A scientist wants to find out what type of mineral she has. What is the best way for her to find out?
She could look at the weight, hardness, and color.
She could look at the color, hardness, and luster.
She could look at the color, weight, and size.
She could look at the shape, size, and hardness.
Answers
Answer:
she could look at the color, hardness, and luster
Related Questions
Chemical bonding in metals is
a. the same as ionic bonding
b. the same as covalent bonding.
C. a combination of ionic and covalent bonding
different from ionic or covalent bonding
Answers
Answer:
different from ionic or covalent bonding
Explanation:
a 45 gram marble is dropped into a graduated cylinder with 30ml of water. The water level increases to 45.0 ml. What is the density of the marble?
Answers
Answer:
3 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume}\\\)
From the question we have
volume = final volume of water - initial volume of water
volume = 45 - 30 = 15 ml
We have
\(density = \frac{45}{15} = 3 \\ \)
We have the final answer as
3 g/mLHope this helps you
How much heat is given off when 16 g liquid methanol (CH3OH) at its freezing point
changes to solid methanol? (methanol AH+ = 3.16 kJ/mol)
A. 0.79 kJ
B. 0.18 kJ
C. 1.58 kJ
D. 0.36 kJ
Answers
Answer:
c. 1.58 KJ
Explanation:
I took the test:)
Answer:
1.58 KJ
Explanation:
What is the density of an unknown compound in g/ml if 1.28 pounds of the compound has a volume of 4.50L
Answers
1.28 pounds * 453.59 grams/pound = 580.61 grams
Next, we can use the formula for density:
Density = Mass / Volume
Density = 580.61 grams / 4.50 L
Density = 128.91 g/L
Therefore, the density of the unknown compound is 128.91 g/L or 0.12891 g/mL (since there are 1000 mL in 1 L).
When copper is changed to copper(II) nitrate by the nitric acid, it is an example of a(n) ______-_______ reaction. (two words, include the hyphen with no spaces)
Answers
Answer:
oxidation- reduction
Explanation:
where gaining electronic reduces one element and losing them oxidize the other nitric acid is not only strong it is also a oxidizing agent
Oxidize: copper = Cu+2When copper is changed to copper(II) nitrate by the nitric acid, it is an example of an oxidation-reduction.
What is molecule?
Molecule is defined as number of atoms combined together, that shows the most smaller chemical compound's fundamental unit that participate in chemical reaction. In the combination of atoms attractive forces play a vital role and it helps to bound the atoms by a chemical bond.
Liquid consist of small range of order and the reason behind this is intermolecular attractive force which is very strong and due to this reason molecules are packed together tightly. Due to presence of high kinetic energy the molecules present in the liquid move rapidly and fastly with one another.
Water is considered to be the simpler molecule and it consist of hydrogen and oxygen atom bounded together and due to the reason of high electronegativity of the oxygen's atom the bonds present are polar as well as covalent. Due to presence of high kinetic energy the molecules present in the liquid move rapidly and fastly with one another.
Therefore, When copper is changed to copper(II) nitrate by the nitric acid, it is an example of an oxidation-reduction.
Learn more about chemical and physical property here:
https://brainly.com/question/1935242
#SPJ2
The pOH of a solution is 6.0. Which statement is correct?
Use pOH = -log[OH-] and PH+pOH = 14.
The pH of the solution is 20.0.
O The concentration of OH ions is 1.0 x 108 M.
The concentration of OH ions is 1.0 x 106 M.
O The pH of the solution is 8.0.
A
Answers
At pOH value of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
In this question we will apply the formula
pH +pOH = 14 . . . . . . . . . . . . .(1)
where pH = concentration of [\(H^{+}\) ] ion
pOH = concentration of [\(OH^{-}\) ] ion
As per the question
pOH =6.0
Putting the value of pOH in equation (1) we get the value of pH
pH + 6.0 =14
pH = 14 -6.0
pH = 8.0
The value of pH if the pOH value is 6.0 is 8.0
To find the concentration of \(H^{+}\) ion we will use the following formula
This is calculated by the formula
[\(H^{+}\)} = \(10^{-pH}\)
where we will write the values of pH
Hence the concentration of [\(H^{+}\)} ion is \(10^{-8}\)
Therefore at pOH of 6.0 the pH value of the following solution is 8.0 and the concentration of [\(H^{+}\) ] ion is \(10^{-8}\)
Read more about pH
https://brainly.com/question/11300720
The complete question is -
What is the pH value and concentration of [\(H^{+}\) ] ion of the following if the pOH value of the solution is 6.0 ?
A mixture of compounds containing diethylamine, phenol, ammonia, and acetic acid is separated using liquid-liquid extraction as follows: Step 1: Concentrated HCl is added followed by draining the aqueous layer. Step 2: Dilute NaOH is added to the organic layer followed by draining the aqueous layer. Step 3: Concentrated NaOH is added to the organic layer followed by draining the aqueous layer. Which compound would you expect to be extracted into the aqueous layer after the addition of dilute HCl, step 1? Group of answer choices
Answers
Complete Question
The complete question is shown on the first uploaded image
Answer:
The correct option is ammonia
Explanation:
The mixture contains two base compound which are
ammonia,
and diethylamine
Now the addition of HCl which is a strong acid in step 1 will cause the protonation of the two base compound , which makes the soluble hence resulting in them being extracted to the aqueous layer as represented in below
\(NH_3 + HCl\to NH_4 ^{+} + Cl^-\)
and
\((CH 3CH 2) 2NH + HCl \to (CH 3CH 2) 2NH_2^{+} + Cl\)

Why are there few fossils of protists. write a paragraph response
Answers
Answer:
protists that produce a hard outer covering have left many fossils, other protists lack hard parts, so few fossils of these organisms have been found. ... Protists in this group are called plantlike because, like plants, they contain the pigment chlorophyll in chloroplasts and can make their own food.
Explanation:
Hope it helps, some how.
How much 5.50M NaOH must be added to 680.0 mL of a buffer that is 0.0215 M acetic acid and 0.0270 M sodium acetate to raise the pH to 5.75?
Answers
5.50M NaOH must be added to 680.0 mL of a buffer, The amount of NaOH is mathematically given as
V=2.107mL
What is the volume of 5.50M NaOH?Generally, the equation for Chemical Reaction is mathematically given as
CH3COOH+OH^{-} ⇄ CHCOO^{-} + H2O
Therefore
pka=-log(1.8*10{-5})
pKa=5-0.25527
pKa=4.3447
Hence
\(pH=pKa+log\frac{CH3COO}{CH3COOH}\\\\0.75=4.7447+log\frac{0.01564+x}{0.01428-x}\)
x=0.0115898
Volume of NaOH
V=0.0021072L
V=2.107mL
Read more about Chemical Reaction
https://brainly.com/question/11231920
#SPJ1
what happens if you put a cat in the microwave?
Answers
She said that the exposure to the radiation in the microwave would have cooked the animal's internal organs. She said: 'It is a horrific case in the fact that the death of the cat would have been prolonged and it is unimaginable what it would have gone through taking some time to die.
If you have 2m of hydrochloric acid and also 2m of vinegar which one is weak and strong and why?
Answers
Prepare one solution that has 0.12 M of FeCl3 and 0.40 M of HCl with the reagents 3 M HCl and Solid FeCL3 * 6H20. Provide the calculations and protocol to make the solution in a lab.
Answers
To prepare a 0.12 M solution of FeCl₃, the amount of solid FeCl₃ to be dissolved in a given volume of solvent will be 9.72 grams.
Given,
Molarity of FeCl₃ (M)= 0.12 M
The molecular weight (m) of FeCl₃ is = 162 gm
The volume of the solution (V) to be prepared is =500 ml
The amount of FeCl₃ to be dissolved to make a 0.12 M solution is= x
So,
MV= x ÷ m × 1000
0.12× 500 = x ÷ 162 × 1000
x = 60 × 162 ÷ 1000
x= 9.72 gm
So 9.72 grams of FeCl₃ is dissolved to make 500 ml of 0.12 M solution.
For preparing 0.4 M HCl from 4M HCL:
If we need to make 500 ml of solution with 0.4M of HCL, then we use the formula:
M₁V₁= M₂V₂
0.4 × 500= 4 × x
x= 50 ml
So 50 ml of 4M HCL is taken to make 0.4 M HCL.
To learn more about FeCl₃, refer to the link:
https://brainly.com/question/32098087
#SPJ1
What will happen when pressure on a reactant mixture at equilibrium and with fewer moles on the reactant side is increased
Answers
when pressure of the reactant mixture at the equilibrium and with the fewer moles in reactant side will be increased and the equilibrium will be shift to the side in the reaction where the fewer moles of the gas.
According to the Le Chartelier, when the reaction is in the equilibrium phase and the one of the constraints which will affect the rate of the reactions, and the equilibrium will be shift to the cancel out this effect that the constraint had.
Therefore, If the pressure of the system or the reaction is in the equilibrium is change, the equilibrium of the reaction will be change that is depending on the side of the reaction with the highest number of the molecules.
To learn more about equilibrium here
https://brainly.com/question/31328735
#SPJ1
How much water needs to be added to a 8.6317 M NaCl solution to obtain a 1.0421 M solution, if you start with 4.7869 liters of solution?
Answers
The amount of water needed to be added to a 8.6317 M NaCl solution to obtain a 1.0421 M solution starting with 4.7869 liters of solution would be 34.8629 L.
DilutionThe problem here has to do with dilution. The dilution principle states that the number of moles of solute in a solution is constant before and after dilution.
In other words, the dilution principle can be mathematically expressed as:
\(m_1v_1\) = \(m_2v_2\).
Where \(m_1\) and \(m_2\) are molarities before and after dilution; \(v_1\) and \(v_2\) are volumes before and after dilution.
In this case: \(m_1\) = 8.6317 M, \(m_2\)= 1.0421 M, \(v_1\)= 4.7869 L. We are to determine v2.
\(v_2\) = \(m_1v_1\)/ \(m_2\)
= 8.6317x4.7869/1.0421
= 39.6498 L
If the final volume of the solution is 39.6498 L and the starting volume is 4.7869, then the amount of water needed can be calculated as:
39.6498 - 4.7869 = 34.8629 L
Thus, the amount of water that will be added to the original solution in order to arrive at the diluted solution is 34.8629 L.
More on dilutions can be found here: https://brainly.com/question/21323871
#SPJ1
Determine the number of carbon atoms in 1.00 kg of carbon dioxide.
____X 10__atoms(Enter your answer in scientific notation.)
Determine the number of oxygen atoms in 1.00 kg of carbon dioxide.
____X 10__atoms (Enter your answer in scientific notation.)
Calculate the mass of potassium nitrate that contains 1.00 mol of oxygen atoms.
_____g
—Pls show steps!! I’m so confused and need help—
Answers
Answer:
1.37x10²⁵atoms of carbon
2.74x10²⁵ atoms of oxygen.
33.7g of KNO₃
Explanation:
To answer this question you must use molar mass of carbon dioxide (44g/mol) and 1 mole are 6.022x10²³atoms.
1.00kg are 1000g of CO₂. Moles are:
1000g CO₂ * (1mol / 44g) = 22.73 moles of CO₂ = 22.73 moles of carbon.
In atoms:
22.73 moles C * (6.022x10²³atoms / 1mole) = 1.37x10²⁵atoms of carbon
There are 22.73 moles of CO₂ * 2 = 45.45 moles of oxygen are present in the carbon dioxide. In atoms:
45.45 moles Oxygen * (6.022x10²³atoms / 1mole) = 2.74x10²⁵ atoms of oxygen.
1 mole of Potassium nitrate, KNO₃, contains 3 moles of oxygen. 1 mol of oxygen are:
1.00 mol O * (1mol KNO₃ / 3 moles O) = 0.33 moles of KNO₃
As molar mass of KNO₃ is 101.1g/mol:
0.33 moles of KNO₃ * (101.1g / mol) = 33.7g of KNO₃
Answer:
1. 1.37 * 10^25 atoms of carbon
2. 2.74 * 10^25 atoms of oxygen
3. 33.67g of Potassium Nitrate
Explanation:
2. Firstly, we want to know the number of atoms of oxygen in 1 kg of carbon dioxide.
Firstly, we will need to know the number of moles of carbon iv oxide in 1kg of carbon iv oxide
Mathematically;
number of moles = mass/molar mass
molar mass of carbon iv oxide is 44 g/mol
mass here is 1000g (1kg is 1000g)
So the number of moles of CO2 in 1kg of CO2 will be; 1000/44 = 22.73 moles
Now 1 mole of CO2 contains 2 atoms of oxygen, thus 1 mole of CO2 contains 2 moles of oxygen
So the number of moles of oxygen in 1kg CO2 will be 2 * 22.73 = 45.46 moles
Mathematically, 1 mole contains 6.02 * 10^23 atoms
So 45.46 moles will contain =
6.02 * 10^23 * 45.46 = 2.74 * 10^25 atoms of oxygen
1. 1 mole of co2 contains 1 atom of carbon , thus, 1 mole of CO2 will
contain 1 mole of carbon
From above, 1kg of carbon iv oxide contains 22.73 moles , thus 1kg of carbon iv oxide will contain 22.73 moles of carbon too
So the number of atoms will be 22.73 * 6.02 * 10^23 = 1.37 * 10^25 atoms
3. Mass of KNO3 that contains 1 mole of oxygen atom
From the formula of the compound, we can see that 1 mole of KNO3 contains 3 atoms of oxygen which translates to 3 moles of oxygen
So 1 mole of oxygen will translate to 1/3 mole of KNO3
Mathematically;
mass = number of moles * molar mass
Molar mass of KNO3 = 39 + 14 + 3(16) = 39 + 14 + 48 = 101 g/mol
So the mass will be = 1/3 * 101 = 33.67g
Part C
Based on your observations, which liquid is the unknown liquid? Give reasons for your answer PLEASE HELP
Answers
When comparing various liquids, a liquid can be recognized by its behavior on paper, which is a defining attribute of the liquid.
On two distinct paper surfaces, we will test four known liquids and an unknown liquid. we will identify an unidentified liquid using their observations. We will learn that we can effectively identify an unidentified liquid by combining the findings of two experiments. On a coffee filter dipped in green food coloring, we will also add water and salt water. we can tell a big difference in how each liquid separates the hues of the green food coloring. When comparing various liquids, a liquid can be recognized by its behavior on paper, which is a defining attribute of the liquid. The equal quantity of each liquid should be applied to the paper in the same location for the test to be fair.
Learn more about Liquids here-
https://brainly.com/question/4509437
#SPJ9
Answer:
Alcohol?
Explanation:
(for Plato)
Which of the four models represents a solid
Answers
Answer:
is there an image
Explanation:
Which interaction occurs when light bounces off of a surface?(2 points)
Absorption
Reflection
Refraction
Separation
HELLLLLPPP
Answers
Answer:
Reflection
Explanation:
Very smooth surfaces such as mirrors reflect almost all incident light.
\(\underline\mathtt\colorbox{gold}{Reflection}\)
Explanation:
Bouncing back of light from a surface is called reflection.
A 100 g block of a substance requires 0.5 kJ of heat to raise its temperature from 25.0°C to 37.8°C.
What is the substance?
A) iron
B) aluminum
C) gold
D) copper
Answers
The metal can be identified using its specific heat capacity. Here, the specific heat capacity of the substance calculated from calorimetric equation is 0.39 J/g °C , that is the specific heat of copper metal. Hence, option D is correct.
What is specific heat capacity ?The specific heat capacity of a substance is the heat energy required to raise the temperature of the substance by one degree Celsius per on gram. It is characteristic to the substance.
The calorimetric equation connecting the heat energy q, with mass of the substance m, specific heat c and temperature difference ΔT is given below:
q = m c ΔT.
Given, m = 100 g
q = 0.5 KJ = 500 J
ΔT = 37.8 - 25 °C = 12.8 °C
then c = q/mΔT
c = 500 J/12.8 °C × 100 g = 0.39 J/g °C
this specific heat corresponds to the copper metal.
Therefore, the block is made of copper metal.
Find more on specific heat:
https://brainly.com/question/11297584
#SPJ2
How can I prepare 7.5mm Cocl2.6H2O?
I want to use it as hypoxic agent for fish.
I'll be waiting for your kind response.
Answers
800 mL of deionized water is added to 1.782 g of Cocl2.6H2O and then more deionized water is added until the total volume reaches 1000 mL.
To prepare a 7.5mm solution of Cocl2.6H2O, you will need to follow these steps:
Calculate the molecular weight of Cocl2.6H2O, which is 237.93 g/mol.
Weigh out 1.782 g of Cocl2.6H2O on a scale and add it to a 1000 mL volumetric flask.
Add approximately 800 mL of deionized water to the flask and swirl to dissolve the compound.
Once the Cocl2.6H2O is dissolved, add more deionized water until the total volume reaches 1000 mL.
Mix the solution thoroughly to ensure homogeneity.
To use this solution as a hypoxic agent for fish, you will need to determine the appropriate dosage for your specific application.
The concentration of Cocl2.6H2O used will depend on the size and species of fish, as well as other factors such as water temperature and oxygen demand. It is important to carefully monitor the fish during treatment to ensure that they are not experiencing any adverse effects.
For more such questions on deionized water
https://brainly.com/question/30529207
#SPJ11
Write the nuclear equation for the alpha decay of \(^{231} _{91} Pa\)
Answers
The nuclear equation is written as:
²³¹₉₁Pa ⇒ ²²⁷₈₉A₀ + ⁴₂He
What is the alpha decay?Alpha decay is defined as a nuclear decay process where an unstable nucleus is transformed to another element by shooting out a particle composed of two protons and two neutrons.
This particle that is ejected is known as an alpha particle.
The steps of the alpha decay is written as;
Write the mass number and the atomic number of the parent atom.
Find the mass number and the atomic number of the daughter atom.
Mass number , A - 4
Atomic number, Z - 2
Then, we have;
²³¹₉₁Pa ⇒ ²²⁷₈₉A₀ + ⁴₂He
Mass number = 231 - 4 = 227
Atomic number = 91 - 2 = 89
Learn more about alpha decay at: https://brainly.com/question/1898040
#SPJ1
I need help with the second one

Answers
Sorry, your photo isn't clear enough.
A student carried out an investigation to discover the effect of a new factory on a wetland ecosystem. The results of the investigation are in the table. What can be concluded from the results of the investigation? The factory had a negative effect on all the populations in the ecosystem. The factory had a negative effect on the algae and a positive effect on the shellfish and crab populations. The factory had a positive effect on all the populations in the ecosystem. The factory had a positive effect on the algae and a negative effect on the shellfish and crab populations.
Answers
The factory had a negative effect on all the populations in the ecosystem. This is the effect of a new factory on a wetland ecosystem. Therefore, the correct option is option A.
A wetland is a composite ecosystem that also contains ecosystems related to hydrology, soil, vegetation, or biology. The rate of urbanisation in China's national economic growth process is now rising quickly, so by the end of 2021, the country's resident population will have an urbanisation rate of 64.72%. The factory had a negative effect on all the populations in the ecosystem. This is the effect of a new factory on a wetland ecosystem.
Therefore, the correct option is option A.
To know more about wetland ecosystem, here:
https://brainly.com/question/29987463
#SPJ1
Polllination refers to the ___________
Answers
Explanation:
from internet
the act of transferring pollen grains from the male anther of a flower to the female stigma
Answer:
transfer of pollen from anther to stigma.
Explanation:
mark me main brainliest
using hard water when preparing a sanitizing solution
Answers
The bactericidal activity of sanitizers prepared from the water will be reduced if the hardness or pH of the water used to prepare EO water or bleach solutions are increased.
What elements influence a sanitizing solution's efficacy?Temperature, pH, relative humidity, and water hardness are other physical and chemical variables that affect disinfection processes. For instance, most disinfectants become more active as the temperature rises, but there are some exceptions.
What degree of hardness might impact cleaning?The harder your water is, the less effective cleaning products will be since it will be difficult to make a soapy lather. Scale, which are crusty deposits made by hard water, can build up in your dishwasher or washing machine. Detergent works effectively when used with soft water.
Learn more about disinfection processes here:
https://brainly.com/question/28321155
#SPJ4
One time I went to the mountains. I was scared of altitude sickness, so I got cannisters of oxgen (O₂). These cannisters contain 2L of compressed oxygen (O₂). When the oxygen is pressurized, it condenses into its liquid form inside the cannister. How much would this oxygen (0₂) weigh in its liquid form?
2.9 grams
1.6 grams
3.6 grams
4.3 grams
Answers
The 2.9 grams would this oxygen (0₂) weigh in its liquid form.
What is oxygen ?
The sole sort of atom in oxygen makes it a chemical element, which is a substance. An oxygen atom has eight protons in its nucleus, as indicated by its atomic number of 8, which is represented by the letter O in its official chemical formula. At room temperature, oxygen is a gas that lacks all other flavors, smells, and colours. Molecular oxygen can be found in nature.
What is liquid ?
Unlike a solid, which is more rigid than a liquid, a liquid is a kind of substance with unique qualities. The opposite of a solid, which has a defined shape, is a liquid, which can flow. An alternative is that a liquid will take on the shape of the container it is held in.
Therefore, 2.9 grams would this oxygen (0₂) weigh in its liquid form.
Learn more about oxygen from the given link.
https://brainly.com/question/12167380
#SPJ1
. For a particular liquid, raising its temperature from 298 K to 318 K causes its vapor pressure to
double. What is the enthalpy of vaporization of this liquid? (R = 8.31 J/(K- mol))
115 kJ/mol
288 kJ/mol
27.3 kJ/mol
2.53 kJ/mol
270 kJ/mol
a)
b)
c)
d)
e
Answers
Answer:
he enthalpy of vaporization is 27.3 kJ/mol. The correct answer is therefore (C) 27.3 kJ/mol.
Explanation:
To find the enthalpy of vaporization, we can use the Clausius-Clapeyron equation, which relates the vapor pressure of a liquid to its enthalpy of vaporization. The equation is given by:
ln(P2/P1) = (delta Hvap / R) * (1/T1 - 1/T2)
where P1 and P2 are the vapor pressures at temperatures T1 and T2, respectively, delta Hvap is the enthalpy of vaporization, and R is the gas constant. In this case, we are given that the vapor pressure doubles when the temperature is raised from 298 K to 318 K, so P2 = 2 * P1. We can plug this into the equation and solve for delta Hvap to find the enthalpy of vaporization:
ln(2) = (delta Hvap / R) * (1/298 - 1/318)
delta Hvap = R * ln(2) / (1/298 - 1/318)
Plugging in the values, we get delta Hvap = 8.31 J/(K-mol) * ln(2) / (1/298 - 1/318) = 27.3 kJ/mol. The correct answer is therefore (C) 27.3 kJ/mol.
Hello I need some helpI need to find the correct sketch in each set with the follow:Melting point of HCI: -114.8 cBoiling point of HCI: -85.1 coption A says -97c,B says -37c, C says 124 c

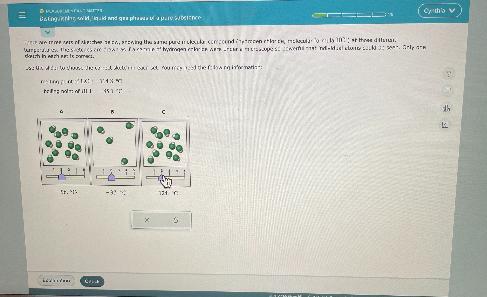



Answers
We have the melting and boiling point of HCl.
The melting point is the temperature at which HCl will change from solid to liquid. Below this temperature, HCl will be solid and the molecules will be very organized and close.
Up to this temperature, HCl will be liquid. The molecules will be a little more disorderly than in the solid state.
The boiling point is the temperature at which HCl will change from liquid to a gaseous state. Below this temperature HCl will be liquid, up to this temperature HCl will be gaseous and the molecules will be very far from each other.
Now, with the given data we have that the states of HCl depending of the temperature will be:
Gaseous state: Temperature> -85.1 C
Liquid state: -114.8 C < Temperature < -85.1 C
Solid state: Temperature < -114.8 C
Now, we have three options given, the state for each option will be:
A. -96 C, HCl will be in Liquid
B. -37 C, HCl will be gaseous (Molecules higly disorganized and far from each other)
C. -124 C, HCl will be solid (Very organized)
Therefore the correct option will be:

Which of these waves has the greatest wavelength? (3 points) Wave shown with 2 wavelengths. Wave shown with 3 wavelengths. Wave shown with 1 wavelength stretch over a short distance. Wavelength shown with 1 wavelength stretched over a long distance.
Answers
The waves that has the greatest wavelength is Wavelength shown with 1 wavelength stretched over a long distance.
Waves explained.A wave could be a disturbance or variety that voyages through a medium or space, carrying vitality without transporting matter. Waves can take different shapes and happen totally different sorts of waves, counting mechanical waves and electromagnetic waves.
Mechanical waves require a medium to propagate, meaning they require a substance like water, discuss, or a strong fabric to transmit the wave. Illustrations of mechanical waves incorporate water waves, sound waves, and seismic waves. In these waves, particles of the medium sway or vibrate in a design, exchanging energy from one molecule to another.
Electromagnetic waves, on the other hand, don't require a medium and can travel through vacuum, such as in space. Electromagnetic waves comprise of electric and attractive areas swaying opposite to each other and to the heading of wave engendering. Illustrations of electromagnetic waves incorporate obvious light, radio waves, microwaves, infrared waves, bright waves, X-rays, and gamma beams.
Learn more about waves below.
https://brainly.com/question/26116832
#SPJ1
A student wants to do scientific research to find new ways to detect toxic
substances in water. What field of science would this research mostly
involve?
OA. Biology
O B. Astronomy
O
OD. Physics
C. Chemistry
SUBMIT
Answers
the field of science which would mostly research is chemistry.