass of KClO₃ is 122.5 g/mol
molar mass of KCl = 74.5 g
molar mass of O₂ = 32 g
Moles of KClO₃ in 5.00 g = 5.00/122.5
moles of KClO₃ = 0.04 molesFor KCl
a. moles: 0.04 moles of KClO₃ will produce 0.04 moles of KCl
b. mass of KCl = 0.04 * 74.5 = 2.98 g KCl
c. number of molecules of KCl = 0.04 * 6.02 * 10²³ = 2.41 * 10²² molecules of KCl
For O₂:
a. moles of O₂; 0.04 moles of KClO₃ will produce = 0.06 moles of O₂.
b. mass of O₂ = 0.06 * 32 g = 1.92 g of O₂
c. number of molecules: 0.06 * 6.02 * 10²³ = 3.61 * 10²² molecules of O₂
Therefore, the amount of products from 5.00 g of KClO₃ is:
0.04 moles of KCl2.98 g KCl2.41 * 10²² molecules of KCl 0.06 moles of O₂.1.92 g of O₂3.61 * 10²² molecules of O₂Learn more about moles and molecules at: https://brainly.com/question/26135244
Ammonia gas can be produced by the reaction of nitrogen and hydrogen gases as shown in the following balanced equation:
N₂ (9) + 3H₂(g) → 2NH3 (9)
Determine the mass of ammonia that can be produced from 40.5 g of
N₂ (9) with excess
H₂ (9).
Mass=
9

Answers
Answer:
mark me brilliant
To determine the mass of ammonia that can be produced from 40.5 g of N₂, we need to use stoichiometry and the balanced chemical equation provided.
From the balanced equation, we can see that 1 mole of N₂ reacts with 3 moles of H₂ to produce 2 moles of NH₃. Therefore, we need to calculate the number of moles of N₂ in 40.5 g:
Number of moles of N₂ = mass / molar mass = 40.5 g / 28 g/mol = 1.4464 mol N₂
Using the mole ratio from the balanced equation, we can calculate the number of moles of NH₃ produced:
Number of moles of NH₃ = (1.4464 mol N₂) x (2 mol NH₃ / 1 mol N₂) = 2.8928 mol NH₃
Finally, we can use the molar mass of NH₃ to convert the number of moles to mass:
Mass of NH₃ = number of moles x molar mass = 2.8928 mol x 17 g/mol = 49.11 g
Therefore, the mass of ammonia that can be produced from 40.5 g of N₂ with excess H₂ is 49.11 g.
Answer:
Explanation:
The first step is to determine the limiting reactant. In this case, we have 40.5 g of N₂ and excess H₂. We can use the molar mass of each substance to determine the number of moles of each reactant:
N₂: 40.5 g / 28.01 g/mol = 1.45 mol
H₂: 40.5 g / 2.016 g/mol = 20.1 mol
Since we have less moles of N₂ than H₂, N₂ is the limiting reactant. This means that the amount of ammonia produced will be determined by the amount of N₂.
We can use the balanced equation to determine the mass of ammonia produced:
1 mol N₂ → 2 mol NH₃
1.45 mol N₂ → 2.90 mol NH₃
2.90 mol NH₃ * 17.03 g/mol = 50.4 g NH₃
Therefore, 50.4 g of ammonia can be produced from 40.5 g of N₂ with excess H₂.
Two examples of potential energy are ________________________ and ______________________. Examples can include _________________________ and __________________________________.
Answers
Two examples of potential energy are gravitational potential energy and elastic potential energy.
What is Gravitational Potential Energy ?Gravitational potential energy is the energy stored in an object due to its position in a gravitational field. For example, a book placed on a shelf has gravitational potential energy because it has the potential to fall to the ground due to the force of gravity.
What is Elastic Potential Energy ?Elastic potential energy is the energy stored in an object when it is deformed, such as when a spring is compressed or stretched. For example, a compressed spring has elastic potential energy because it has the potential to return to its original shape and release the stored energy when it is released.
Other examples of potential energy include chemical potential energy, which is the energy stored in the bonds between atoms in a molecule, and nuclear potential energy, which is the energy stored in the nucleus of an atom.
To know more about Chemical potential energy , visit :
https://brainly.com/question/11440456
#SPJ1
Abby fills a graduated cylinder with 3 mL of water. She then drops a
small rock into the cylinder, and the water level rises to the 5 ml
mark. What is the volume of the rock?
mL
Answers
Answer: 2ML
Explanation: all you needed to do was to subtract
Enter your answer in the provided box.
Answer the following questions about the fermentation of glucose (C6H12O6, molar mass 180.2 g/mol) to ethanol (C2H6O) and CO2.
C6H12O6(s) → 2 C2H6O(l) + 2 CO2(g) ΔH = −16 kcal/mol
glucose ethanol
How many kilocalories of energy are released from 40.0 g of glucose?
kcal of energy released
Report answer to TWO significant figures.
Answers
Answer:
Explanation:
40/ 180.2 x (-16 / 1 mole glucose)=-3.6 KJ
How does physical properties differ from chemical properties? 1.Chemical properties are observed using your senses. 2.Chemical properties can only be observed using your taste sense. 3.Physical properties can be observed and chemical properties cannot. 4.Physical properties cannot be observed and chemical properties can.
Answers
Answer:
3. Physical properties can be observed and chemical properties can't
which environment would best support large land animals like giraffes elephants and lions
Answers
The savanna grassy plain environment would best support large land animals like giraffes elephants and lions.
A dry climate with a distinct wet and dry season, as well as moderate to high temperatures is observed in savannas. Grass species which can withstand seasonal droughts and frequent fires, predominate in savanna vegetation. Although they are more widely spaced than in other forested environments, trees and shrubs are still present.
Large land animals thrive in the savanna because it offers a variety of food sources, such as grasses, leaves and fruits, as well as plenty of room to move around and find resources. Animals can see each other clearly and communicate effectively in the open grasslands and the scatted trees and shrubs provide shelter from the sun and wind.
Learn more about environment at:
brainly.com/question/29244782
#SPJ1
When 1-bromobutane is exposed to magnesium, followed by acetone and then aqueous acid, what is the resulting product
Answers
The product of the reaction when 1-bromobutane is exposed to magnesium, followed by acetone and then aqueous acid is 1-pentanol.
What is Grignard reagent?
A Grignard reagent is an alkyl magnesium halide which can be converted to different products via chemical reactions. The Grignard reagent is a synthetic route to many compounds.
The product of the reaction when 1-bromobutane is exposed to magnesium, followed by acetone and then aqueous acid is 1-pentanol.
Learn more about grignard reagent: https://brainly.com/question/9309478
Answer:
2-Methyl-2-hexanol
Explanation:
1-bromobutane when reacted with Magnesium in ether forms a Grignard reagent.
The Grignard reagent reacts with carbonyl compounds to produce corresponding alcohols.
Primary alcohols are formed when the carbonyl compound is formaldehyde.
Secondary alcohols are formed when the carbonyl compound is any aldehyde other than formaldehyde.
Tertiary alcohols are formed when Grignard reagent is reacted with ketones.
In given question the carbonyl compound is Acetone (ketone) hence the final product will be a tertiary alcohol.

PLEASE HELP!!!! WILL GIVE BRAINLIEST!!!

Answers
Answer:
im gonna say b
Explanation:
How does a plant get and use energy?
Drag and drop the steps of the process to show the correct order.

Answers
The steps of how plants get energy in the correct order is as follows:
Sunlight shines on the leaves of a plant (option B). Cells in the leaves perform photosynthesis (option A). Glucose and other sugars travel throughout the plant (option D). Plant cells break apart the sugars to release their energy (option C) What is photosynthesis?Photosynthesis is the process by which plants and other photoautotrophs convert light energy into chemical energy.
Photosynthesis is carried out by the cells of green plants to synthesize their food in form of sugar powered by the energy from sunlight.
The cells in the leaf use the energy from the sun (light energy) and produce sugars (chemical energy). After which, the sugars are broken down to release energy for use by the cells in a process called cellular respiration.
Learn more about photosynthesis at: https://brainly.com/question/29764662
#SPJ1
Why are cells important to an organisms survival
Answers
Answer:
Cells are the basic structures of all living organisms. Cells provide structure for the body, take in nutrients from food and carry out important functions. ... These organelles carry out tasks such as making proteins?, processing chemicals and generating energy for the cell
Answer: I absolutely love this question! Biology is so interesting, so I always love to answer the curiosity of others regarding biology, such as that!
Cells are simply the basic structures of all organisms, that are living, of course! Cells provide structure for the body, and they also take in nutrients that your body needs from food and they carry out important functions. These organelles carry out tasks such as making proteins, processing chemicals, and generating energy for the cell. Isn’t that cool?
Hope this helped! <3
<
A 83.5 g sample of a nonelectrolyte is dissolved in 291.7 g of water.
The solution is determined to have a boiling point of 102.3 °C. What is
the molar mass of the compound? (Kb for water is 0.510 °C/m).
Answers
The molar mass of the compound is = 1.42g/ mol
Calculation of compound molar mass∆Tb = kB × molarity
∆Tb = 102.3°C
Kb = 0.510 °C/m
Molarity= electrolyte mass × water solubility/ Mw× mass of water.
That is,
102.3°C= 0.51× 83.5×1000/Mw × 291.7
Make Mw the subject of formula,
Therefore,
Mw = 0.51 × 83.5×1000/ 102.3 × 291.7
Mw= 42,585/29,840.91
Mw= 1.42g/mol
Learn more about molarity here:
https://brainly.com/question/14469428
#SPJ1
The Xs show the location of beanbags a student tossed at a target. The
student was aiming for the center circle. Which words best describe the
student's results?
A. Low precision, low accuracy
O B. High precision, low accuracy
O C. High precision, high accuracy
O D. Low precision, high accuracy
Answers
Answer:
It is D low precision, high accuracy
Explanation:
I got it correct
The Xs show the location of beanbags a student tossed at a target. The student was aiming for the center circle. Low precision, high accuracy best describe the student's results. Therefore, option D is correct.
What is precision ?There are two ways to assess observational error: accuracy and precision. Precision measures how closely two measurements are to one another, whereas accuracy measures how close a group of measurements is to its actual value. In other words, precision is a measure of statistical variability and a description of random errors.
The proximity of two or more measurements to one another is referred to as precision. Using the aforementioned example, your measurement is extremely accurate if you weigh a certain substance five times and obtain 3.2 kg each time. Accuracy is not necessary for precision.
Regardless of whether or whether two or more measurements are correct, precision is described as "the property of being exact" and relates to how close two or more measurements are to one another.
Thus, option D is correct.
To learn more about the precision, follow the link;
https://brainly.com/question/28336863
#SPJ5
The best name for H2S when it is classified as an acid is _____(i)_______; the best name for N2O when named as a molecular compound is _____(ii)_________; and the best name for Cs2O is ______(iii)_________.
O (i) hydrosulfuric acid; (ii) dinitrogen monoxide; (iii) cesium oxideO (i) dinitrogen monoxide; (ii) hydrosulfuric acid; (iii) cesium oxideO (i) dinitrogen monoxide; (ii) cesium oxide ; (iii) hydrosulfuric acidO (i) hydrosulfuric acid; (ii) cesium oxide ; (iii) dinitrogen monoxide
Answers
(i)hydrosulfuric acid; (ii) dinitrogen monoxide; (iii) cesium oxide are the correct answers.
What are compounds ?
Compounds are substances made up of two or more different types of atoms that are chemically bonded together in a specific ratio. The atoms in a compound are held together by chemical bonds, which are formed when the atoms share or transfer electrons in order to achieve a more stable electron configuration.
There are many different types of compounds, including covalent compounds, ionic compounds, and metallic compounds.Covalent compounds are formed when atoms share electrons in order to complete their valence electron shells. Ionic compounds are formed when electrons are transferred from one atom to another, resulting in the formation of positive and negative ions that are attracted to each other by electrostatic forces.
To learn more about the compounds, click the given link ;
https://brainly.com/question/26487468
#SPJ4
A parasitic way of life can be best demonstrated by the feeding adaptations of the spider.
TRUE OR FALSE
Answers
The given statement "A parasitic way of life can be best demonstrated by the feeding adaptations of the spider." is False. Because, While some spiders are parasites, not all spiders are parasitic.
Additionally, many spiders are not even true parasites, as they typically do not harm their host organism. Spiders are typically classified as predators, as they feed on other insects and arthropods. While some spiders may occasionally feed on the blood of larger animals, such as birds or mammals, this behavior is not typically considered parasitic, as it does not involve a long-term relationship between the spider and the host organism.
To know more about parasites, here
brainly.com/question/22589174
#SPJ1
< Question 27 of 27 > You decide it is time to clean your pool since summer is quickly approach chlorine, Cl₂, concentration of the pool should be between 1 and 3 ppin. you send a sample of pool water to a chemist for analysis of the Cl₂ conte 3.71 × 10–5 M. Convert the concentration of Cl, to parts per million (ppm). Macmillan Learning concentration:
Answers
To kill bacteria, chlorine is added to the water. However, it does not function immediately away and kill CDC.
Thus, When handled correctly, free chlorine* can destroy the majority of bacteria in a matter of minutes.
The CDC advises maintaining a pH of 7.2–7.8 and free chlorine levels of at least 1 ppm in swimming pools and 3 ppm in hot tubs and spas.
The CDC advises a pH of 7.2–7.8 and a free accessible chlorine content of at least 2 ppm in swimming pools when using cyanuric acid, a chlorine stabilizer, or chlorine products containing cyanuric acid (for instance, products generally known as dichlor or trichlor. The CDC advises against using cyanuric acid or chlorine products containing it in hot tubs or spas.
Thus, To kill bacteria, chlorine is added to the water. However, it does not function immediately away and kill CDC.
Learn more about Chlorine, refer to the link:
https://brainly.com/question/19460448
#SPJ1
Identify the polar solvent. Identify the polar solvent. toluene carbon tetrachloride diethyl ether acetone hexane
Answers
Answer:
Acetone.
Explanation:
Hello,
In this case, we can distinguish between polar solvent and nonpolar solvent by the nature of the bonds present in the compound. Thus, since the bonds C-Cl, C-C, C-H and C-O are nonpolar, which are contained in the toluene, carbon tetrachloride, diethyl ether and hexane, they are discarded as polar.
Nevertheless, since the carbonyl group contained in the acetone is a polar because of the formed positive and negative charges, it is actually the polar solvent, acting as an exception. This is substantiated by the fact the acetone is soluble in water whereas the other substances not,
Regards.
Find the pressure of 5 L of a gas 27oC if number of moles is 0.8?

Answers
ANSWER
The pressure of the gas is 3.9 atm
EXPLANATION
Given information
The volume of the gas = 5L
The temperature of the gas = 27 degrees Celcius
The number of moles = 0.8 moles
To find the number of moles, we will need to apply the below formula
\(PV\text{ = nRT}\)Where
P = pressure
V = volume
n = number of moles
R = gas constant
T = Temperature
Recall, that the gas constant is 0.082005 L atm mol^-1 K^-1
The next step is to convert the temperature from degrees Celcius to Kelvin
\(\begin{gathered} T\degree\text{ K = t + 273.15} \\ T\degree K\text{ = 27 + 273.15} \\ T\degree K\text{ = 300.15} \end{gathered}\)The next step is to substitute the given data into the formula in order to find the pressure of the gas
\(\begin{gathered} P\text{ }\times\text{ 5 = 0.082005 }\times\text{ 0.8 }\times\text{ 300.15} \\ 5P\text{ = 19.6910} \\ \text{ Divide both sides by 5} \\ \frac{5P}{5}\text{ = }\frac{19.6910}{5} \\ P\text{ = 3.9 atm} \end{gathered}\)Hence, the pressure of the gas is 3.9 atm
The generic metal hydroxide M(OH)2 has Ksp = 8.05×10−12. (NOTE: In this particular problem, because of the magnitude of the Ksp and the stoichiometry of the compound, the contribution of OH− from water can be ignored. However, this may not always be the case.)
a) What is the solubility of M(OH)2 in pure water?
b) What is the solubility of M(OH)2 in a 0.202 M solution of M(NO3)2?
Answers
Answer:
a. in pure water Solubility (x) = 1.26 x 10⁻⁴M
b. in 0.202M M⁺² Solubility (x) = 9.963 x 10⁻¹²M
The large drop in solubility is consistent with the common ion effect.
Explanation:
a. Solubility in pure water
Given: M(OH)₂ ⇄ M⁺² + 2OH⁻
I --- 0 0
C --- x 2x
E --- x 2x
Ksp = [M⁺²][OH⁻]² = (x)(2x)² = 4x³ => x = CubeRt(Ksp/4)
solubility in pure water = x = CubeRt(8.05 x 10⁻¹²/4) = 1.26 x 10⁻⁴M
b. Solubility in presence of 0.202M M⁺² as common ion.
Given: M(OH)₂ ⇄ M⁺² + 2OH⁻
I --- 0.202M 0
C --- +x +2x
E --- 0.202M + x 2x
≈ 0.202M
Ksp = [M⁺²][2x]² = (0.202)(2x)² = (0.202)(4x²) = 8.05 x 10⁻¹²
=> x = (8.05 x 10⁻¹²)/(0.202)(4) = 9.963 x 10⁻¹²M
A certain bimolecular reaction at 40 °C at an activation energy of 30 kJ/mol. The addition of a catalyst reduces the activation energy by a factor of 2. How much faster does the catalyzed occur?
Select one:
OA. 318.63
OB. 358.63
C. 338.63
OD. 378.63
Answers
k = Ae^(-Ea/RT)
where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the gas constant (8.314 J/mol·K), and T is the temperature in Kelvin.
First, we need to convert the temperature from Celsius to Kelvin:
40°C + 273.15 = 313.15 K
Now, let's find the ratio of the rate constants for the catalyzed and uncatalyzed reactions:
k_catalyzed / k_uncatalyzed = e^((Ea_uncatalyzed - Ea_catalyzed) / RT)
Since the catalyst reduces the activation energy by a factor of 2:
Ea_catalyzed = 30 kJ/mol / 2 = 15 kJ/mol
Convert the activation energies to J/mol:
Ea_uncatalyzed = 30,000 J/mol
Ea_catalyzed = 15,000 J/mol
Now, plug in the values:
k_catalyzed / k_uncatalyzed = e^((30,000 J/mol - 15,000 J/mol) / (8.314 J/mol·K × 313.15 K))
k_catalyzed / k_uncatalyzed ≈ 358.63
The catalyzed reaction occurs approximately 358.63 times faster. So, the correct answer is OB. 358.63.
2. Which number is not a coefficient in the equation,
2C6H14+ 19O2,-- 12CO2,+ 14H2O?
Answers
Answer:
2, 19, 12 and 14 are the coefficients.
How much energy is contained in the six-cookie serving size recommended on the label?
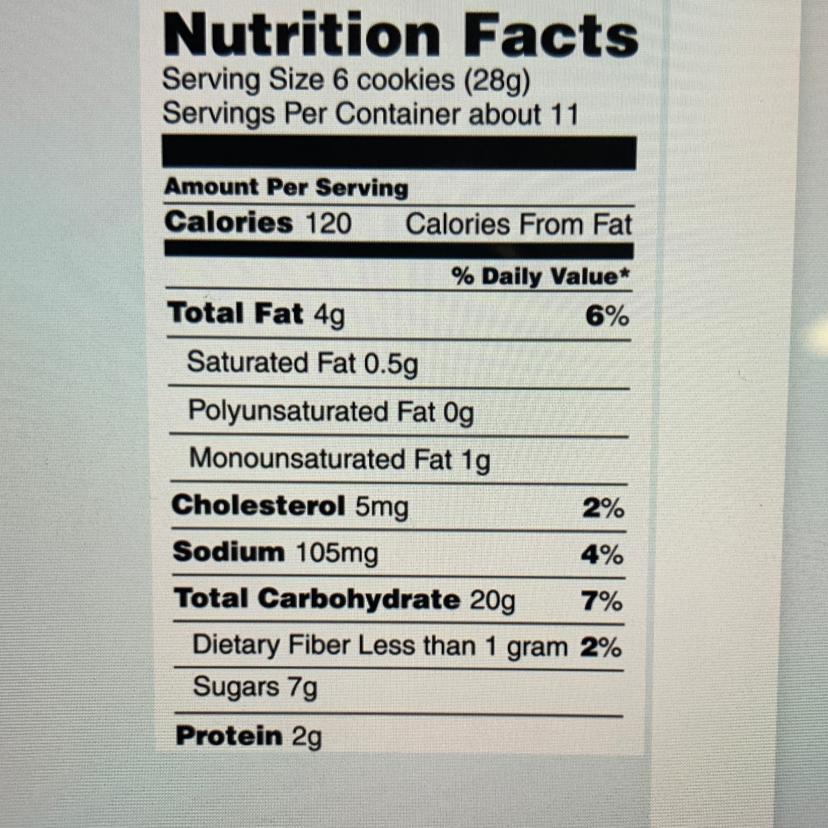
Answers
Monounsaturated Fat 1g
The correct answer for the following calculation where 43 and 7 are counted numbers and 2,310 and 0.370 are measured numbers is which of the following? 43 X 2.310 7 X 0.370 a) 38.35 b) 38.4 Oc) 38 O d) 40
Answers
The actual result of the calculation is 101.920. Therefore, none of the options provided in the question matches the correct answer.
To calculate the given expression: (43 × 2.310) + (7 × 0.370), we perform the multiplication first and then add the results.
Multiplying the counted numbers:
43 × 2.310 = 99.330
Multiplying the measured numbers:
7 × 0.370 = 2.590
Now, we add the results:
99.330 + 2.590 = 101.920
The correct answer is not provided in the given options: a) 38.35, b) 38.4, c) 38, or d) 40.
The actual result of the calculation is 101.920. Therefore, none of the options provided in the question matches the correct answer.
It's important to note that when performing calculations, it is crucial to accurately follow the order of operations (multiplication before addition) and ensure precision when dealing with decimal numbers.
In this case, the correct answer is not among the options provided, and the accurate result is 101.920.
For more such questions on calculation visit:
https://brainly.com/question/28902645
#SPJ8
a category of classification below a kingdom? A phylum B domain C species
Answers
Answer:
Phylum
Explanation:
After that it goes class, order, family, genus, and finally species. In that order, BTW before kingdom is domain.
Answer:
Phylum
Explanation:
Activation energy is:
A. The energy needed to begin breaking the bonds of reactants.
B. None of these.
C. The maximum amount of energy reactants can hold.
D. The energy needed to begin breaking the bonds of products.
Answers
Activation energy is the energy needed to begin breaking the bonds of reactants. Hence, option A is correct.
What is activation energy?Activation energy is defined as the minimum amount of energy necessary to initiate a chemical reaction.
Hence, activation energy is the energy needed to begin breaking the bonds of reactants.
Learn more about activation energy here:
https://brainly.com/question/2410158
#SPJ5