A concentrated reagent is sold as a solution that is 60. 0% solute by mass. If the molar mass of the solute is 120 g/mol and the density of the solution is 1. 70 g/ml, what is the molarity, m, of the solution?.
Answers
To calculate the molarity of a concentrated reagent, we need to use the mass percentage, molar mass and density of the solution. We use the formula given below to find the molarity:Molarity, M = (mass percentage/ molar mass) × (1000/density)Substitute the given values into the above formula:Molarity, M = (60%/120 g/mol) × (1000/1.70 g/ml)Molarity, M = 25.6 M.
The molarity of the given concentrated reagent is 25.6 M.The given information shows that the mass percentage is 60%, which means that 100 g of the solution contains 60 g of the solute. Since the molar mass of the solute is 120 g/mol, we can calculate the number of moles of solute in 1000 g (1 L) of the solution as follows:Number of moles of solute = (60 g/120 g/mol) = 0.5 molesMolarity, M = (0.5 moles/1 L) = 0.5 MHowever, this is not the answer to the given question, as the solution is not 100% solute by mass. Therefore, we need to calculate the density of the solution to find the actual volume of the solution containing 0.5 moles of solute.Using the density of the solution, we can find the volume of the solution that contains 1 L of the solution by dividing the mass of 1 L of the solution by its density. The mass of 1 L of the solution is equal to the sum of the mass of the solute and the solvent. If the mass of 1 L of the solution is m (g), then :m = mass of solute (g) + mass of solvent (g)Since the solution is 60% solute by mass and the molar mass of the solute is 120 g/mol, the mass of solute in 1 L of the solution is equal to 600 g. Therefore:m = 600 g + mass of solvent (g)Now, we can write the density of the solution as follows:density = (mass of solute + mass of solvent) / volume of the solution density = (600 g + mass of solvent) / Vwhere V is the volume of the solution (L).Since the density of the solution is given as 1.70 g/ml, we can write:1.70 g/ml = (600 g + mass of solvent) / V Substituting V = 1000 ml, we get:1.70 g/ml = (600 g + mass of solvent) / 1000 mlSolving for the mass of the solvent, we get:mass of solvent = 1070 gNow, the volume of the solution that contains 0.5 moles of solute can be calculated as follows:0.5 moles × 120 g/mol = 60 g of solute in the solution The mass of the solvent in 1 L of the solution is equal to 1070 g, so the mass of the solvent in V L of the solution is equal to 1070V g. The mass of 1 L of the solution is equal to 600 g + 1070 g = 1670 g.Therefore, the volume of the solution that contains 0.5 moles of solute is given by:V = 1670 g / 1.70 g/ml = 982.4 ml ≈ 0.98 LThus, the molarity of the solution is given by :M = 0.5 moles / 0.98 L = 25.6 M.
For more information on molarity visit:
brainly.com/question/31545539
#SPJ11
Related Questions
Calculate the pH of a 0.25 M solution of NaNO2 (Ka(HNO2) = 4.5 x 10^-4) (1.97)
a) pH = 3.35
b) pH = 4.45
c) pH = 5.55
d) pH = 6.65
Answers
The pH of a 0.25 M solution of NaNO2= 6.65.
Given the concentration of NaNO2, we can find the concentration of NaOH and HNO2 as follows:
NaNO2 = 0.25 MNaOH = HNO2 = x
(since they have equal concentrations due to the stoichiometry of the reaction)
Thus, we can write the equilibrium constant expression as:
Ka = x^2/0.25
Now, let's solve for x:
x^2 = 0.25 x 4.5 x 10^-4x = √(0.25 x 4.5 x 10^-4) = 0.015
This value represents the concentration of both HNO2 and NaOH. Since we are interested in pH, we need to find the concentration of H+ ions using the following equation:
Kw = [H+][OH-]
Since we have found the concentration of OH- (which is the same as the concentration of NaOH),
we can solve for H+:
Kw = 1.0 x 10^-14[H+][0.015] = 1.0 x 10^-14[H+] = 6.7 x 10^-13
Finally, we can find pH:
pH = -log[H+]pH = -log(6.7 x 10^-13)pH = 6.65
Therefore, the correct option is d) pH = 6.65.
learn more about pH here
https://brainly.com/question/172153
#SPJ11
how many grams of naoh are needed to give a ph of 11.5 in a 14.5 l tank of water?
Answers
1.84 x 10^(-9) g (or 1.84 ng) of NaOH to give a pH of 11.5 in a 14.5 L tank of water.
What mass of NaOH is required to achieve a pH of 11.5 in a 14.5 L water tank?
To find the mass of NaOH needed to give a pH of 11.5 in 14.5 L of water, we first need to calculate the concentration of hydroxide ions (OH-) required to achieve that pH.
The pH of a solution is defined as the negative logarithm of the hydrogen ion (H+) concentration, so we can write:
\(pH = -log[H+]\)
We can rearrange this equation to solve for [H+]:
\([H+] = 10^(-pH)For a pH of 11.5, we have:[H+] = 10^(-11.5) = 3.16* 10^(-12) M\)
Since NaOH is a strong base that completely dissociates in water, we know that the concentration of hydroxide ions will be the same as the concentration of NaOH we add.
Therefore, we can calculate the amount of NaOH needed using the following equation:
\(moles of NaOH = volume of water (in L) * desired [OH-] concentration (in M)moles of NaOH = 14.5 L * 3.16 * 10^(-12) M = 4.59 * 10^(-11) mol\)
Finally, we can convert moles of NaOH to grams using the molar mass of NaOH:
\(mass of NaOH = moles of NaOH * molar mass of NaOHmass of NaOH = 4.59 x 10^(-11) mol* 40 g/mol = 1.84 * 10^(-9) g\)
Therefore, we need 1.84 x 10^(-9) g (or 1.84 ng) of NaOH to give a pH of 11.5 in a 14.5 L tank of water.
Learn more about Mass of NaOH
brainly.com/question/1416379
#SPJ11
What is the radius of this circle if the circumference is 18 pi cm?
Answers
Answer: 2.86 inches
Explanation: To find the radius, then, we insert 18 in for the circumference. So 18=2∏r. Solving for r gives 9/∏, or approximately 2.86 inches.
Answer:
2.86 inches
Explanation:
What is the final temperature of the water went 100 mL of 30°C water is mixed with 500 mL of 60°C water
Answers
The final temperature of the water resulting from the mixing of 100 mL of 30°C water with 500 mL of 60°C water would be 55°C.
Temperature calculationIn order to calculate the final temperature of a mixture of two different temperatures of water, we can use the following formula:
\(T_{(final)} = (m_1T_1 + m_2T_2) / (m_1 + m_2)\)
where:
T(final) is the final temperature of the mixturem1 and m2 are the masses of water in milliliters (mL) or grams (g)T1 and T2 are the initial temperatures of water in degrees Celsius (°C).In this case, we have 100 mL of 30°C water and 500 mL of 60°C water. We can convert mL to grams using the density of water which is approximately 1 g/mL2. Therefore:
m1 = 100 g T1 = 30°C m2 = 500 g T2 = 60°C
Thus:
T(final) = (100x30) + (500x60) / (100 + 500) T(final) = (3000 + 30000) / 600 T(final) = 55°CTherefore, the final temperature of the mixture is 55°C.
More on temperature can be found here: https://brainly.com/question/11464844
#SPJ1
Which of the following is an adaptation of organisms in avoiding unfavorable condition due to a change in abiotic factor?
A. Migration of birds due to change in seasons.
B. Hibernation of a snake from September to December.
C. Plants die due to lack of water and high temperature.
D. Body temperature regulation of polar bears during winter
Volcanic eruption is considered as one of the changes in abiotic factors in the ecosystem. Which of the following is/are the effect/s of volcanic eruptions in the ecosystem?
A. I and II only
B. I and III only
C. II and III only
D. I, II, and III
Help plsssssss

Answers
The effects of volcanic eruption include acid rain and destruction of lives and property.
What are abiotic factors?The term abiotic factors refers to those factors that are non living such as rainfall, topography, etc. The adaptation of organisms in avoiding unfavorable condition due to a change in abiotic factor is hibernation of a snake from September to December.
A volcanic eruption is a sudden outburst of molten magma from the earth's crust. The effects of volcanic eruption include acid rain and destruction of lives and property.
Learn more about volcanic eruptions: https://brainly.com/question/1622004
Georgia's Coastal Plains region includes about 60% of the state. Long ago this area was part of the Atlantic Ocean and completely covered by
water. An important feature of Georgia's coast is the salt marsh. Georgia's salt marshes play a unique role in maintaining the delicate balance of
nature so vital along our coastal estuaries.
In 1970, the State of Georgia established the Coastal Marshlands Protection Act to protect the marsh and estuarine areas, and to regulate the
activities within these public trust lands that is held for the citizens of Georgia.
Georgia's salt marshes have been identified as one of the most extensive and productive marshland systems in the United States. It is production
almost beyond comprehension, producing nearly twenty tons to the acre; four times more productive than the most carefully cultivated corn. This is
so very important for ALL BUT ONE of the reasons listed. That is:
Answers
The fires keep the Carolina Bay habitat from becoming overgrown with vegetation.
What is the exception?Peat is an organic material consisting of leftover parts after plant breakdown. This substance has a brown color that can be either light or dark depending on the variation and is especially rich in charcoal.
It serves as a substrate and is primarily used in gardening, but it is also useful in natural settings because it promotes soil preservation, the supply of organic matter, increased water porosity, and improved soil retention.
The removal of this excess peat is what explains the advantage of natural fires occurring in the Carolina Bay habitat, the advantage is that fires prevent the Carolina Bay habitat from being overgrown.
Learn more about Coastal Marshland:https://brainly.com/question/17573035
#SPJ1
Can you please help resolve and explain?
Answers
The Ka of an acid indicates how strong this acid is. This means that greater acidity constant Ka corresponds to stronger acids, and lower Ka corresponds to weakest acids. I the example the acetic acid (CH3COOH) has an acidity constant 6 order of magnitude greater than the HCO3- there fore acetic acid is stronger than HCO3-
Hi! This is a science question...
Which of the following is NOT an example of acceleration?
speeding up
slowing down
remaining at rest
stopping
Answers
Answer:
C: remaining at rest
Explanation:
Acceleration (a) is the change in velocity (Δv), remaining at rest is not changing the velocity.
when mtbe (shown) is reacted with hbr, carbons a and b will undergo nucleophilic substitution to yield two alkyl halides. by what mechanism of substitution will each c-o bond be cleaved?
Answers
when mtbe (shown) is reacted with hbr, carbons a and b will undergo nucleophilic substitution to yield two alkyl halides then by SN2 mechanism each c-o bonds will be cleaved.
When MTBE (methyl tert-butyl ether) is reacted with hydrogen bromide (HBr), the two C-O bonds in the molecule are expected to undergo an S N 2 substitution mechanism. This is because the reaction conditions are favorable for a bimolecular, concerted mechanism, where the nucleophilic (HBr) and substrate (MTBE) interact with each other to produce the products.
In the S N 2 mechanism, the C-O bonds in MTBE are cleaved via backside attack by the nucleophile, HBr. This involves the simultaneous formation of a new C-Br bond and breaking of the existing C-O bond. The attack takes place in a stereospecific manner, so that the orientation of the substrate and nucleophile determines the stereochemistry of the products. The S N 2 mechanism is typically favored under conditions where the substrate is a good leaving group and the nucleophile is a strong base.
In summary, both of the C-O bonds in MTBE are expected to be cleaved via an S N 2 mechanism when reacting with HBr.
Learn more about nucleophilic here:
https://brainly.com/question/28325919
#SPJ4
Each "100-mg" tablet of Imitrex contains 140 mg of sumatriptan succinate equivalent to 100 mg sumatriptan base. If the molecular weight of sumatriptan succinate is 413.5, calculate the molecular weight of sumatriptan base.
Answers
The molecular weight of sumatriptan base is 295.4
What is molecular mass?The molecular mass is the mass of a given molecule: it is measured in daltons or atomic mass.
It can also be defined as the sum of the atomic masses of all atoms in a molecule. The molecular mass of hydrogen, oxygen carbon are 1, 16, 12 respectively.
The mass ratio of Sumatriptan succinate and Sumatriptan base is
140 : 100
= 7 : 5
therefore;
7/5 = 413.5/x
413.5 × 5 = 7x
2067.5 = 7x
x = 2067.5/7
x= 295.4
Therefore the molecular weight of Sumatriptan base is 295.4
learn more about molecular mass from
https://brainly.com/question/837939
#SPJ4
6 points
Question #2: Identify the acid in this equation below: *
HBr + NH 3 →NH 4 + +Br -
4+
HBr
NH 3
A.
B.
NH 4*
Br-
O
C.
O
D.
Need quick answers !!!!!!
Answers
Answer:
HBr
Explanation:
Acids have Hydrogen as their first/leading element.
6. In which of the following will the bulb glow?
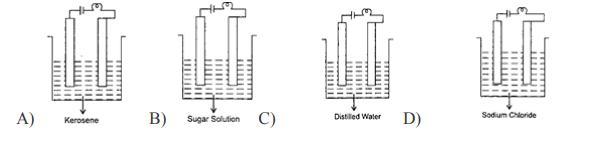
Answers
Answer:
Kerosene
Explanation:
You use process of elimination in this question
None of them except for Kerosene can power a bulb
Explanation:
sodium chloride
thank me later
2. If I have an unknown volume of gas held at a temperature of 115. K in a container with a pressure of 60.0 kPa with 1.50 mol of particles. If by increasing the temperature to 225. K, decreasing the pressure to 30.0 kPa, and allowing some gas to escape until 1.00 moles remain causes the volume of the gas to be 29.0 liters, how many liters of gas did I start with?
Answers
The initial volume of the gas was approximately 14.5 liters. The molar volume of a gas expresses the volume occupied by 1 mole of that respective gas under certain temperature and pressure conditions. This answer supports our expectation from Charles law.
To find the initial volume, we can use the Ideal Gas Law formula, which is PV = nRT. Here, P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant (8.314 J/mol·K), and T is the temperature in Kelvin. Step 1, Convert the given pressure from kPa to Pa by multiplying by 1000. So, 60.0 kPa * 1000 = 60000 Pa. Step 2, Plug the values into the Ideal Gas Law formula: 60000 Pa * V = 1.50 mol * 8.314 J/mol·K * 115 K. Step 3, Solve for V: V = 1.50 mol * 8.314 J/mol·K * 115 K / 60000 Pa. Step 4, Calculate the volume: V ≈ 14.5 liters.
So, you started with approximately 14.5 liters of gas. Finally, we can use the fact that the initial number of moles of particles was 1.50, and that only 1.00 moles remained after some gas escaped, to calculate the initial volume of the gas, so the correct number is 14.5 liters.
To know more about gas visit:
https://brainly.com/question/14812509
#SPJ11
which is more likely to be thermodynamically favored, the forward reaction or the reverse reaction?
Answers
Answer: The thermodynamic favorability of a chemical reaction can be determined by comparing the change in free energy (ΔG) between the reactants and products. A negative value of ΔG indicates that the reaction is thermodynamically favored, meaning that it is energetically favorable for the reaction to proceed in the forward direction. Conversely, a positive value of ΔG indicates that the reaction is not thermodynamically favored, and the reverse reaction is energetically favorable.
If the ΔG of the forward reaction is negative, the forward reaction will be thermodynamically favored, and the reverse reaction will not be. If the ΔG of the forward reaction is positive, the reverse reaction will be thermodynamically favored, and the forward reaction will not be. If the ΔG of the forward reaction is zero, the reaction is at equilibrium and neither the forward nor the reverse reaction is thermodynamically favored.
Therefore, whether the forward or reverse reaction is thermodynamically favored depends on the magnitude and sign of ΔG for that reaction.
Explanation:
rank a series of molecules by expected solubility in water based on polarity and hydrogen bonding. some slightly soluble compounds are included in this exercise. rank the organic compounds from most soluble to least soluble. to rank items as equivalent, overlap them.
Answers
The solubility in water based on polarity and hydrogen bonding is that polar compounds dissolves in the water due to the hydrogen bonding.
The polar compounds easily soluble in the water as they makes hydrogen bond with the water. Let us take an example : The order of solubility in some compounds is given as :
CH₃CH₂CH₂COOH > CH₃CH₂CH₂CH₂OH > CH₃CH₂OCH₂CH₂ > CH₃CH₂CH₂CH₃
Carboxylic acid is more soluble in the water as it males more hydrogen bond with water as compared to the alcohol. The last one is not able to make the hydrogen bond with the water . The two types of hydrogen bonding are : intermolecular hydrogen bonding and intermolecular hydrogen bonding.
To learn more about solubility here
https://brainly.com/question/14367567
#SPJ4
Concentrations of Chemical Species Graded Question Consider sample of Sr(OH)2(aq) that was made by dissolving 0.305 g Sr(OH),(s) in enough water to make 200.0 mL of solution at 25°C. What is the concentration of Sr?+ (aq)? M What is the concentration of OH(aq) M What is the pH of the solution? report to at least 2 places after the decimal What is the pOH of the solution? report to at least 2 places after the decimal
Answers
Sr2+(aq) and OH(aq) concentrations are, respectively, 0.01255 M and 0.0251 M. The solution has a pH of 12.40 and a pOH of 1.60.
We must first determine the moles of Sr(OH)2(s) that are present in the solution in order to determine the concentration of Sr2+ (aq).
We may convert the mass of the solid to moles using the molar mass of Sr(OH)2 (121.63 g/mol):
0.00251 mol Sr(OH) is equal to 0.305 g Sr(OH)2(s) x (1 mol Sr(OH)2 / 121.63 g Sr(OH)2).2
The amount of moles of Sr2+ (aq) is also 0.00251 mol since the stoichiometry of the reaction is 1:1 for Sr2+ (aq) and Sr(OH)2(s) as well.
We divide the quantity of moles by the litres of the solution's volume to determine the concentration:
0.2000 L / 0.00251 mol Sr2+ (aq) = 0.0125 M Sr2+ (aq)
Sr2+ has an aqueous concentration of 0.0125 M.
Similarly, by taking into account the dissociation of Sr(OH)2(s) in water, we may determine the concentration of OH- (aq):
Sr(OH)2(s) transforms to Sr2+ (aq) + 2OH- (aq).
The number of moles of OH- (aq) in the solution is because the stoichiometry indicates that two moles of OH- (aq) are created for each mole of Sr(OH)2(s).
0.00502 mol OH- (aq) is equal to 2 x 0.00251 mol.
dividing by the solution's liter-volume:
0.0251 M OH- (aq) = 0.00502 mol OH- (aq) / 0.2000 L.
OH- (aq) has a concentration of 0.0251 M.
We must first determine the pOH in order to determine the solution's pH:
pOH = -log(0.0251) = -log(OH- (aq)] = 1.60
Then, we can use the equation:
pH + pOH = 14
pH + 1.60 = 14
pH = 12.40
The pH of the solution is 12.40.
To know more about concentration visit:
https://brainly.com/question/10725862
#SPJ11
What happens when the value of the mass number to atomic number ratio as stble isotopes become heavier?
Answers
While the mass number of higher elements is greater than twice that of lighter elements, the mass number of lighter elements is equal to two times the atomic number.
What is the Mass Number and Isotopes?The number of protons in an element's nucleus, which is equivalent to the number of electrons in the neutral, non-ionized version of the atom, is represented by the element's atomic number. Each element has a distinct identity thanks to its atomic number, but an isotope does not. This is due to the large variety of neutron counts that one atom of a particular element may have. The total amount of protons and neutrons (nucleons) in an atom's nucleus determines its mass. However, the mass number of each isotope of a certain element varies.
Isotopes are two or more varieties of an element's atoms that share the same atomic number (number of protons) and place in the periodic table but have different numbers of nucleons (protons + neutrons). Their varied masses cause their nuclei to contain varying numbers of neutrons, which is why this occurs. Since all isotopes of a given element have the same atomic number, their chemical characteristics are nearly identical. However, because of various atomic masses, they have different physical characteristics.
To learn more about Isotopes visit:
https://brainly.com/question/11680817
#SPJ4
What is the concentration of Cl- ions in a .1M solution of calcium chloride?
Well the chemical formula is important.. it is CaCl2 so there are twice as many Cl ions so the concentration would be 0.2M
Answers
The concentration of Cl⁻ ions in a 0.1M solution of calcium chloride (CaCl2) is 0.2 M.
Calcium chloride (CaCl2) dissolves into three ions:
one Ca²⁺ ion and two Cl⁻ ions. So, in a 0.1M solution of CaCl2, we have the following concentrations:
1Ca²⁺= 0.1 and 2Cl⁻ = 0.2
Therefore, the concentration of Cl⁻ ions in a 0.1M solution of calcium chloride (CaCl2) is 0.2 M.
Learn more about Calcium chloride:
https://brainly.com/question/12216405
#SPJ11
The concentration of Cl- ions in a 0.1M solution of calcium chloride is 0.2M, as for each molecule of calcium chloride, there are two chloride ions present.
In chemistry, the concentration of ions in a solution is typically determined by the molarity of the compound and the stoichiometry of the ions in the compound.
For example, in the case of a 0.1M solution of calcium chloride (CaCl₂), there are two chloride (Cl-) ions for every molecule of calcium chloride.
Therefore, the concentration of Cl- ions in the solution would be double the molarity of the calcium chloride, or 0.2M.
Learn more about Concentration here:https://brainly.com/question/31665413
#SPJ12
Gallium is a metallic element in Group III. It has similar properties to aluminium.
(a) (i) Describe the structure and bonding in a metallic element.
Answers
Metallic elements exist in a solid-state and they are opaque, have a shiny surface, good conductors of electricity and heat, malleable and ductile, and are dense. The structure of metals is formed by atoms that are held together by metallic bonds. These atoms have loosely bound valence electrons that can be shared between the neighboring atoms.
Therefore, the outermost shells of these atoms are incomplete due to the sharing of valence electrons, forming a lattice structure known as a metallic bond.Metallic elements have a unique crystal structure that occurs in two forms. The most common type of metal crystal structure is the body-centered cubic structure where the atoms are arranged in a cube with one atom located at the center of the cube. The other type of metal crystal structure is the face-centered cubic structure, where each corner of the cube is an atom and there is an additional atom at the center of each face of the cube .Metallic bonding occurs due to the delocalized electrons that exist in the metal structure. The valence electrons from each atom are free to move throughout the entire metal lattice. Therefore, these electrons form a "sea of electrons" that is shared by all the atoms in the lattice. This results in the metal structure having high thermal and electrical conductivity.Metals are known for their ductility and malleability properties. These properties are due to the metallic bonding that exists in the metal structure. Since the valence electrons are shared, they can easily move past one another, allowing the metal to be hammered into different shapes without breaking.The properties of metals vary depending on their structure and bonding. Gallium, being a metallic element in Group III, has similar properties to aluminum. Therefore, it has a similar metallic bond structure with delocalized electrons that provide the metal with its unique properties.For such more question on valence electrons
https://brainly.com/question/371590
#SPJ8
1.) Draw a diagram of the chemical structure of oil-based paint.
With an explanation
Answers
The specific composition of oil-based paint can vary depending on the brand and formulation.
Oil-based paint typically consists of three main components: pigments, binders, and solvents. The pigments provide the color and opacity to the paint, while the binders are responsible for holding the pigment particles together and adhering them to the painted surface. The solvents help to adjust the paint's consistency and facilitate its application.
The binder in oil-based paint is commonly a natural oil, such as linseed oil or tung oil. These oils are composed of fatty acid molecules, which contain a long carbon chain with a carboxyl group (-COOH) at one end. The carboxyl group can react with oxygen in a process called oxidation, forming a cross-linked network of molecules that harden over time, creating a durable paint film.
The solvents in oil-based paint are typically organic compounds, such as mineral spirits or turpentine. These solvents dissolve the binder and pigments, making the paint flowable and easy to apply. As the solvent evaporates, the paint gradually dries and the binder undergoes the oxidation process, resulting in the formation of a solid paint film.
To know more about composition refer here
https://brainly.com/question/32502695#
#SPJ11
s: How many moles of fluorine atoms are in 5.50 moles of Freon (CCl₂F2)?
Answers
The number of moles of fluorine atoms are in 5.50 moles of Freon is 11 moles.
What are the moles of a substance?The mole is the amount of a substance that contains exactly as many molecules, atoms, radicals, ions, or electrons as there are in 12 grams of 12C.
The moles of atoms of an element present in a compound is obtained from the formula of the compound.
The moles of fluorine atoms present in 5.50 moles of Freon (CCl₂F₂) is obtained as follows:
From the formula of Freon, there are 2 moles of fluorine atoms in 1 mole of Freon.
In 5.50 moles of Freon, there will be 5.5 * 2 moles of fluorine = 11 moles of fluorine atoms.
Learn more about moles of atoms at: https://brainly.com/question/15383578
#SPJ1
Draw the Lewis structure for CO32- including any valid resonance structures. Which of the following statements is TRUE? A) The CO32- ion contains one C-O single bond and two C=O double bonds. B) The CO32- ion contains two C-O single bonds and one C=O double bond. C) The CO32- ion contains three C-O double bonds. D) The CO32- ion contains two C-O single bonds and one C=0 triple bond. E) None of the above are true. 14) How many of the following elements can form compounds with an expanded octet? Pb Kr Si B A) 0 B) 1 C) 2 D) 3 E) 4
Answers
The Lewis structure for CO32- has one carbon atom bonded to three oxygen atoms, with a negative charge on the ion. One of the oxygen atoms is singly bonded to the carbon atom, while the other two oxygen atoms are double bonded to the carbon atom. There are two valid resonance structures for CO32-, where the double bonds are shifted between the different oxygen atoms.
Therefore, the answer is A) The CO32- ion contains one C-O single bond and two C=O double bonds.
Elements in the third period or higher, such as sulfur, phosphorus, and chlorine, can form compounds with an expanded octet. However, elements in the second period, such as boron and carbon, typically do not form compounds with an expanded octet.
Therefore, the answer is B) 1 (only the element Pb can form compounds with an expanded octet).
Learn more about oxygen atoms:
https://brainly.com/question/28009615
#SPJ11
Why does the drink in the regular cup warm
up?
The drink in the regular cup warms up because
Answers
Answer:
Energy transfer between the particles in solids, liquids, and gases can explain how warm matter outside a cup can cause a cold liquid inside the cup to warm up.
Explanation:
Answer:
The drink in the regular cup warms up.
Explanation:
The reason why it warms up is because the cup is colder than room temperature and melts another reason why the drink in the regular cup warms up is because when we breathe out hot air is coming out soo that also makes the drink warmer.
A pH strip was used to test the pH of a glass of water. The image shows the results.
Use the scale below to determine the pH value of the water, and determine whether the water is acidic, alkaline, or
neutral. Then predict what will happen to the pH if someone were to place a straw into the water and blow.

Answers
The pH strip is used to test the pH of a solution. The pH of water is neutral which is around 7.
What is pH?The pH is known as the power of hydrogen. The pH is used to measure the degree of basicity and acidity of a solution. The amount of hydrogen ion concentration in a solution determines the pH of the solution. Mathematically, pH is given by the formula:
pH -= -log [H⁺]
The pH strip is a strip of litmus paper with which a person can measure the pH value of a liquid solution. The substance in the pH paper causes the paper to show a different color at different acidity values. The official pH scale is between the pH values of 0 to 14, where 0 is very acidic and 14 very alkaline and 7 is neutral pH.
Learn more about pH here:
https://brainly.com/question/15289714
#SPJ1
Answer:
The pH value of the water is 7. And I don't exactly know what would happen if you put a straw into it and blew into it, but if I had to make a guess then I would guess that the pH value would go down because the water is moving around.
please help asap I will give brainiest

Answers
Answer:
12 = Lead
13 = red
Explanation:
12:
Given data:
Mass of metal = 143 g
Volume of metal = 12.6 cm³
Identity of metal = ?
Solution:
To check which metal is this we will calculate the density.
Formula:
d = m/v
d = 143 g/ 12.6 cm³
d = 11.3 g/cm³
The metal is lead.
13:
First of all we will calculate the densities of all liquid. The liquid with the highest density will form the bottom layer.
Blue:
d = m/v
d = 12 g/100 mL
d = 0.12 g/mL
Clear:
d = 10 g/ 100 mL
d = 0.1 g/mL
Red:
d = m/v
d = 15 g/100 mL
d = 0.15 g/mL
Yellow:
d = m/v
d = 13 g/ 100 mL
d = 0.13 g/mL
The density of red liquid is highest from all thus it will form bottom layer.
A sample of O2 with an initial temperature of 50.0 oC and a volume of 105 L is cooled to -25 oC. The new pressure is 105.4 kPa and the new volume is 55.0 L. What was the initial pressure of the sample? Show your work, using the G.U.E.S.S. method.
Answers
Answer:
71.92 kPa
Explanation:
Using the combined gas law equation;
P1V1/T1 = P2V2/T2
Where;
P1 = initial pressure (kPa)
P2 = final pressure (kPa)
V1 = initial volume (L)
V2 = final volume (L)
T1 = initial temperature (K)
T2 = final temperature (K)
According to the information provided in this question;
T1 = 50°C = 50 + 273 = 323K
V1 = 105L
T2 = -25°C = -25 + 273 = 248K
P2 = 105.4 kPa
P1 = ?
V2 = 55.0 L
Using P1V1/T1 = P2V2/T2
P1 × 105/323 = 105.4 × 55/248
105P1/323 = 5797/248
0.325P1 = 23.375
P1 = 23.375 ÷ 0.325
P1 = 71.92 kPa
Calculate the pH of a buffer solution created by reacting 100 mL of 0.1 M NH3 with 90 mL of 0.1 M HNO3. (Remember, you can find Ka and Kb values on gchem!)
Answers
This question is asking for the pH of a buffer solution between ammonia and nitric acid, with given volumes and concentrations. At the end, the result turns out to be 10.488.
BuffersIn chemistry, buffers are known as substances attempting to hold a relatively constant pH by mixing and acid and a base (weak and strong). In such a way, for the substances given, the first step will be to calculate the consumed moles as they are mixed:
\(n_{NH_3}=0.1L*0.1mol/L=0.01mol\\\\n_{HNO_3}=0.09L*0.1mol/L=0.009mol\)
Now, since ammonia is in a greater proportion, one can calculate how much of it is left after being consumed by the nitric acid:
\(n_{NH_3}^{left}=0.01mol-0.009mol=0.001mol\)
And its new concentration:
\([NH_3]=\frac{0.001mol}{0.1L+0.09L} =0.00526M\)
Next, with ammonia's ionization:
\(NH_3+H_2O\rightleftharpoons NH_4^++OH^-\)
We set up the equilibrium expression based on ammonia's Kb:
\(Kb=\frac{[NH_4^+][OH^-]}{[NH_3]}\)
Which can be solved by introducing x and using ammonia's Kb:
\(1.8x10^{-5}=\frac{x^2}{0.00526M}\\ \\\)
Then, we solve for x which is also equal to the concentration of ammonium and hydroxide ions in the solution:
\(x=\sqrt{0.00526*1.8x10^{-5}}=0.000308M\)
Ultimately, we calculate the pOH and then turn it into pH with:
\(pOH=-log(0.00308)=3.512\\\\pH=14-3.512=10.488\)
Learn more about buffers: https://brainly.com/question/24188850
A 0.25 M solution of a salt NaA has pH 9.29. What is the value of Ka for the parent acid HA?
Answers
The Ka value for the parent acid of Ha is \(Ka = 6.576 \times 10^{-6}\).
Overall equation for this reaction can be given as :
Na+ (aq) + A-(aq) + H2O(l) → Ņa+(aq) + HA(aq) + OH-(aq)
Net ionic equation will be :
\(A^{-}(aq) + H_{2}O(l) \rightarrow HA(aq) + OH^{-}(aq)\\\\K_{b} = \frac{[HA][OH^{-}]}{[A^{-}]}\)
Given, pH = 9.29
The pH of the solution is the negative logarithm of hydrogen ion concentration in aqueous solution.
So, pOH = 14 - 9.29 = 4.71
So, \([OH^-] = 10-4.71 = 1.95 \times 10^-{5}\)
M = [HA]
\(K_{b} = \frac{[HA][OH^{-}]}{[A^{-}]}or, K_{b} = \frac{[1.95 \times 10^{-5}]^{2}}{[0.25]}or, K_{b} = 1.521 \times 10^{-9}\)
Now, we know that;
\(K_{a} \times K_{b} = 10^{-14}or, K_{a} = \frac{10^{-14}}{1.521 \times 10^{-9}}or,\mathbf{ K_{a} = 6.576 \times 10^{-6}}\)
Therefore, the value of \(Ka = 6.576 \times 10^{-6}\).
To learn more about pH check the link below-
https://brainly.com/question/172153
#SPJ4
identify ways to reduce the risk of liquid bumping while heating.
Answers
The most common way of preventing bumping is by adding one or two boiling chips to the reaction vessel. However, these alone may not prevent bumping and for this reason it is advisable to boil liquids in a boiling tube, a boiling flask, or an Erlenmeyer flask.
The half-life of sodium-24 is 15 hours. How much sodium-24 will remain after 3.75 days, if the initial sample
was 20.0 grams
Answers
After 3.75 days, 6.25 grams of sodium-24 will remain.