A group of cells is called an organ true or false quick pls :)
Answers
Answer: Flase
Explanation: The answer would be tissue
Related Questions
A weather foreshows 35 m/h on the weather map. Which weather component does this number represent
Answers
Answer:
this is the minutes and hours this is representing
A 50. 6 grams sample of magnesium hydroxide (Mg(OH)2) is reacted with 45. 0 grams of hydrochloric acid (HCl). What mass of MgCl2 is produced?
Answers
82.67 grams of MgCl₂ are produced when 50.6 grams of Mg(OH)₂ and 45.0 grams of HCl are reacted.
The balanced chemical equation for the reaction between magnesium hydroxide and hydrochloric acid is:
Mg(OH)₂ + 2HCl → MgCl₂ + 2H₂O
To find the mass of MgCl₂ produced, we need to determine which reactant is limiting. This can be done by calculating the number of moles of each reactant and comparing them to the stoichiometric ratio in the balanced equation.
Number of moles of Mg(OH)₂ = 50.6 g / 58.32 g/mol = 0.868 mol
Number of moles of HCl = 45.0 g / 36.46 g/mol = 1.235 mol
According to the balanced equation, 1 mole of Mg(OH)₂ reacts with 2 moles of HCl. Therefore, Mg(OH)₂ is the limiting reactant, since only 0.868 moles of Mg(OH)₂ are available to react with HCl.
From the balanced equation, we know that 1 mole of Mg(OH)₂ produces 1 mole of MgCl₂. Therefore, the number of moles of MgCl₂ produced is also 0.868 moles.
The molar mass of MgCl₂ is 95.21 g/mol. Therefore, the mass of MgCl₂ produced is:
Mass of MgCl₂ = 0.868 mol x 95.21 g/mol = 82.67 g
Therefore, approximately 82.67 grams of MgCl₂ are produced when 50.6 grams of Mg(OH)₂ and 45.0 grams of HCl are reacted.
Learn more about magnesium hydroxide
https://brainly.com/question/21904397
#SPJ4
A solution is
Question 6 options:
a homogeneous mixture.
a compound.
chemically separable.
a heterogeneous mixture.
Answers
Answer:
the first one. a homogeneous mixture.
Explanation:
hope this helps :)
in a nucleic acid, adjacent nucleotides are bound to each other in what way?
Answers
The adjacent nucleotides are bound to each other through a phosphodiester bond in a nucleic acid.
What is nucleic acid?Nucleic acid is a biopolymer made up of nucleotide monomers that make up nucleic acid chains. The nucleotide's three components are a five-carbon sugar, a phosphate group, and a nitrogenous base. Nucleic acids are present in all living cells, including viruses and bacteria, and they play a critical role in storing, transmitting, and expressing genetic information. RNA and DNA are two types of nucleic acids.
The phosphate group in one nucleotide forms a phosphodiester bond with the hydroxyl group on the sugar molecule of the next nucleotide in line in nucleic acids. This reaction is carried out by removing a molecule of water, resulting in a strong covalent bond between two nucleotides. These bonds make up the sugar-phosphate backbone of a nucleic acid chain, which is fundamental to its structure.
Learn more about Nucleic acid: https://brainly.com/question/17701344
#SPJ11
A gas has a volume of 350 ml at 45oC. If the volume changes to 400. ml, what is the new temperature in oC
Answers
Answer:
It should be 28 degrees C
Explanation:
Answer:
28°C
Explanation:
each body atom is fully inside the boundaries of the cube. what fraction of each corner atom is inside the boundaries of the cube?
Answers
The fraction of each corner atom inside the boundaries of the cube is \($\frac{a^3 - \pi r^3}{\pi r^3}$\). This fraction will be greater as the size of the cube increases compared to the size of the atoms.
Assuming that we have a cube where each body atom is fully inside the boundaries, it means that the cube's edges are at least twice the size of the atoms' radii. In such a scenario, the corner atoms will have only a fraction of their volumes inside the boundaries of the cube.
To find out the fraction of each corner atom inside the boundaries of the cube, we can first calculate the volume of the cube, and then subtract the volume outside the cube, which will give us the volume inside the boundaries.
Let's assume that the length of the cube's edge is 'a'. The volume of the cube will be given by a^3. The corner atoms are located at the eight corners of the cube. Since the atoms are spherical, the volume of each atom will be \($ \frac{4}{3} \pi r^3 $\), where 'r' is the radius of the atom.
Now, the distance from the center of the corner atom to any of the cube's faces is 'a/2 + r'. Thus, the distance from the center of the corner atom to the opposite corner of the cube is √3(a/2 + r).
Therefore, the fraction of each corner atom inside the boundaries of the cube will be \($\frac{a^3 - 8 \cdot \frac{1}{8} \cdot \frac{4}{3} \pi r^3}{\frac{4}{3} \pi r^3}$\). This simplifies to \($\frac{a^3 - \pi r^3}{\pi r^3}$\).
To learn more about atoms
https://brainly.com/question/30898688
#SPJ4
What separates the inner planets from the outer planets in our solar system?
()Comet Belt
()Asteroid Belt
()Their differences
()Distance
Help plss!!
Answers
Answer:
the answer is B Astroid Belt
What is the hydronium ion concentration of a solution with a pOH of 7.20?
A - 1.56 × 10^−6 M
B - 1.79 × 10^−6 M
C - 1.58 × 10^−7 M
D - 2.78 × 10^−7 M
I think the correct answer is C, can anyone confirm this?
Answers
Answer:
C - 1.58 × 10^−7 M
Explanation:
The hydroxide ion concentration can be found from the pOH by the reverse mathematical operation employed to find the pOH. [OH-] = 10-pOH or [OH-] = antilog ( - pOH) Page 2 Example: What is the hydroxide ion concentration in a solution that has a pOH of 5.70? On a calculator calculate 10-5.70, or "inverse" log (- 5.70).
The concentration of hydronium ion C) - 1.58 × 10^−7 M
The concentration of hydronium ionif pOH is 7.20 than pH = 14-7.20∴pH=6.80
The hydroxide ion concentration can be found from the pOH by the reverse
How do you calculate concentration from pH?To calculate the pH of an aqueous solution we need to know the concentration of the hydronium ion in moles per liter (molarity).
The pH is then calculated using the expression:
pH = - log [H3O+]
-log[6.80]
- 1.58 × 10^−7 M
learn more about hydronium ion here: https://brainly.com/question/1396185
#SPJ2
Which two events will happen if more H2 and N2 are added to this reaction after it reaches equilibrium?
3H2 + N2 to 2NH3
Answers
If more \(H_{2}\) and \(N_{2}\) are added to the reaction 3\(H_{2}\) + N2 → 2\(NH_{3}\) after it reaches equilibrium, two events will occur Shift in Equilibrium and Increased Yield of \(NH_{3}\)
1. Shift in Equilibrium: According to Le Chatelier's principle, when additional reactants are added, the equilibrium will shift in the forward direction to consume the added reactants and establish a new equilibrium. In this case, more \(NH_{3}\) will be produced to counteract the increase in \(H_{2}\) and \(N_{2}\).
2. Increased Yield of \(NH_{3}\): The shift in equilibrium towards the forward reaction will result in an increased yield of \(NH_{3}\). As more \(H_{2}\) and \(N_{2}\) are added, the reaction will favor the production of \(NH_{3}\) to maintain equilibrium. This will lead to an increase in the concentration of \(NH_{3}\) compared to the initial equilibrium state.
It is important to note that the equilibrium position will ultimately depend on factors such as the concentrations of \(H_{2}\), \(N_{2}\), and \(NH_{3}\), as well as the temperature and pressure of the system. By adding more reactants, the equilibrium will adjust to achieve a new balance, favoring the formation of more \(NH_{3}\).
Know more about Le Chatelier's principle here:
https://brainly.com/question/2943338
#SPJ8
Particles in a liquid can __________.
Answers
Answer:
Slide past each other
Explanation:
allows oxidation of co (carbon monoxide) to less-harmful co₂ (carbon dioxide) allows oxidation of hc (unburned hydrocarbons) to co₂ (carbon dioxide) and h₂o (water)
Answers
The oxidation of co (carbon monoxide) to less-harmful co₂ (carbon dioxide) and the oxidation of hc (unburned hydrocarbons) to co₂ (carbon dioxide) and h₂o (water).
What is meant by an oxidation reaction?Oxidation is that the loss of electrons during a reaction by a molecule, atom or ion. Oxidation occurs when the oxidation number of a molecule, atom or ion is increased. the other process is called reduction, which occurs when there's a gain of electrons or the oxidation state of an atom, molecule, or ion decreases.
Why is it called an oxidation reaction?
The term oxidation was first employed by Antoine Lavoisier to signify the reaction of a substance with oxygen. Much later, it had been realized that the substance, upon being oxidized, loses electrons, and therefore the meaning was extended to include other reactions in which electrons are lost, no matter whether oxygen was involved.
Learn more about oxidation reaction:
brainly.com/question/25886015
#SPJ4
Proteco Oils Pressed Purity are a range of cold pressed oils ideal for cooking. The high quality oils are extracted from nuts, fruit and seeds. They are flavoursome and are naturally chemical and preservative free. Pressed Purity are one of the few oils on mainstream supermarket shelves which is 100% Australian. They offer a wider range variants than any other oil manufacturer in Australia. Proteco Oils’ state of the art refinery in Kingaroy, South East Queensland is uniquely equipped. With highly specialized equipment for complete oil processing on a large scale. Now, exporting into China and throughout Asia Pacific, this family owned company has grown with the help of Evolve Brand Design
Market Mostly females, 25-60+ years, with a contemporary cooking attitude. These consumers are health conscious, seeking natural and chemical free options for themselves and their family. The secondary target audience are men and women of all ages. This group consider themselves to be gourmet home chefs and are open to new tastes. Communication of the product concept was critical with the initial brand name development. Evolve Brand Design presented a range of concepts and the brand name ‘Pressed Purity’ was chosen. This concept was the winner as it implied the chemical free processing of the raw crops into edible oils. Likewise, the design for the brand is an analogy for pressing the oil from the fruit, nut or seeds using a vice. The Pressed Purity distinctive edge is threefold. Chemical free, 100% natural ingredients and genuinely Australian. Export opportunities have risen due to the third, very important, unique selling point (USP). In addition, they have a wide range of flavours with applications tailored to a range of food preparation methods. From flavourful salad dressing oils to baking and high heat applications like stir-frying and barbeques, Pressed Purity has a solution
Q.2.1 With the use of examples applicable to the case study, explain human resource forecasting. (10)
Q.2.2 Explain the concept of product differentiation in the context of Pressed Purity. (5)
Q.2.3 Recommend a work-study method for Proteco Oil’s refinery. (10)
Q.2.4 Identify and explain the criteria Proteco Oil used for market segmentation. (10)
Q.2.5 Identify the operational process used by Proteco Oil. Justify your choice. (10)
Answers
Q.2.1 With the use of examples applicable to the case study, explain human resource forecasting. Human resource forecasting refers to the process of estimating and planning for the future staffing needs of an organization.
Q.2.3 One work-study method that could be recommended for Proteco Oil's refinery is the method of time and motion study.
Q.2.1 It involves analyzing the current workforce, identifying future workforce requirements, and developing strategies to meet those needs. In the case of Proteco Oil's Pressed Purity, human resource forecasting would involve predicting the number and types of employees needed to support the company's growth and expansion.
For example, as Pressed Purity expands its export operations into China and throughout the Asia Pacific region, they would need to forecast the additional human resources required to manage international logistics, distribution, and marketing. This may include hiring employees with expertise in international trade, language skills, and knowledge of the target markets. Human resource forecasting would also consider the need for additional staff at the state-of-the-art refinery in Kingaroy to handle increased production and quality control.
Q.2.2 Product differentiation refers to the process of distinguishing a product from its competitors by highlighting unique features, benefits, or characteristics. In the context of Pressed Purity, product differentiation is evident in several aspects of their offerings.
One example of product differentiation is their focus on being 100% Australian. This sets them apart from other oil manufacturers in Australia who may rely on imported ingredients. By promoting their Australian origin, Pressed Purity appeals to consumers who prioritize supporting local businesses and value the quality associated with Australian products.
Additionally, Pressed Purity emphasizes being chemical and preservative-free. This addresses the growing consumer demand for natural and healthier food options. By positioning their oils as naturally chemical-free, Pressed Purity differentiates themselves from competitors who may not have such a strong emphasis on natural and chemical-free products.
Q.2.3 One work-study method that could be recommended for Proteco Oil's refinery is the method of time and motion study. Time and motion study involves analyzing and improving work processes by observing and measuring the time required to complete specific tasks or activities.
In the context of the refinery, a time and motion study could be conducted to identify any inefficiencies or bottlenecks in the oil processing operations. This could involve observing workers as they perform tasks and measuring the time taken for each step of the process. By analyzing the data collected, the refinery management can identify areas where time can be saved, processes can be streamlined, and productivity can be improved.
For example, the time and motion study may reveal that certain equipment or machinery in the refinery is causing delays or requiring excessive manual labor. Based on these findings, the management can make informed decisions on investing in more efficient equipment or implementing process improvements to optimize productivity and reduce costs.
Q.2.4 Proteco Oil used the following criteria for market segmentation:
Demographic segmentation: The primary target audience for Pressed Purity is mostly females, aged 25-60+ years, with a contemporary cooking attitude. These consumers are health-conscious and seek natural and chemical-free options for themselves and their families. The secondary target audience includes men and women of all ages who consider themselves gourmet home chefs and are open to new tastes.
Psychographic segmentation: Pressed Purity targets consumers who prioritize natural and chemical-free products. By emphasizing the use of 100% natural ingredients and being genuinely Australian, Pressed Purity appeals to health-conscious consumers who value the quality and authenticity of the products they consume. They also cater to gourmet home chefs who are looking for unique and flavorful cooking options.
Geographic segmentation: Initially, Proteco Oil focused on the domestic market in Australia.
To know more about Demographic segmentation
https://brainly.com/question/22401136
#SPJ11
Look at the diagram. Which shows the correct arrangement of electrons in a hydrogen chloride molecule?

Answers
Answer:
D............................
There are two types of chemical compound one is covalent compound and another is ionic compound, covalent compound formed by sharing of electron and ionic compound formed by complete transfer of electron. The correct representation is option D.
What is chemical Compound?Chemical Compound is a combination of molecule, Molecule forms by combination of element and element forms by combination of atoms in fixed proportion.
Covalent bond is present in molecule HCl. Hydrogen has 1 electron in its outermost shell. Chlorine has 7 electrons in its valence shell. So one one electron from each element is shared between them to form a covalent bond.
Therefore, the correct representation of arrangement of electrons in a hydrogen chloride molecule is option D.
To learn more about chemical compound, here:
https://brainly.com/question/26487468
#SPJ2
what is the element symbol

Answers
Answer:
Oxygen
Explanation:
Oxygen because it has 8 neutros and 8 protons
Ollie has a mass of 45 kilograms. What is his weight in newtons?
Answers
Answer:
646.8
Weight in Newtons can be found by multiplying the mass times the acceleration of gravity.
The mass of the man is 66 kilograms on Earth.
Earth has an acceleration of gravity that is 9.8 meters per square second.
Substitute the values into the formula.
Multiply.
1 kg * m/s² is equal to 1 Newton, therefore our answer of 646.8 kg *m/s² is also equal to 646.8 Newtons, or N.
The man's weight in Newtons in 646.8 Newtons
............................................................
What measurements should you use for large amounts of energy transfer
Answers
Identify 3 physical conditions that can optimize rate of diffusion of a gas across a membrane, and relate these to Fick’s Law of Diffusion. Please describe 3 ways animal respiratory systems have evolved in order to maximize the exchange of O2 and CO2 across their membranes.
Answers
Physical conditions that can optimize rate of diffusion of a gas across a membrane and relate these to Fick’s Law of Diffusion are The partial pressure difference of gases, The surface area of the membrane, The thickness of the membrane, Ventilation and Increased surface area
Physical conditions that can optimize rate of diffusion of a gas across a membrane and relate these to Fick’s Law of Diffusion are the following:
The partial pressure difference of gases: It is the main driving force behind gas exchange. Fick's Law of Diffusion states that the rate of diffusion of a gas is directly proportional to the pressure gradient. The greater the partial pressure difference, the faster the rate of gas diffusion.
The surface area of the membrane: Fick's Law of Diffusion states that the rate of gas diffusion is proportional to the surface area of the membrane. The more surface area available for gas exchange, the faster the rate of gas diffusion.
The thickness of the membrane: Fick's Law of Diffusion also states that the rate of gas diffusion is inversely proportional to the thickness of the membrane. The thinner the membrane, the faster the rate of gas diffusion.
Animal respiratory systems have evolved in order to maximize the exchange of O2 and CO2 across their membranes in the following ways:
Ventilation: The movement of air or water over the respiratory surface increases the partial pressure gradient of gases. This increases the rate of diffusion of gases across the membrane.
Increased surface area: Respiratory surfaces have evolved to have a large surface area to increase the rate of diffusion of gases across the membrane.Thin respiratory surfaces: Respiratory surfaces have evolved to be thin to reduce the diffusion distance for gases. This increases the rate of diffusion of gases across the membrane.
To know more about Ventilation visit:
https://brainly.com/question/31440202
#SPJ11
the melting point of scandium fluoride is 1552°c, explain why scandium fluoride has a high melting point.
Answers
Answer:
Scandium(III) fluoride, ScF3, is an ionic compound. It is slightly soluble in water but dissolves in the presence of excess fluoride to form the ScF63− anion.
hope it will help you......
A high melting point is caused by a high fusion heat, a low fusion entropy, or a blend of the two. The crystal phase of highly symmetrical molecules is densely packed with many efficient intermolecular interactions, resulting in a higher enthalpy change on melting.
What is intermolecular force?An intermolecular force is a force that mediates the interaction of molecules, including electromagnetic forces of attraction or repulsion that act between atoms and other types of neighboring particles, such as atoms or ions.
The intermolecular forces can be in order of strength, ion-dipole, hydrogen bonding, dipole-dipole, and Van der Waals forces.
Because it is the power of attraction or repulsion between atoms or molecules rather than the ability to share or give/take electrons.
ScF3, or Scandium(III) fluoride, is an ionic compound. It is only slightly soluble in water, but in excess fluoride it dissolves to form the ScF63 anion.
Thus, due to the strong intermolecular forces, it have high melting point.
For more details regarding intermolecular forces, visit:
https://brainly.com/question/9007693
#SPJ2
Hey.... If I got a question in my exams of which metal does not react with hydrochloric acid and I write gold as the answer, will I get full marks on that questiona dn the whole exam paper in total????
Answers
Answer:
I think you would pretty much get the answer wrong.
You won´t get full credit. sorry for your wrong test answer, :(
Explanation:
The correct answer would be copper and mercury do NOT react with hydrochloric acid.
On a hot sunny day, why do people sprinkle water on the roof or open
ground?
Answers
When water is sprinkled on the roof top or ground it evaporates because of the latent heat of vaporisation leaving behind a cooling effect. in case of ground, this evaporation causes cooling of the surrounding area after some time; whereas in case of roof top the room beneath is cooled.
The latent heat of vaporization causes water sprinkled on the ground or roof to evaporate, producing a cooling effect.
What is vaporization?Vaporization is defined as the process of changing a substance's liquid or solid state into its gaseous (vapor) state. Boiling and evaporation are the two processes that cause vaporization. When water is exposed to sunlight, evaporation takes place when the water turns into vapour and rises into the atmosphere. Boiling is the process of vaporizing a liquid when the environment permits the development of vapor bubbles inside the liquid.
After a while, this evaporation in the case of the ground cools the space around it, whereas in the case of the roof, the room below is chilled. The significant latent heat of vaporization of water contributes to cooling the hot surface, which is why evaporation of water has a cooling effect. The water quickly evaporates from the hot road surface, removing heat from it in the process.
Thus, the latent heat of vaporization causes water sprinkled on the ground or roof to evaporate, producing a cooling effect.
To learn more about vaporization, refer to the link below:
https://brainly.com/question/14578189
#SPJ2
A statement that’s accuracy can be tested by experiments and through observations is formally called a _______________.
Answers
Answer:
A hypothesis
Explanation:
how do particles differ after a physical change?
Answers
Answer:
They are still the same particals just. packed together diffrently depending on what stae of matter it's in.
Explanation:
Help!! I’ll mark you Brainliest.!

Answers
Answer:
For 5 pts? gimme that
Explanation:
Answer:
Explanation:
if you know what those phrases mean, then you can look them up and draw those pictures you find, as well as add the definition of each.
Draw the neutral organic product that results from the given reaction. Include all hydrogen atoms. CH_3CH_2CH_2OH(I) H_2SO_4 (conc) /200^∘C
Answers
The neutral organic product obtained from the reaction is propene (CH3CH=CH2)
What is the role of concentrated sulfuric acid in the dehydration of 1-propanol?The given reaction involves the dehydration of 1-propanol (CH3CH2CH2OH) in the presence of concentrated sulfuric acid (H2SO4) at 200°C. Dehydration is the removal of a water molecule from the alcohol compound. In this case, 1-propanol loses a water molecule to form propene (CH3CH=CH2), which is an alkene.
The neutral organic product resulting from this reaction is propene (CH3CH=CH2). The reaction occurs as the hydroxyl group (-OH) of 1-propanol is protonated by the acidic H2SO4, leading to the loss of a water molecule. This creates a double bond (C=C) between the second and third carbon atoms, resulting in the formation of propene.
Thus, the neutral organic product obtained from the reaction is propene (CH3CH=CH2).
Learn more about neutral organic product brainly.com/question/17313223
#SPJ11
Given the following balanced equation, determine the limiting reagent when the following quantities of reactants are mixed with \( 2 \mathrm{Cr}+3 \mathrm{Cl}_{2} \rightarrow 2 \mathrm{CrCl}_{3} \). 1
Answers
A limiting reagent is a substance that limits the reaction's extent since it is used up in the reaction. The excess reactant is the reagent that does not get entirely consumed in a reaction. Consider the balanced equation given as \(\(2 \mathrm{Cr}+3 \mathrm{Cl}_{2} \rightarrow 2 \mathrm{CrCl}_{3}\).\)
The amount of each reactant provided to us is not given explicitly. However, based on the balanced equation, we can determine the stoichiometric ratio of the reactants and products.
There are two chromium atoms and three chlorine molecules in this equation reacting to form two chromium trichloride molecules. As a result, 2 moles of Cr react with 3 moles of \(Cl2\). This means that the limiting reagent will be whichever reactant is not supplied in the appropriate stoichiometric ratio.
According to the stoichiometric ratio, 2 moles of Cr react with 3 moles of \(Cl2\); thus, \(Cl2\) is the limiting reagent if there are less than 1.5 moles of \(Cl2\) available. On the other hand, Cr is the limiting reagent if there is less than 1 mole of Cr available.
If the number of moles of each reactant supplied is greater than or equal to the number of moles needed for the balanced equation, no reactant is limiting, and the reactants are present in excess. Therefore, based on the stoichiometry of the balanced equation, we can calculate the limiting reagent's amount and the excess reactant's amount.
To know more about limiting reagent here
https://brainly.com/question/31171741
#SPJ11
Can somebody plz help me with some grade7science?
Answers
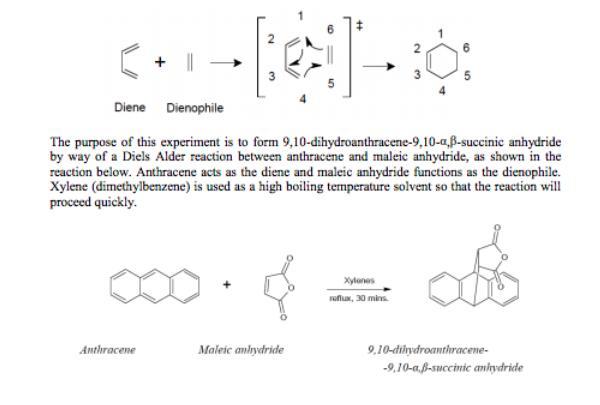
Consider the Diels-Alder reaction of anthracene and maleic anhydride. What is the role of each of the reactants
Answers
The role of each of the reactants are:
The o=benzene (extra line+C)=o acts as the [Dienophile]The three benzene rings acts as the [diene]What is the Diels-Alder reaction?
The Diels–Alder reaction is known to be a kind of reaction that is said to exist between what we call a conjugated diene and that of an alkene (dienophile).
Note that the union of both leads to a unsaturated six-membered rings and because the reaction is one that entails the formation of a cyclic product through what we call a cyclic transition state, it is said to be known as a "cycloaddition".
Note that from the experiment, The role of each of the reactants are:
The o=benzene (extra line+C)=o acts as the [Dienophile]The three benzene rings acts as the [diene]Learn more about Diels-Alder reaction from
https://brainly.com/question/14496475
#SPJ1
Which compound can inadvertently be created through the distillation process and can be fatal if consumed
Answers
Answer:
lWhich compound can inadvertently be created through the distillation process and can be fatal if consumed?
Methanol
for the following reaction, 3.34 grams of iron are mixed with excess oxygen gas. the reaction yields 4.40 grams of iron(iii) oxide. 4fe (s) 3o2 (g) 2fe2o3 (s) (1) what is the theoretical yield of iron(iii) oxide? grams (2) what is the percent yield for this reaction? %
Answers
The theoretical yield of iron(iii) oxide55.85 g/mol and the percent yield for this reaction 110.55%.
The balanced chemical equation is given by:4 Fe(s) + 3 O2(g) → 2 Fe2O3(s)
The chemical reaction between iron and oxygen gas produces iron oxide.
According to the problem, 3.34 grams of iron are mixed with excess oxygen gas to yield 4.40 grams of iron (III) oxide. Thus,
Theoretical yield of iron (III) oxide can be calculated using the following equation:
Theoretical Yield of Fe2O3 = Mass of Fe * (1 mole of Fe / Atomic Mass of Fe) * (2 moles of Fe2O3 / 4 moles of Fe) * (Formula Weight of Fe2O3 / 1 mole of Fe2O3)
The atomic weight of Fe is 55.85 g/mol.
The formula weight of Fe2O3 is (2*55.85) + (3*16) = 159.7 g/mol
Substituting the given values,
Theoretical Yield of Fe2O3 = 3.34 * (1/55.85) * (2/4) * (159.7/1) = 3.98 g
Therefore, the theoretical yield of iron (III) oxide is 3.98 g.
The percentage yield is given by the following formula:
% yield = (Actual Yield / Theoretical Yield) * 100
The actual yield of iron (III) oxide is given as 4.40 g.
Substituting the given values in the formula:
% yield = (4.40 / 3.98) * 100% yield
= 110.55%
Therefore, the percent yield of iron (III) oxide is 110.55%.
To know more about Theoretical visit :
brainly.com/question/1446190
#SPJ11
number 5 plz! what ideas do you have about why there are patterns in earthquake and valcano data?

Answers
Answer:
Explanation:
The interaction along plate boundaries results in an increased frequency of earthquakes at those locations. ... Additionally, stronger earthquakes are more likely to occur along active plate boundaries. Strong earthquakes are more common at transform and convergent plate boundaries.