a monodentate ligand binds to a metal atom through only one atom.
Answers
A monodentate ligand is a type of ligand that binds to a metal atom through only one atom.
Monodentate ligands are commonly used in coordination chemistry to form coordination complexes. They have only one binding site, which allows them to form a single bond with a metal atom. Examples of monodentate ligands include halides (e.g. Cl-, Br-), water (H2O), and ammonia (NH3). These ligands play an important role in biological systems, such as in the binding of oxygen to iron in hemoglobin, which involves the monodentate ligand, dioxygen (O2). The use of monodentate ligands in coordination chemistry allows for the control of the geometry and reactivity of the resulting coordination complexes.
To learn more about Monodentate ligands:
https://brainly.com/question/31980760
#SPJ11
Related Questions
Please help with this question!

Answers
Answer:
The answer is 1:1
Explanation:
Calculate the freezing point of a solution containing 60 g of glucose (Molar mass = 180 g mol ^−1) in 250 g of water. (Kf of water =1.86 K kg mol ^−1).
Answers
The freezing point of the solution containing 60 g of glucose in 250 g of water is -2.48°C.
To calculate the freezing point of the solution, we need to use the formula:
ΔTf = Kf × m
Where ΔTf is the change in freezing point, Kf is the freezing point depression constant of water, and m is the molality of the solution.
First, we need to calculate the molality of the solution:
Moles of glucose = 60 g / 180 g mol^-1 = 0.333 mol
Molality (m) = moles of solute / mass of solvent in kg
= 0.333 mol / 0.250 kg
= 1.332 mol kg^-1
Now we can substitute the values into the formula:
ΔTf = Kf × m
ΔTf = 1.86 K kg mol^-1 × 1.332 mol kg^-1
ΔTf = 2.48 K
So the freezing point of the solution is lowered by 2.48 degrees Celsius compared to pure water. To find the actual freezing point of the solution, we need to subtract this value from the freezing point of water (0 degrees Celsius):
Freezing point of solution = 0°C - 2.48°C
= -2.48°C
Therefore, the freezing point of the solution containing 60 g of glucose in 250 g of water is -2.48°C.
Learn more about freezing point here
https://brainly.com/question/3121416
#SPJ11
What approximate volume of reaction solution was used in the experiments described in the passage if 0.5 mg of MgCl2 were added to the reaction mixture?
10mM OF MgCl2 added
Answers
The approximate volume of reaction solution used in the experiments described in the passage if 0.5 mg of MgCl₂ were added to the reaction mixture 10 mM of MgCl₂ was 525 µL.
To calculate the approximate volume of reaction solution used in the experiments where 0.5 mg of MgCl₂ was added, you need to consider the concentration of MgCl₂ provided, which is 10mM.
First, convert the mass of MgCl₂ (0.5 mg) to moles using the molar mass of MgCl₂ (95.21 g/mol):
= 0.5 mg × (1 g / 1000 mg) × (1 mol / 95.21 g)
= 5.25 × 10⁻⁶ mol
Next, use the concentration of MgCl₂ (10mM) to determine the volume of the solution:
= 5.25 × 10⁻⁶ mol / 10 mM
= 5.25 × 10⁻⁴ L
To give the answer in a more convenient unit, convert the volume to microliters (µL):
= 5.25 × 10⁻⁴ L × (1,000,000 µL / 1 L)
= 525 µL
So, approximately 525 µL of reaction solution was used in the experiments described in the passage where 0.5 mg of MgCl₂ was added to the reaction mixture.
Learn more about solution: https://brainly.com/question/25291588
#SPJ11
What is the measure for the amount of disorder in a system?
Answers
Answer: Entropy
Explanation: Entropy. A measure of the level of disorder of a system is entropy, represented by S
Hoped this helped!!!!
What is the full name of the chemical in marijuana that is the mind altering/psychoactive ingredient that can impact memory, coordination and addiction?.
Answers
Answer: delta-9-tetrahydrocannabinol also known as THC
Explanation: according to dea.gov
Which explains the charge of an ion of calcium (Ca)?
A. 2 electrons are gained by the atom.
B. 2 electrons are removed from the atom.
C. 2 protons are removed from the atom.
D. 2 protons are gained by the atom.
Answers
Answer:
B. 2 electrons are removed from the atom
Explanation:
The charge of an ion of calcium (Ca) is +2, which means that the calcium atom has lost 2 electrons.
Therefore, the correct answer is B. 2 electrons are removed from the atom.
The correct option is B. 2 electrons are removed from the atom.
Explanation:
Calcium (Ca) has an atomic number of 20, which means it has 20 electrons in its neutral state, arranged in shells around the nucleus. The electronic configuration of neutral calcium is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s².
When calcium loses two electrons from its outermost shell (4s²), it forms a calcium ion (Ca²⁺) with a noble gas electron configuration of argon. This is achieved by removing the two valence electrons from the 4s² subshell. The loss of two electrons from the neutral calcium atom leaves behind 18 electrons, but still 20 positively charged protons in the nucleus, giving the calcium ion a 2+ charge.
uppose you were confused about which of the two containers held standard NaOH and which held vinegar. Which of the following would be the safest way to decide the contents of each diluted container? a. Feel each solution. b. Taste each solution. c. Add one or two drops of phenolphthalein indicator to small samples of each solution.
Answers
which of the following statements about the mass of an object is correct
a. mass changes with location
b. mass remains constant
c. mass changes with altitude
d. mass changes with gravity
Answers
Answer: Mass remains constant
Explanation:
What is produced at the anode in the electrolysis of molten CuF2? O A. F2 O B. H2 O C. cu D.02
Answers
The produced at the anode in the electrolysis of molten CuF2 is A. F₂.
During electrolysis, CuF₂ will separate into Cu₂⁺ cations and F⁻anions. Cu₂⁺ will be reduced to copper metal, which will deposit on the cathode, whereas F⁻ anions will be oxidized to fluorine gas on the anode in the electrolysis of molten CuF₂. Hence, the answer is option A. Fluorine gas (F₂) is generated at the anode in the electrolysis of molten CuF₂.
Therefore, during the electrolysis of molten CuF₂, Cu₂⁺ is reduced to copper metal, which deposits on the cathode, and F⁻ anions are oxidized to fluorine gas on the anode, which is produced at the anode. The chemical reactions taking place during electrolysis of CuF₂ are given below: At the cathode, Cu₂⁺ cations get reduced to copper metal. Cu₂⁺ + 2e⁻ ⟶ Cu. At the anode, F⁻ anions get oxidized to fluorine gas. 2F⁻ ⟶ F₂ + 2e⁻. Therefore, option A is correct.
Learn more about electrolysis at:
https://brainly.com/question/12994141
#SPJ11
ll of the following metals will corrode if immersed in fresh water except (a) copper (b) nickel (c) chromium (d) iron
Answers
All of the following metals will corrode if immersed in freshwater except chromium. Therefore, the correct answer is option (C)
The metal chromium is very resistant to corrosion and does not corrode in freshwater. Chromium is a potent oxidising agent, which means it can readily provide electrons to other substances, creating a shield of protective oxide that prevents corrosion of the metal.
The metal is protected from the atmosphere by this extraordinarily tough oxide layer, which also serves to shield the metal from further oxidation. Chromium can also create a solid connection with oxygen, which increases its resistance to corrosion.
Chromium does not interact with other chemicals in freshwater, hence it does not corrode. To maintain corrosion resistance, chromium is frequently utilised in products like plumbing fixtures, tools, and automobile components.
Complete Question:
All of the following metals will corrode if immersed in freshwater except
(a) copper
(b) nickel
(C) chromium
(D) aluminum
To learn more about Metals visit:
https://brainly.com/question/4701542
#SPJ4
If a new method for obtaining oil from dry oil fields is found, then we will see a. the AS curve shift to the left. b. a movement to the left along the AD curve. c. the AD curve shift to the left. d. the AD curve shift to the right. e. the A5 curve shift to the right.
Answers
If a new method for obtaining oil from dry oil fields is found, then we would expect to see an increase in oil production, which would lead to a leftward movement along the AD curve as demand for oil is met more easily.
However, if this increase in supply is significant enough, it could also lead to a shift of the AS curve to the right, indicating an increase in potential output. Therefore, the correct answer would be b. a movement to the left along the AD curve, and potentially a shift of the AS curve to the right. The AD curve represents the relationship between aggregate demand and the price level, while the AS curve represents the relationship between aggregate supply and the price level.
If a new method for obtaining oil from dry oil fields is found, then we will see e. the AS (Aggregate Supply) curve shift to the right. This is because the new method would increase the availability of oil, resulting in a larger supply of goods and services at the same price level, which causes the AS curve to shift to the right.
Visit here to learn more about AD curve:
brainly.com/question/29610075
#SPJ11
17. A cast iron skillet is used to fry bacon. For optimal frying, the pan must be heated to about 178 °C
from a room temperature of 22.0 °C. It is known that 1.58 x 10 J of heat energy are absorbed by the
pan to reach the desired temperature and the specific heat of iron is 0.450 J/g °C. What must the mass
of the skillet be?
A. 12.7 kg
B. 2.25 kg
C. 110 kg
D. 1.97 kg
Answers
According to specific heat capacity,the mass of the skillet is 226.52 kg.
What is specific heat capacity?Specific heat capacity is defined as the amount of energy required to raise the temperature of one gram of substance by one degree Celsius. It has units of calories or joules per gram per degree Celsius.
It varies with temperature and is different for each state of matter. Water in the liquid form has the highest specific heat capacity among all common substances .Specific heat capacity of a substance is infinite as it undergoes phase transition ,it is highest for gases and can rise if the gas is allowed to expand.
It is given by the formula ,
Q=mcΔT
Substitution in above formula gives m=1.58×10/0.450×155=0.2265 g or 226.5 kg.
Thus, the mass of the skillet is 226.52 kg.
Learn more about specific heat capacity,here:
https://brainly.com/question/29766819
#SPJ1
Your question is incomplete, but most probably your full question was,A cast iron skillet is used to fry bacon. For optimal frying, the pan must be heated to about 178 °C
from a room temperature of 22.0 °C. It is known that 1.58 x 10 J of heat energy are absorbed by the
pan to reach the desired temperature and the specific heat of iron is 0.450 J/g °C. What must the mass
of the skillet be?
A. 12.7 kg
B. 2.25 kg
C. 110 kg
D. 1.97 kg
E.226.52 kg
Task
01
Perform a literature
survey to find out the methods of simulating general aerofoil shape
including experimental and numerical techniques. Student may use
about 2000 words (± 10%) to elaborate
Answers
The methods of simulating general aerofoil shape include experimental techniques such as wind tunnel testing, and numerical techniques such as Computational Fluid Dynamics (CFD) simulations.
Simulating general aerofoil shapes involves both experimental and numerical techniques. Experimental methods include wind tunnel testing, where scaled-down models of the aerofoil are tested in controlled airflow to measure aerodynamic forces. Numerical techniques, such as Computational Fluid Dynamics (CFD) simulations, involve solving fluid flow equations on a computer to analyze flow characteristics and aerodynamic forces. CFD simulations are cost-effective, flexible, and can handle complex aerofoil shapes, but require accurate modeling and validation. A combination of experimental and numerical methods enhances our understanding of aerofoil aerodynamics and helps optimize their design.
To know more about Computational Fluid Dynamics (CFD) click here,
https://brainly.com/question/30578986
#SPJ11
When the carbonic acid sodium bicarbonate buffer pair buffers lactic acid?
Answers
When the carbonic acid sodium bicarbonate buffer pair buffers lactic acid, the following reaction occurs:$$\text{HCO}_3^- + \text{H}^+ \leftrightarrow \text{H}_2\text{CO}_3$$The carbonic acid/bicarbonate buffer system is one of the most important in human blood. When the pH of the blood decreases (becomes more acidic), the amount of bicarbonate ions in the blood decreases, and the concentration of hydrogen ions increases.
A buffer is a solution of a weak acid and its conjugate base that prevents changes in pH when small amounts of strong acid or base are added. Buffer systems protect organisms from pH changes by regulating and neutralizing acids and bases that enter or are produced by cells.
When the carbonic acid sodium bicarbonate buffer pair buffers lactic acid, the following reaction occurs:$$\text{HCO}_3^- + \text{H}^+ \leftrightarrow \text{H}_2\text{CO}_3$$The carbonic acid/bicarbonate buffer system is one of the most important in human blood. When the pH of the blood decreases (becomes more acidic), the amount of bicarbonate ions in the blood decreases, and the concentration of hydrogen ions increases. To balance the excess hydrogen ions, carbonic acid (H2CO3) is formed from carbon dioxide (CO2) and water (H2O). Carbonic acid then decomposes to form bicarbonate ions and hydrogen ions, and the pH of the blood is returned to normal. The bicarbonate ions act as a base, neutralizing the excess hydrogen ions that cause the blood to become more acidic. This is called the bicarbonate buffer system. Lactic acid is produced during intense exercise when the body doesn't get enough oxygen to meet its energy needs. The buildup of lactic acid in muscles can cause fatigue and muscle soreness. The carbonic acid/bicarbonate buffer system can also help to buffer the excess lactic acid produced during exercise, preventing the blood from becoming too acidic.
To know more about sodium bicarbonate visit :
brainly.com/question/8506770
#SPJ11
which detail about globalr clusters does photograhp on page 1 mke clear
Answers
Answer:
Globular clusters are densely packed collections of ancient stars. Roughly spherical in shape, they contain hundreds of thousands, and sometimes millions, of stars. Studying them helps astronomers estimate the age of the universe or figure out where the center of a galaxy lies.
Explanation:
a 25.0 ml sample of a saturated c a ( o h ) 2 solution is titrated with 0.030 m h c l , and the equivalence point is reached after 38.1 ml of titrant are dispensed. based on this data, what is the concentration (m) of c a ( o h ) 2 ?
Answers
Calculations have been made about OH- and Ca(OH)2 concentrations.
Let's start by converting H+ and OH- ions to the following equation:
C1 x V1 equals C2 x V2C1 x 25.
= 0.03*38.1C1
= 0.04571 M
The OH- ion concentration is thus 0.04571 M.
Ca(OH)2 concentration, for example
= 0.5*[OH-]
[Ca(OH)2]
= 0.5*0.04571
= 0.02285 M
As a result, the concentrations of Ca(OH)2 and OH- have both been computed.
What exactly is focus?
The amount of solute that has dissolved in a given volume of solvent or solution is indicated by the concentration of the solution. A solution is referred to as concentrated when a large amount of dissolved solute is present in it.
Hence, the answer the OH- ion concentration is thus 0.04571 M.
As a result, the concentrations of Ca(OH)2 and OH- have both been computed.
Further information about concentration, click
https://brainly.com/question/17206790
#SPJ4
The concentration of OH- ion is 0.04571 M and concentration of Ca(OH)₂ = 0.02285 M
Equating concentration :
The Concentration of both OH- and Ca(OH)₂ is evaluated :
Equating H+ ions and OH- ions as follows:
C₁ × V₁ = C₂ × V₂C₁ × 25 = 0.03 × 38.1C₁ = 0.04571 M
Then, concentration of OH- ion is 0.04571 M
For, concentration of Ca(OH)₂ = 0.5 × [OH-][Ca(OH)₂]
= 0.5 × 0.04571
= 0.02285 M
What is a solution?A solution is a homogeneous combination of one or further solutes dissolved in a solvent.solvent the material in which a solute dissolves to produce a homogeneous combination solute the substance that dissolves in a solvent to produce a homogeneous mixture.Many dissimilar kinds of solutions live. For illustration, a solute can be a gas, a liquid or a solid. Solvents can also be feasts, liquids or solids.
The succeeding figuring show the bitsy geste of several distinctive kinds of solutions. Note that in each case, the solute particles are slightly distributed among the solvent particles.
Therefore, concentration of both OH- and Ca( OH)₂ is calculated.
To learn more about Solutions,
brainly.com/question/25275758
#SPJ4
Exercise 2:
The thermic decomposition of potassium chlorate (KClO3
) is a method used at laboratory to produce oxygen (O2
) and produce potassium
chloride (KCl).
1. Write the balanced equation of the reaction
2. Calculate :
3.1. The molar mass of KClO3
3.2. The number of moles of oxygen gaz produced during the decomposition of
0,5mol de KClO3
(s)
3.3. The mass of potassium chloride obtained from 30,6 g KClO3 solid
M(K)= 39 g.mol-1
; M(Cl)= 35 g.mol-1
; M(O)= 16 g.mol-1
Answers
Answer:
1. KCLO3------>KCL + 3/2O2(g)
2. 122.5g/mol
3. 0.2mol
4. 18.5g
What is the number of moles of glucose (C₆H₁₂O₆) in 0.500 L of a 0.40 M solution?
Answers
Answer:
0.20 moles
Explanation:
In order to solve this problem it is necessary to keep in mind the definition of molarity:
Molarity = moles / litersIf we input the data given by the problem we're left with:
0.40 M = moles / 0.500 LMeaning that we can proceed to calculate the number of moles:
moles = 0.40 M * 0.500 Lmoles = 0.20 molesThe number of moles of glucose (\(C_6H_{12}O_6\)) in 0.500 Liters of a 0.40 M solution is 0.2 moles.
Given the following data:
Molarity of solution = 0.40 MVolume of solution = 0.500 LTo determine the number of moles of glucose (\(C_6H_{12}O_6\)) in 0.500 Liters of a 0.40 M solution:
Mathematically, the molarity of a solution is given by the formula:
\(Molarity = \frac{number\;of\;moles}{Volume \;in\;liters}\)
Making number of moles the subject of formula, we have:
\(Number\;of\;moles = Molarity \times Volume\)
Substituting the given parameters into the formula, we have;
\(Number\;of\;moles = 0.40 \times 0.500\)
Number of moles = 0.2 moles.
Read more: https://brainly.com/question/13750908
300mL of 0.83mol/L acetic acid reacts with 12.0g of sodium carbonate at 21 C and 100.3kPa. What volume of dry carbon dioxide is released in this reaction? Water vapour pressure at this temperature is 2.49 kPa.
Answers
Answer:
The volume that carbon dioxide release is 2.83L
Explanation:
The reaction of acetic acid (CH₃COOH) with sodium carbonate (Na₂CO₃) is:
2 CH₃COOH + Na₂CO₃ →Na₂(CH₃COO)₂ + CO₂ + H₂O
Moles of acetic acid and sodium carbonate (Molar mass: 105.99g/mol) in the reaction are:
Acetic acid: 0.300L ₓ (0.83mol / L) = 0.249 moles.
Sodium carbonate: 12g ₓ (1mol / 105.99g) = 0.113 moles.
Based on the chemical equation, 2 moles of acetic acid reacts per moles of sodium carbonate. For a complete reaction of sodium carbonate you need:
0.113 moles Na₂CO₃ ₓ (2 moles CH₃COOH / 1 mole Na₂CO₃) = 0.226 moles of CH₃COOH
As you have 0.249 moles, Na₂CO₃ is limitng reactant.
As 1 mole of sodium carbonate produce 1 mole of CO₂, from 0.113 moles of Na₂CO₃ you obtain 0.113 moles of CO₂
Using PV = nRT, it is possible to find the volume that a gas occupies, thus:
V = nRT / P
n = 0.113 moles
R = 8.314 kPa×L/mol×K
T = 21°C + 273.15 = 294.15K
P = 100.3kPa - 2.49kPa = 97.81kPa
The vapor pressure is subtracted because is the pressure that water exerted.
Replacing:
V = 0.113mol×8.314 kPa×L/mol×K×294.15K / 97.81kPa
V = 2.83L
The volume that carbon dioxide release is 2.83LWhich one of the following substances is an element?
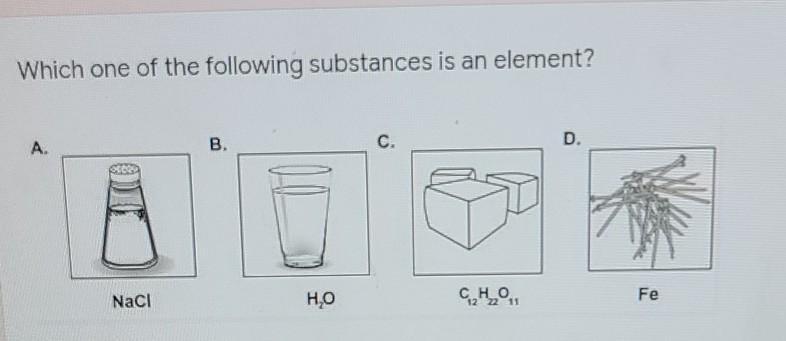
Answers
Answer: Fe
Explanation: Fe (Iron) is the only element since it involves only 1 atom. The other options are compounds, since there are more than 2 atoms bonded together.
a molecule of n2o4has twice the mass as a molecule of no2.what do you notice about the partial pressure exerted by 2 moles of no2compared to the partial pressure exerted by 2 moles of n2o4
Answers
The partial pressure exerted by 2 moles of NO₂ compared to the partial pressure exerted by 2 moles of N₂O₄ is less due to it's low molecular weight.
Pressure is defined as the force applied on an object perpendicular to it's surface per unit area over which it is distributed.Gauge pressure is a pressure which is related with the ambient pressure.
There are various units by which pressure is expressed most of which are derived units which are obtained from unit of force divided by unit of area . The SI unit of pressure is pascal .
Learn more about pressure,here:
https://brainly.com/question/18431008
#SPJ4
How many grams of molecular chlorine will be required to completely react with 0.0223 moles of sodium iodide according to the following reaction?
2Nal + Cl2 ---> 2NaCl + I2
A. 1.57 104 grams
B. 3.16 grams
C. 0.0112 grams
D. 0.791 grams
Answers
The amount of chlorine will be 0.791 gram required to completely react with 0.0223 moles of sodium iodide .
The given chemical reaction is :
2Nal + \(Cl_{2}\) → 2NaCl + \(I_{2}\)
It is given that:
Amount of sodium iodide = 0.0223 mol.
Atomic mass of chlorine = 35.45 g.
The amount of chlorine can be calculated as:
0.0223mol. NaI × (1mol. Cl₂/2mol. NaI) × (35.45g/1mol. Cl2)
=0.791 grams
Therefore, the correct answer will be option (D).
To know more about sodium chloride.
https://brainly.com/question/20273068
#SPJ1
The solid dissolves quickly is a chemical or physical reaction
Answers
Answer:
i think it is chemical wait no physical
Explanation:
_____of gas molecules with an object is the cause of
pressure by a gas.
Answers
Answer:
Gas Pressure
Explanation:
Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects (Figure 5.2. 1). Although the force of each collision is very small, any surface of appreciable area experiences a large number of collisions in a short time, which can result in a high pressure.
Answer:
Collisions
Explanation:
It says that it was the answer.
Can somebody plz help answer both questions correctly thank you!!
WILL MARK BRAINLIEST WHOEVER ANSWERS FIRST :D

Answers
Answer:
answer #1 used throughout the world to power devices, appliances and methods of transportation utilized in daily life. To make things operate, electrical energy must be emitted from energy sources such as power plants, to enable an object to consume the power it needs to function. ((if you want to cut it down short use the two first sentences))
answer #2 We get solar heat energy from the sun, and sunlight can also be used to produce electricity from solar
Explanation:
Ethane (C2H6) is burned with 20% excess air during a combustion process. Assuming complete
combustion and a total pressure of 100 kPa, determine:
1. the A/F ratio.
2. the dew point temperature of the products.
Answers
To solve this problem, we need to use the principles of stoichiometry and combustion thermodynamics. The A/F ratio is the air-to-fuel ratio by mass, and it is defined as the mass of air required to completely combust one unit mass of fuel. The dew point temperature is the temperature at which the water vapor in the combustion products begins to condense, and it depends on the composition and temperature of the products.
The A/F ratio can be calculated as follows:
First, we need to write the balanced chemical equation for the combustion of ethane:
C2H6 + 3.5 O2 + (0.2 x 3.5) O2 -> 2 CO2 + 3 H2O + (0.2 x 3.5) N2
Here, the 3.5 O2 represents the stoichiometric amount of oxygen required to completely combust one unit mass of ethane, and the 0.2 x 3.5 O2 represents the excess oxygen supplied (20% excess air). The (0.2 x 3.5) N2 represents the corresponding amount of nitrogen in the excess air.
The molecular weights of the reactants and products are:
C2H6: 2 x 12.01 + 6 x 1.01 = 30.07 g/mol
O2: 2 x 16.00 = 32.00 g/mol
CO2: 1 x 12.01 + 2 x 16.00 = 44.01 g/mol
H2O: 2 x 1.01 + 16.00 = 18.02 g/mol
N2: 2 x 14.01 = 28.02 g/mol
Using these values, we can calculate the mass of air required to combust one unit mass of ethane:
A/F = (mass of O2 + mass of N2) / mass of C2H6
= [(3.5 x 32.00) + (0.2 x 3.5 x 28.02)] / 30.07
= 12.53
Therefore, the A/F ratio is 12.53 by mass.
To determine the dew point temperature of the products, we need to calculate the mole fractions of the water vapor and nitrogen in the products, and then use a psychrometric chart or equations to find the dew point temperature. Alternatively, we can use the following simplified equation:
Tdp = (243.12 x ln(RH/100) + 17.62 x T) / (17.62 - ln(RH/100) - T)
Here, Tdp is the dew point temperature in °C, RH is the relative humidity of the products, and T is the temperature of the products in °C.
Assuming the combustion products are initially at a temperature of 25°C and a relative humidity of 100% (i.e., the products are fully saturated with water vapor), we can calculate the mole fractions of the water vapor and nitrogen as follows:
Mole fraction of H2O = (n H2O) / (n H2O + n N2)
= (3 x 0.2) / [(3 x 0.2) + (3.5 x 0.2)]
= 0.3
Mole fraction of N2 = (n N2) / (n H2O + n N2)
= (3.5 x 0.2) / [(3 x 0.2) + (3.5 x 0.2)]
= 0.35
Using these values
For more questions like Ethane visit the link below:
https://brainly.com/question/19539045
#SPJ11
What are two factors that can change the rate of a chemical?
Answers
Answer:
the surface area of a solid reactant.
temperature.
presence/absence of a catalyst.
Atom is a smallest particle of an element which have all the chemical properties of that element.What is the origin of Atom.
Answers
Answer:
ORIGIN OF ATOM.Sometimes peoples like to say that atom is like a cell in living things,is it true?comment below,letss goo!!
Explanation:
A greek philosopher known as Democritus was the first to consider the idea that matter is made up of small particles ,about 400BC.
Since there was no experimental evidence to support his idea,it was not accepted.
About 2000 years later an English man called John Dalton revived the discussion.He used experimental evidence to support his idea to convince people that matter is made up of particles called atoms.It accepted!.
An English man called John Dalton revived the discussion.He used experimental evidence to support his idea to convince people that matter is made up of particles called atoms.It was accepted ,he conclude the following theories:
Matter is made up of tiny particles called atom which cannot be splitted into simpler substancesAtom cannot be created nor destroyedWhat does it mean for a unit to be "derived"?
Answers
Answer:
you differentiate to find the derivative
Explanation:
Answer:
A derived unit is a unit that results from a mathematical combination of SI base units.
OR
A unit of measurement obtained by multiplication or division of the base units of a system without the introduction of numerical factors.
Explanation:
i dont know if that answered your question
Calculate the concentrations of hydronium ion and hydroxide ion at 25°C in: (a) 0.10 M HCl, (b) 1.4 × 10–4 M Mg(OH)2, a strong base. answer with steps please
Answers
Ai. The concentration of hydronium ion, [H₃O⁺], is 0.10 M
Aii. The concentration hydroxide ion, [OH⁻] is 1×10⁻¹³ M
Bi. The concentration of hydronium, ion [H₃O⁺], is 3.57×10⁻¹¹ M
Bii. The concentration hydroxide ion, [OH⁻] is 2.8×10¯⁴ M
A. How do i determine [H₃O⁺] and [OH⁻] of 0.10 M HCl?i. The concentration of hydronium ion, [H₃O⁺] can be obtained as follow:
HCl(aq) + H₂O <=> H₃O⁺(aq) + Cl⁻(aq)
From the above equation,
1 mole of HCl contains 1 mole of H₃O⁺
Therefore,
0.10 M HCl will also contain 0.10 M H₃O⁺
Thus, the concentration of hydronium ion, [H₃O⁺] is 0.10 M
ii. The concentration of hydroxide ion, [OH⁻] can be obtained as follow:
Concentration of hydronium, ion [H₃O⁺] = 0.10 MConcentration hydroxide ion, [OH⁻] =?[H₃O⁺] × [OH⁻] = 10¯¹⁴
0.10 × [OH⁻] = 10¯¹⁴
Divide both side by 3.02×10⁻¹⁰
[OH⁻] = 10¯¹⁴ / 0.10
[OH⁻] = 1×10⁻¹³ M
Thus, concentration of hydroxide ion, [OH⁻] is 1×10⁻¹³ M
B. How do i determine [H₃O⁺] and [OH⁻] for 1.4×10¯⁴ M Mg(OH)₂?First, we shall obtain concentration hydroxide ion, [OH⁻]. Details below:
Mg(OH)₂(aq) <=> Mg²⁺(aq) + 2OH⁻(aq)
From the above equation,
1 mole of Mg(OH)₂ is contains 2 mole of OH⁻
Therefore,
1.4×10¯⁴ M Mg(OH)₂ will contain = 1.4×10¯⁴ × 2 = 2.8×10¯⁴ M OH⁻
Thus, concentration hydroxide ion, [OH⁻] is 2.8×10¯⁴ M
Now, we shall obtain the concentration of hydronium, ion [H₃O⁺]. Details below:
Concentration of hydroxide ion, [OH⁻] = 2.8×10¯⁴MConcentration of hydronium, ion [H₃O⁺] = ?[H₃O⁺] × [OH⁻] = 10¯¹⁴
[H₃O⁺] × 2.8×10¯⁴ = 10¯¹⁴
Divide both side by 2.8×10¯⁴
[H₃O⁺] = 10¯¹⁴ / 2.8×10¯⁴
[H₃O⁺] = 3.57×10⁻¹¹ M
Thus, the concentration of hydronium, ion [H₃O⁺], is 3.57×10⁻¹¹ M
Learn more about hydroxide ion concentration, [OH⁻]:
https://brainly.com/question/19800885
#SPJ1