a rydberg atom is an atom whose valence electrons are in states with a very large principal quantum number n. this means it has a probability cloud with a large amplitude a large distance from the nucleus. evidence of such atoms has been detected by radio astronomers in the form of radiation from diffuse hydrogen gas in intersellar space. in fact, there is no theoritical limit on the size an atom can attain, provided it is free from outside influences. 1)what is the smallest value of n such that the bohr radius of a single such hydrogen atom would be greater than 7 microns, roughly the size of a typical single-celled organism. ee
Answers
A Rydberg atom is an atom with valence electrons in states with a very high principal quantum number n. The smallest value of n such that the Bohr radius of a single hydrogen atom would be greater than 7 microns is approximately 1,573.
This implies that it has a probability cloud with a high amplitude and is situated at a significant distance from the nucleus. Such atoms' existence has been discovered by radio astronomers through radiation from diffuse hydrogen gas in interstellar space. The largest size an atom can achieve is unlimited, given it is free of external effects.A single-celled organism's typical size is 7 microns. To determine the smallest value of n where the Bohr radius of a single hydrogen atom would be greater than 7 microns,
we will use the following formula for Bohr radius:
r = n²h²/4π²me²k where h is Planck's constant, me is the electron's mass, and k is Coulomb's constant.
We can see that the Bohr radius increases as n². We can therefore express the equation as:n² = 4π²me²k * r / h²When r = 7 µm, we can plug it into the equation and solve for n as:n² = 4π²(9.10938356 × 10^-31 kg) (8.9875517923 × 10^9 N m²/C²)(7 × 10^-6 m) / (6.62607015 × 10^-34 m² kg/s)²n² = 2,469,471.663n ≈ 1,572.90
Therefore, the smallest value of n such that the Bohr radius of a single hydrogen atom would be greater than 7 microns is approximately 1,573.
To know more about Rydberg atom refer to:
https://brainly.com/question/31392723
#SPJ11
Related Questions
How many molecules are in 5.06L of oxygen gas?
Answers
If a laser operating at a wavelength of 488 nm and a power of 123.0 mW is turned on for 18.73 minutes, how many photons has it emitted?
Answers
Answer:
3.39e+20
Explanation:
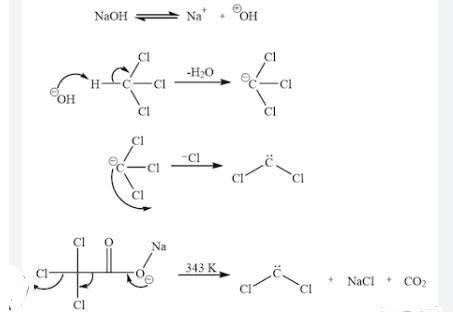
When dichlorocarbene is generated in the presence of an atkene. a dichlorocyclopropane is formed. Write the complete stepwise mechanism for the formation of dichlorocarbene, CCl2. Show all intermediate structures and show all electron flow with arrows. Draw the complete Lewis electron dot structure for dichlorocarbene, CCl2.
Answers
Dichlorocarbene is an intermediate species which is formed from the reaction of trichloromethane with a base. The intermediate CCl₃ further loss a Cl formes CCl₂.
What is dichlorocarbene?The reactive intermediate with the chemical formula CCl₂ is called dichlorocarbene. Despite not having been isolated, this chemical species is a typical intermediate in organic chemistry since it is produced from chloroform. This twisted diamagnetic molecule enters other bonds quickly.
Carbenes contains two electrons in their valence shell and they are highly reactive and therefore used in many synthetic reactions.
CHCl₃ on reaction with a strong base such as NaOH produce the intermediate anion CCl₃⁻ by the elimination of water molecule. This trichlorocarban further eliminates one Cl forms dichlorocarbene.
To find more on dichlorocarbene, refer here:
https://brainly.com/question/28202225
#SPJ4
6. Which of the following elements is most likely to form covalent bonds when a molecule is
formed?
A. He
B. Al
C. CI
D. Na
7. Which of the following elements are NOT likely to form chemical bonds?
A. Group 1 elements
B. Group 2 elements
С. Group 17 elements
D. Group 18 elements
8. All the following atoms can form negative ions in their compounds EXCEPT
A. Fluorine
B. Sulfur
C. Oxygen
D. Magnesium
Answers
Answer: hydogen
Explanation:
each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. This is actually one of the chemical properties of metals and nonmetals: metals tend to form cations, while nonmetals tend to form anions
Answer:
6.C
7.D
8.D
Explanation:
6. Chlorine is a non-metal, so is helium is inert hence Chlorine is the correct answer.
7.Group 18 elements are noble gases with maximum electron configuration hence they do not bond further
8.During bonding metals transfer electrons i.e. they become less negative and more positive thus having a positive ion
how does a tiny seed turn into a tree overtime?
Answers
Answer:
A group of cells ready to form roots a stem and the first leaves
is p2 polar or no polar
Answers
Answer:
Non-polar
Explanation:
The way to determine if something is polar is by subtracting their electronegativity values. The two atoms in this molecule are both phosphorous which have equal electronegativity values (since they are both phosphorus). Anything minus itself will equal 0. If the final difference between the electronegativity values is less than 0.4, it will be non-polar. Since the result is 0, P2 is non-polar.
In P₂ molecule there is absence of partial charges, thus the molecule has been nonpolar.
The molecules have been differentiated based on polarity as polar and nonpolar molecules. The molecules that have been comprised of the difference in the electronegativity of the elements and develop partial charges have been termed polar compounds.
The compounds with no difference in electronegativity and absence of partial charges are termed nonpolar molecules. In the P₂ molecule, there have 2 phosphorus molecules with the same electronegativities.
Since there has been an absence of polarity in the molecule, the molecule of P₂ is nonpolar.
For more information about polar molecules, refer to the link:
https://brainly.com/question/24775418
Please the app isn’t working and I can’t find other questions that got answered

Answers
Given:
Sum of masses of two isotopes = 371.9087 u
Re-185 natural abudance = 37.40%
Re-187 natural abudance = 62.60%
Known:
atomic weight of Re = 186.207 u
Atomic mass of Re-185:
To find the atomic mass of Re-185, take the total mass given and subtract atomic weight.
abundance of Re-185 = 37.40% = 0.3740
(371.9087 - x) = atomic weight of Re-187 in u
To find mass of Re-187:
abundance of Re-187 = 62.60% = 0.6260
Solution:
Step 1. Multiply x times the abundance of Re-185 and multiply (371.9087 - x) times the abundance of Re-187.
Re-185: (0.3740)(x) = 0.3740x
Re-187: (0.6260)(371.9087 - x) = 232.8148462 - 0.6260x
Step 2. Add the results and set them equal to 186.207.
0.3740x + 232.8148462 - 0.6260x = 186.207
Step 3. Solve for x by subtracting 232.8148462 from both sides and then divide both sides by -0.2520.
0.3740x + 232.8148462 - 0.6260x - 232.8148462 = 186.207 -
232.8148462
0.3740x - 0.6260x = -46.6078462
-0.2520x = -46.6078462
-0.2520x/-0.2520x = -46.6078462/-0.2520
x = 184.9517706 u
Step 4. Atomic weights of Re-185 and Re-187.
x = 185.0 u = the atomic weight of Re-185
(371.9087 - 184.9517706) = 186.9569294 = 187.0 u = the atomic weight of Re-187
Therefore the atomic weight of Re-185 is 185.0 u, and the atomic weight of Re-187 is 187.0 u.
the distance between two successive peaks is called
Answers
the distance between two successive peaks on adjacent waves is its..
NEED HELP NOW!! Scientists study two kinds of jellyfish. One lives at the surface of the ocean; the other lives in the deep region of the ocean. Which adaptations would you be more likely to find in the jellyfish that lives in the deeper waters of the ocean?
A: Develop fins and a muscular tail
B: Adapt to lower temperatures
C: Develop photosynthesis
D: Develop bioluminescence
Answers
Develop bioluminescence would you be more likely to find in the jellyfish that lives in the deeper waters of the ocean.
What is Bioluminescence?Light generated by a chemical reaction inside a living thing is known as bioluminescence. A type of chemiluminescence known as bioluminescence is simply a chemical process that results in the production of light.
A "cold light" is what bioluminescence is. Less than 20% of a light's energy is used to produce thermal radiation, or heat.
The ocean is the most common habitat for bioluminescent creatures. Fish, bacteria, and jellies are among the marine creatures that glow bioluminescent. Fireflies and fungus are two examples of the bioluminescent creatures that can be found on land.
Therefore, Develop bioluminescence would you be more likely to find in the jellyfish that lives in the deeper waters of the ocean.
To learn more about bioluminescence refer to the link:
https://brainly.com/question/8897369
#SPJ2
How would you turn oxygen gas into solid oxygen
Answers
Answer:
Oxygen gas would be converted into solid oxygen by decreasing the temprature with the increase in preassure. It will bring its molecules together, which would result in the formation of solid oxygen
Answer:
Oxygen gas would be converted into solid oxygen by decreasing the temprature with the increase in preassure. It will bring its molecules together, which would result in the formation of solid oxygen.
The equation represents the decay of a polonium nucleus to form a lead nucleus. An alpha particles is emitted. (a) What is the value of A ? (b) What is the value of Z ? 1 point for (a) 1 point for (b) * □
4i
(2 Points)
84
210
P
8
→
82
206
Pb+
Z
A
He+energy
Answers
The equation represents the decay of a polonium nucleus to form a lead nucleus. An alpha particles is emitted hence, after summarizing:
(a) The value of A is 210.
(b) The value of Z is 82.
In the given equation, the decay of a polonium nucleus to form a lead nucleus is represented. An alpha particle is emitted. Let's break down the information provided:
(a) The value of A represents the mass number, which is the total number of protons and neutrons in the nucleus.
From the equation, we can see that the initial nucleus, polonium (Po), has a mass number of 210 (the superscript number before the element symbol).
Therefore, the value of A is 210.
(b) The value of Z represents the atomic number, which is the number of protons in the nucleus.
From the equation, we can see that the resulting nucleus, lead (Pb), has an atomic number of 82 (the subscript number after the element symbol).
Therefore, the value of Z is 82.
Learn more about alpha particles from the link given below.
https://brainly.com/question/24276675
#SPJ4
3. Draw each structure and determine how many configurational isomers are possible each A. 4-chloro-3-hexen-2-ol B. 2,4-hexadiene C. 3-chloro-1,4-pentadiene 4. Determine for each of the following isomeric pairs whether they are constitutional, isomers or stereoisomers. OH OH OH and OH OH OH and OH and
Answers
The structures provided for the isomeric pairs. Isomers are pairs of compounds with the same molecular formula but different structures.
To determine the number of configurational isomers for each compound, first draw each structure:
4-chloro-3-hexen-2-ol:
The structure of 4-chloro-3-hexen-2-ol contains a double bond between carbons 3 and 4 and an OH group at carbon 2. Since the double bond can have two possible configurations (cis or trans), there are two configurational isomers for this compound.
2,4-hexadiene:
The structure of 2,4-hexadiene contains two double bonds, one between carbons 2 and 3, and another between carbons 4 and 5. Each double bond can have two possible configurations (cis or trans), so there are a total of 2 x 2 = 4 configurational isomers for this compound.
3-chloro-1,4-pentadiene:
The structure of 3-chloro-1,4-pentadiene has a double bond between carbons 1 and 2, another double bond between carbons 4 and 5, and a Cl atom at carbon 3. Since the two double bonds are not adjacent, there are no cis or trans configurations to consider. Therefore, there is only one configurational isomer for this compound.
It seems that the structures provided for the isomeric pairs.
Learn more about isomeric pairs: brainly.com/question/14063244
#SPJ11
Is a doctor of medicine a scientist or an engineer?
Answers
Answer:
scientist
Explanation:
Answer:
A doctor of medicine
Explanation:
This is answer in my opinion.
Volcanoes can be destructive LOCALLY causing all of the following immediate effects EXCEPT:
O New growth in forests
O Personal damage
O Lack of breathable air
O Disruption of clean water
O Death
Answers
Answer:
New growth of trees is an exception
Explanation:
when volcanoes erupt, they release hot magma that is destructive to the environment causing personal damage, lack of breathable air and death.
heat produce cannot in any way help in growth of trees
after giving an oil-retention enema, the person is left in the
Answers
After giving an oil-retention enema, the person is left in the left lateral position for 30 minutes. The patient should be advised to hold the oil enema for at least 30 minutes before expelling.
Doing so would be beneficial to the patient's health. Below are some additional details about oil-retention enemas. An oil retention enema is a form of treatment that is intended to cure bowel blockages. It aids in the removal of solid feces from the colon and is commonly used to treat constipation.
In addition, the oil serves as a lubricant, making it simpler to expel feces. The goal of the enema is to encourage defecation, which helps to relieve symptoms such as stomach pain, bloating, and gas. In some cases, the procedure might be unpleasant. Patients may feel the urge to go to the bathroom, or they may experience cramps or pain. As a result, it's critical to ensure that patients are as comfortable as possible during the procedure.
To know more about enema visit:
https://brainly.com/question/30638137
#SPJ11
True or False: Quantitative data is about a description or observation and qualitative data is about measurements and numbers. Please help in under 20 min,
Answers
Answer:
False
Explanation:
Quantitative data is measured using numbers and Qualitative data is descriptive
The reaction between between common salt and concentrated tetraoxosulphate(vi) acid will liberate
A. sulphur (iv) oxide
B. oxygen and chloride
C. Hydrogen chloride gas
D. Hydrogen sulphide gas
Answers
most prolly option D
Explanation:
NaCl + H2SO4 -> Na2SO4 + HCl[g]
The reaction between between common salt and concentrated tetraoxosulphate(vi) acid will liberate Hydrogen chloride gas .
What is a Chemical reaction?
This is the type of reaction in which two or more elements/compounds react together to form a new substance.
The reaction between common salt and concentrated tetraoxosulphate(vi) acid can be seen below:
NaCl + H2SO4 ⇒ Na2SO4 + HCl(g)
Read more about Chemical reaction here https://brainly.com/question/11231920?source=archive
When you break an iron magnet into two pieces, you get ____.
two north poles
two south poles
two north poles and two south poles
a piece of iron that is no longer magnetic
Answers
Answer:
two north poles and two south poles
Explanation:
A single magnet has a north pole and a south pole. If it is broken into two pieces, then each of the two pieces will have a north pole and a south pole.
No matter how many times or into how many pieces a magnet is broken, the resulting pieces will have two poles each.
Answer:
Each piece will still have a north pole and a south pole.
Explanation:
In an NF3 molecule, how many electrons does the nitrogen have?
A. 3
B. 5
C. 8
D. None of the above
Answers
Answer:
B
Explanation:
because I've just guessed and you've seen this but when you get this wrong and you see you'll wish you put that down
So it would have 5 but share 3 to equal 8
5. Looking at the following 5 substances, Rank them in order of increasing solubility (the ability to dissolve in water). Briefly explain the basis of your ranking. (A3)
Br2 – Bromine, IF – iodine monofluoride , SCl2 –Sulfur dichloride,
PF3 -Phosphorus Trifluoride, C2F4 -Tetrafluoroethylene
Answers
Answer
:just switch the first ones around
Explanation:
i d0nt know how to explain sorry
give the formula for a potassium solution that could be mixed with a solution of silver nitrate to form silver chromate (as shown below). be sure to include a phase label with your formula.
Answers
Silver chromate (Ag2CrO4) is a solid precipitate that is created when potassium dichromate and silver nitrate combine. Silver chromate is a substance that is solid, as indicated by the phase label "(s)".
The formation of silver chromate from a potassium solution and a solution of silver nitrate involves a double displacement reaction. The balanced chemical equation for this reaction is:
2KNO₃(aq) + Ag₂CrO₄(aq) → 2AgNO₃(aq) + K₂CrO₄(aq)
In this equation, the phase labels indicate that the compounds are dissolved in an aqueous solution (denoted by "(aq)"). The potassium solution (represented by KNO₃) reacts with silver chromate (Ag₂CrO₄) to yield silver nitrate (AgNO₃) and potassium chromate (K₂CrO₄).
It's important to note that the formation of silver chromate requires an additional step, where silver nitrate is mixed with potassium dichromate (K₂Cr₂O₇) to produce silver chromate. The balanced equation for this step is:
2AgNO₃(aq) + K₂Cr₂O₇(aq) → Ag₂CrO₄(s) + 2KNO₃(aq)
Here, the reaction between silver nitrate and potassium dichromate produces a solid precipitate of silver chromate (Ag₂CrO₄). The phase label "(s)" indicates that silver chromate is a solid compound.
For more such questions on potassium solution
https://brainly.com/question/25380525
#SPJ4
Which part of homeostasis is this adorable puppy balancing out with his little stuffed animal buddy?
Body Temperature
Energy
Waste
Water Level
Cuteness
Plzzz help

Answers
Answer:
body temperature and energy
help me i waisted 60 points but bots keep answering someone else answer
it says its answered but its a bot i swear

Answers
Answer:
mid-ocean ridge.
Explanation:
What coefficient is missing in C2H4 + (?)O2 → 2CO2 + 2H2O?
Answers
Answer:
3
Explanation:
you must multiply everything out till everything is equal on both sides
A bond between two sugar molecules is formed by which type of reaction?
Answers
Disaccharides form when two monosaccharides undergo a dehydration reaction (a condensation reaction); they are held together by a covalent bond. Sucrose (table sugar) is the most common disaccharide, which is composed of the monomers glucose and fructose.
A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate ( sugar ) molecule to another group, which may not be another carbohydrate.
A 5.0 g sample of metal was heated to a temperature of 100.0 °C then placed into a calorimeter containing 35 mL of water at 20.0°C.
The final temperature was 26.5°C What is the specific heat capacity of the metal?
Answers
The specific heat capacity of the metal : c = 2.59 J/g °C
Further explanationGiven
5 g sample
35 ml water = 35 g
Required
The specific heat capacity of the metal
Solution
Heat can be formulated :
Q = m.c.Δt
Q in = Q out
Q absorbed = Q released
Q water = Q metal
35 g x 4.184 J/g °C x (26.5-20)= 5 x c x (100-26.5)
951.86 J = 367.5 x c
c = 2.59 J/g °C
Judge Joe is looking over a list of expert witnesses that must be summoned to court. Which one of the following actions would he take pretrial to ensure they appear? A. Issue an arrest warrant. B. Conduct a jury poll. C. Send out subpoenas. D. Interpret case law.
Answers
if you collect oxygen over water at 763.0 torr total pressure and 22.3 degrees celsius, what is the partial pressure of the oxygen
Answers
The partial pressure of oxygen when it is collected over water at 763.0 torr total pressure and 22.3 degrees Celsius is 741.9 torr.
To solve for the partial pressure of oxygen when it is collected over water at 763.0 torr total pressure and 22.3 degrees Celsius, we need to use Dalton's Law of Partial Pressures.
According to Dalton's Law, the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases. Thus, we can write:
\(P_{\text{total}} = P_{\text{oxygen}} + P_{\text{water vapor}}\)
where \(P_{\text{total}}\) is the total pressure of the gas mixture, \(P_{\text{oxygen}}\) is the partial pressure of oxygen, and \(P_{\text{water vapor}}\) is the partial pressure of water vapor.
We can rearrange this equation to solve for \(P_{\text{oxygen}}\) as follows:
\(P_{\text{oxygen}} = P_{\text{total}} - P_{\text{water vapor}}\)
To use this equation, we need to find the partial pressure of water vapor at 22.3 degrees Celsius.
We can do this using a water vapor pressure chart or table. At 22.3 degrees Celsius, the vapor pressure of water is 21.1 torr.
Now, we can substitute this value and the given total pressure of 763.0 torr into the equation above:
\(P_{\text{oxygen}} = 763.0 \, \text{torr} - 21.1 \, \text{torr} = 741.9 \, \text{torr}\)
To know more about Partial pressure here: https://brainly.com/question/32820495
#SPJ11
In a given chemical reaction, the energy of the products is less than the energy of the reactants. Which statement is true for this chemical
reaction?
A Energy is absorbed in the reaction.
B.
Energy is released in the reaction.
О с.
There is no transfer of energy in the reaction.
D. Energy is lost in the reaction.
Answers
Which atom has the largest atomic radius? *
K
Fr
Cs
Rb