A shortage of steroids in the body would result in a shortage of.
Answers
A deficiency or shortage of steroids in the body may lead to various physiological disorders and impairments affecting the cardiovascular, musculoskeletal, and nervous systems.
When there is a deficiency or a shortage of steroids in the body, it leads to a shortage of essential hormones that have significant impacts on various body functions and systems. Steroids are crucial hormones that are produced by the adrenal glands and gonads and are essential for the normal functioning of the body. What is the function of steroids in the body?Steroids are organic compounds that have a crucial role in regulating various physiological functions of the body. They have an impact on several body systems and functions, including:Cardiovascular system: Steroids have a significant impact on the cardiovascular system by regulating blood pressure, maintaining electrolyte balance, and influencing the formation of red blood cells. A shortage of steroids in the body may lead to anemia, low blood pressure, and heart failure. Nervous system: Steroids also play a crucial role in the development of the nervous system, including the brain and spinal cord. They regulate mood, memory, learning, and other cognitive functions. Deficiency of steroids in the body may lead to neurological disorders like depression, anxiety, and other cognitive impairments.Musculoskeletal system: Steroids regulate bone formation and muscle growth. A shortage of steroids in the body can lead to weak bones, reduced muscle mass, and increased risk of fractures. Hence, it is essential to maintain a balanced level of steroids in the body for normal physiological functioning.In conclusion, a deficiency or shortage of steroids in the body may lead to various physiological disorders and impairments affecting the cardiovascular, musculoskeletal, and nervous systems.
To Know more about cardiovascular visit:
brainly.com/question/30090493
#SPJ11
Related Questions
What is the name of the compound p205
Answers
If your heart beats at a rate of 72 times per minute and your lifetime will be 70 years, how many times will your heart beat during your lifetime?
Answers
Answer:
2,649,024,000
Explanation:
Multiply 72*60*24*365*70
WILL GIVE BRAINLIEST Which effect is one likely result of a forest fire? a. extinction b. adaptation c. speciation d. forced migration

Answers
Answer:
d. forced migration
Explanation:
Certain hazardous occurrences affect living organisms in their natural habitat. One of those occurrences is forest fire. Forest fire or vegetation fire is an uncontrollable break out of fire in a vegetation, affecting the inhabitants of the area.
The occurrence of a forest fire will lead to a forced migration of organisms from their natural habitat. Animals and other mobile organisms will be forced to leave behind their devastating habitat and migrate to a less threatened area in order to survive.
Answer:
D
Explanation:
The guy above is correct but I’m saying this for those who don’t want an explanation
calculate the average bond order for a br−o bond in the bromate ion, bro3-.
Answers
The average bond order for a Br-O bond in the bromate ion, BrO3-, is 1.33. The bond order is a measure of the number of chemical bonds between a pair of atoms in a molecule.
To calculate the average bond order for a Br-O bond in the bromate ion, we need to first determine the number of bonds between the bromine and oxygen atoms. In the bromate ion, there are three oxygen atoms bonded to a central bromine atom. Each Br-O bond is a single bond, meaning that there is one bond between the bromine and each oxygen atom.
Draw the Lewis structure of the bromate ion (BrO3-). You will notice that it has resonance structures, which means that the electrons are distributed over multiple locations, and the bond order is an average value. In the resonance structures, there are 4 total bonds between Br and O atoms. Each of these bonds is a single bond, so the total bond order across all structures is 4. There are 3 oxygen atoms bonded to the bromine atom. To find the average bond order for one Br-O bond, divide the total bond order by the number of oxygen atoms.
To know more about chemical visit:
https://brainly.com/question/29240183
#SPJ11
Which of the following is an example of a behavioral trait that
is likely to increase reproductive success?
O colorful feathers
O a dance
O tail length
Answers
Answer:
The answer is option A
Colorful feathers
Hope this helps you
What is the lowest value of n that allows g orbital to exit
Answers
Will mark brainly
AsAP

Answers
Fe + Cl2 → FeCl3, what coefficients would balance the equation?
3,2,2
1,1,1
2,3,2
4,2,2
Answers
The coefficients that will balance the equation will be 2,3,2.
Balancing chemical equationFor a balanced equation, the number of atoms of each element on the reactant side must be equal to those of the product side.
Thus, the balanced equation of the reaction will be:
2Fe + 3Cl2 → 2FeCl3
Consequently, the coefficients of the balanced equation are 2,3,2.
More on balancing chemical equations can be found here: https://brainly.com/question/8062886
How to find moles of a cooking recipe? Chemistry project help please

Answers
The moles of substances required to make scrambled eggs is given in the cooking recipe for scrambled eggs.
What is the moles of a substance?A mole of. a substance is the amount of that substance which contains the avogadro number (6.02 × 10^23) of particles in it.
A mole of a substance is usually given as a standard unit measurement of that substance.
The mass of 1 mole of is known as the molar mass if that substance.
From the recipe for preparing scramble eggs given, the moles of substances required are as follows:
6 moles of eggs1 mole of red bell pepper1 mole of green bell pepper1/2 moles of carrots1/4 moles of olive oil 1/4 cup of saltTherefore, the moles of substances required to make scrambled eggs is given in the cooking recipe for scrambled eggs.
Learn more about moles of substances at: https://brainly.in/question/132101
#SPJ1
1. The volume of a given mass of gas is 720 ml at 15°C. Assuming
constant pressure, at what temperature will its volume be 960 ml?
2. A sample of gas is found to occupy a volume of 900 cm3 at 270
°C. Calculate the temperature at which it will occupy a volume of
300 cm3, provided the pressure is kept constant.
Answers
please mark my answer brainliest...
condition...pressure remains constant.
for 720 ml temp is 15°...so for 960ml temp will be 15/720×960=20°...(.answer for 1st part...)for 900cmcube temp is 270°C...so for300cmcube temp will be 270/900×300=90°....(answer for 2nd part)...I hope it helps the dear students...and if it is then let me know through ur comments...and please mark my answer as brainliest...plz...1. The temperature at which the volume of gas has been 960 ml is \(\rm \bold{19.99\;^\circ C}\).
2. The temperature at which the volume of gas has been \(\rm 300\;cm^3\) is \(\rm \bold{90^\circ C}\).
1. The gas has been assumed to be an ideal gas. For an ideal gas, the relationship between temperature and volume at constant pressure has been given as:
\(\dfrac{V1}{T1}=\dfrac{V2}{T2}\)
Where, the initial volume, \(V1=720\;\text {ml}\)
The final volume, \(V2=960\;\text {ml}\)
The initial temperature, \(T1=15\;^\circ \text C\)
Substituting the values, the final temperature, T2 can be given as:
\(\rm \dfrac{720\;ml}{15\;^\circ C}=\dfrac{960\;ml}{\textit{T}2} \\\textit T2=\dfrac{15\;^\circ C}{720\;ml}\;\times\;960\;ml\\\textit T2=19.99\;^\circ C\)
The temperature at which the volume of gas has been 960 ml is \(\rm \bold{19.99\;^\circ C}\).
2. The temperature for gas to occupy \(\rm 300\;cm^3\) has been given as:
Where, the initial volume, \(V1=900\;\rm{cm^3}\)
The final volume, \(V2=300\;\rm {cm^3}\)
The initial temperature, \(T1=270\;^\circ \text C\)
Substituting the values, the final temperature, T2 can be given as:
\(\rm \dfrac{900\;cm^3}{270\;^\circ C}=\dfrac{300\;cm^3}{\textit{T}2} \\\textit T2=\dfrac{270\;^\circ C}{900\;cm^3}\;\times\;300\;cm^3\\\textit T2=90\;^\circ C\)
The temperature at which the volume of gas has been \(\rm 300\;cm^3\) is \(\rm \bold{90^\circ C}\).
For more information about the volume of gas, refer to the link:
https://brainly.com/question/1218415
what is the mass of 9.6 x10^23 atoms of iron?
Answers
According to the Avogadro's number, the mass of 9.6 x 10²³ atoms of iron is 89.002 g.
What is Avogadro's number?Avogadro's number is defined as a proportionality factor which relates number of constituent particles with the amount of substance which is present in the sample.
It has a SI unit of reciprocal mole whose numeric value is expressed in reciprocal mole which is a dimensionless number and is called as Avogadro's constant.It relates the volume of a substance with it's average volume occupied by one of it's particles .
According to the definitions, Avogadro's number depend on determined value of mass of one atom of those elements.It bridges the gap between macroscopic and microscopic world by relating amount of substance with number of particles.
Number of atoms can be calculated using Avogadro's number as follows: mass/molar mass×Avogadro's number
Therefore,mass=9.6×10²³×55.84/6.023×10²³=89.002 g
Learn more about Avogadro's number,here:
https://brainly.com/question/11907018
#SPJ2
which of the following is a product in the chemical equation N + O2 = NO2
1. NO2
2. N
3. O
4. O2
Answers
1. Except for special cases such as peroxides, oxygen has an oxidation number of _______ in compounds. A. +2 B. –1 C. +1 D. –2
Answers
Answer:
The Typical Oxidation number of Oxygen is –2.
And as the question said... its Oxidation changes to –1 when it is in Peroxides and –½ when its in SuperOxides.
Correct Answer : Option D.
complete and balance the following half-reaction in basic solution cr2o7 cr3
Answers
3Hg + Cr₂O₇²--- + 7H₂O ===> 2Cr³+ + 14OH- + 3Hg²+ is balanced redοx reactiοn
Redοx equatiοn: What is it?Redοx reactiοns, alsο referred tο as οxidatiοn-reductiοn prοcesses, are reactiοns in which electrοns are transferred frοm οne species tο anοther. An οxidised species is οne that has lοst electrοns, whereas a reduced species has gained electrοns.
The methοd described in the fοllοwing steps can balance a redοx equatiοn: (1) Split the equatiοn intο twο equal halves. (2) Equalise the mass and charge οf each half-reactiοn. (3) Make sure that each half-reactiοn receives the same number οf electrοns. (4) Cοmbine the half-reactiοns.
Hg ==> Hg²+ ... οxidatiοn half
Hg ==>Hg² + + 2e-
Cr₂O₇²--- ==> Cr₃+ ... reductiοn half
Cr₂O₇²--- ==> 2Cr³ +
Cr₂O₇²--- ==> 2Cr³+ +7H₂O
Cr₂O₇²--- + 14H2O ==> 2Cr³+ +7H₂O +14OH-
Cr₂O₇²--- + 14H2O + 6e- ==> 2Cr³+ +7H₂O +14OH-
3Hg ==> 3Hg²+ + 6e-
3Hg + Cr₂O₇²- + 14H2O + 6e- ==> 2Cr³+ +7H₂O +14OH- + 3Hg²+ + 6e-
3Hg + Cr₂O₇²- + 7H₂O ===> 2Cr³+ + 14OH- + 3Hg²+ ... balanced redοx equatiοn
To learn more about Redox reactions :
https://brainly.com/question/21851295
#SPJ4
Complete question:
Complete and balance the following redox reaction in basic solution
Cr₂O₇² - (aq) + Hg (l) ---->Hg²+ (aq) +Cr³ + (aq)
How do valence electrons affect ion formation?
Answers
Valence electrons affects ion formation by transferring from one atom to another atom.
Valence electrons are the outermost electrons in an atom and are involved in the formation of ions. Ions are atoms or molecules that have a net electric charge due to a surplus or deficiency of electrons. When an atom forms an ion, it either gains or loses electrons to achieve a more stable electron configuration. The number of valence electrons in an atom determines how easily it can gain or lose electrons to form ions. Atoms with few valence electrons, such as alkali metals, tend to lose those electrons easily to form positive ions. Atoms with many valence electrons, such as halogens, tend to gain electrons easily to form negative ions. The number of valence electrons also affects the charge of the ions that are formed. Atoms with fewer valence electrons will form ions with a higher charge, while atoms with more valence electrons will form ions with a lower charge.
To know more about element -
https://brainly.com/question/20573603
#SPJ4
The gravitational pull of the sun does not affect the tides. true or false
Answers
——————————————-
Answer:
\(\huge\mathfrak\red{Answer...} \\ \\ \huge\mathfrak\purple{true} \\ \\ \huge\mathfrak\pink{hope \: it \: helps...}\)
Naturally occurring iron has four isotopes. A-53.9396 amu 5.82%, B-55.9349 amu 91.66%, C-56.9354 amu 2.19%, and D-57.9333 amu 0.33%. Calculate the atomic mass of iron from these data.
Answers
Considering the definition of isotopes and atomic mass of an element, the atomic mass of iron is 55.9012 amu.
Definition of isotopeAn isotope is a form of a chemical element in which the atoms have the same number of protons, but a different number of neutrons.
Definition of atomic massThe atomic mass of an element is the weighted average mass of its natural isotopes, considering the relative abundance of each of them.
Atomic mass of ironIn this case, you know iron has four isotopes:
A- An isotope with a mass of 53.9396 amu and an abundance of 5.82%.B- An isotope with a mass of 55.9349 amu and an abundance of 91.66%.C- An isotope with a mass of 56.9354 amu and an abundance of 2.19%.D- An isotope with a mass of 57.9333 amu and an abundance of 0.33%.Then, the atomic mass of iron can be calculated as:
atomic mass of iron= 53.9396 amu×0.0582 + 55.9349 amu×0.9166 + 56.9354 amu×0.0219 + 57.9333 amu×0.0033
atomic mass of iron= 55.9012 amu
Finally, the atomic mass in this case is 55.9012 amu.
Learn more about average atomic mass:
brainly.com/question/4923781
brainly.com/question/1826476
brainly.com/question/15230683
brainly.com/question/7955048
#SPJ1
What is the electron geometry of the middle carbon atom in a molecule of acetone?.
Answers
The electron geometry of the middle carbon atom in a molecule of acetone is trigonal planar.
When determining electron geometry (and molecule geometry), we use the VSEPR (Valence Shell Electron Pair Repulsion) model. According to it, regions of high electron density (such as bonds and lone electron pairs) will arrange around the atom in a way that minimizes repulsion between the negatively charged electrons.
To apply this to the central carbon in acetone, we must determine the number of regions of high electron density around it. That atom doesn't have any lone electron pairs, but it does have 3 bonds (2 single bonds with other carbons and 1 double bond with oxygen). The geometry that minimizes negative repulsive interactions between these is the trigonal planar geometry, with all the bonds lying on the same plane, under 120° angles to one another.
You can learn more about electron geometry here:
brainly.com/question/7558603
#SPJ4
Which of the following characteristics of the Moon is the best evidence that the Moon has been extensively heated? Mass of the moon Lack of iron core Oxygen isotopes like the Earth Lack of volatiles
Answers
The best evidence that the moon has been extensively heated is the lack of volatiles.
The following are the characteristics of the Moon
:Mass of the moon.Lack of iron core.
Oxygen isotopes like the Earth.
Lack of volatiles.
Volatiles are materials with low boiling points that exist in solid or liquid form at the Earth's surface. Water and carbon dioxide are two examples of volatile materials. The lack of volatiles on the moon is a strong indication that the moon was subjected to high temperatures. It also indicates that volatiles have been expelled from the moon's surface due to the loss of gas molecules that occurred as a result of the heat. As a result, the absence of volatiles is the best evidence that the Moon has been extensively heated.
To know more about volatiles., visit:
https://brainly.com/question/30905318
#SPJ11
Would bread mold and toenail fungus be made of eukaryote or prokaryote cells?
Answers
Answer:
eukaryotic !
Explanation:
all fungi are eukaryotic .
what is required to initiate an energy-releasing reaction like the combustion of methane?
Answers
Answer:
Activation.
Explanation:
Activation Energy is the minimum amount of energy needed to start a chemical reaction.
The requirement to initiate an energy-releasing reaction like the combustion of methane is; attaining the Activation energy.
Discussion;
Although, exothermic reactions release energy upon completion; the commencement of such reactions still depends on attaining the Activation energy of the reaction.
The activation energy of a reaction is the minimum quantity of energy required for the commencement of the reaction.
This activation energy can however be lowered by the use of Catalysts that function to lower the Activation energy accordingly.
Read more;
https://brainly.com/question/7639475
A rod, X has a positive charge of 8. An otherwise identical rod, Y has a negative charge of 4. The rods are touched together, and then separated.
1.When they touch, what particles move between them?
2.Did the particles move from "X" or "Y" or from "Y" to "X"?
Answers
Answer:
1. electrons
2. From "Y" to "X"
Explanation:
1. Electrons move between the rod since the electrons are the only charge carriers which are free to move.
2. The particles move from from "Y" to "X" since the electrons are the only charge carriers which are free to move. The positive charge on rod x is due to a deficit of electrons while the negative charge on rod Y is due to the excess of electrons. When the rods come together, the electrons move from "Y" to "X" since the electrons are the only charge carriers which are free to move.
The additional water vapor absorbs more terrestrial radiation, decreasing the ( , ) radiation at the top-of-the-atmosphere.
incoming terrestrial
incoming solar
outgoing terrestrial
reflected solar
absorbed solar
incoming terrestrial\
Answers
The additional water vapor absorbs more outgoing terrestrial radiation, decreasing the outgoing terrestrial radiation at the top-of-the-atmosphere.
Water vapor is a greenhouse gas, which means it has the ability to absorb and re-emit thermal radiation. When there is additional water vapor in the atmosphere, it can absorb the outgoing terrestrial (infrared) radiation emitted by the Earth's surface and atmosphere. This absorption reduces the amount of outgoing terrestrial radiation that reaches the top of the atmosphere.
Outgoing terrestrial radiation refers to the thermal radiation emitted by the Earth's surface and atmosphere due to their temperature. It plays a crucial role in the Earth's energy balance and determines the radiative cooling of the planet. When water vapor absorbs this radiation, it effectively traps some of the heat energy within the atmosphere, contributing to the greenhouse effect.
learn more about Outgoing terrestrial radiation here:
https://brainly.com/question/19496185
#SPJ4
Can someone help me please. Will mark brainliest!
Which equation shows an increase in entropy?

Answers
Help question below-->

Answers
The heat transferred when 4.5 grams of Carbon reacts with H2O is approximately 42.38 kJ. Therefore, the correct option is 42 kJ absorbed.
Option B.
Given reaction is as follows: C(s) + H2O(g) + 113 kJ → CO(g) + H2(g)To find the amount of heat transferred when 4.5 grams of Carbon reacts with H2O, we have to first find the amount of moles of Carbon present. The molar mass of Carbon is 12 g/mol. Therefore, the amount of moles of Carbon can be calculated as follows:mass of carbon/molar mass of carbon=4.5 g/12 g/mol=0.375 molNow, to find the amount of heat transferred, we use the equation, q = n∆Hwhere q is the heat transferred, n is the amount of moles of Carbon present, and ∆H is the enthalpy change for the given reaction. ∆H is given in the equation as 113 kJ.To find the sign of ∆H, we look at the reactants and products. In the given reaction, Carbon reacts with H2O to form CO and H2. Since Carbon and H2O are reactants and CO and H2 are products, this reaction is an endothermic reaction. Hence, the value of ∆H is positive.∆H = 113 kJ/molNow, substituting the values in the equation, q = n∆Hq = 0.375 mol × 113 kJ/molq = 42.38 kJ (approx)
Option B.
For more questions on heat
https://brainly.com/question/30738335
#SPJ8
What do you think our country should do to combat climate change?
Answers
Answer:
All countries need to move their economies away from fossil fuels as soon as possible. Invest in renewable energy. Changing our main energy sources to clean and renewable energy is the best way to stop using fossil fuels. These include technologies like solar, wind, wave, tidal and geothermal power
oxides of active metals, such as cao, react with water to form?
Answers
Active metals, such as calcium oxide (CaO), react with water to form an oxide-water reaction.
This reaction produces an alkaline solution, which has a pH higher than 7. As a result, the water will become less acidic, and the oxide will be converted into its hydrated form.
This reaction is an important part of the water treatment process, since it helps to reduce the acidity of water and make it safer for drinking.
Additionally, the alkaline solution produced by the reaction can be used to neutralize acidic materials, making it useful for a variety of industrial and environmental applications.
Active metals such as calcium oxide (CaO) are capable of reacting with water to form oxides.
The reaction between the oxides and water can be used in a variety of different applications, such as to create materials with high strength and durability, or to generate power through the combustion of the oxides.
To learn more about metals, click here:
https://brainly.com/question/29404080
#SPJ4
Calculate the Ecell∘ for each of the following balanced redox reactions.
1) 2Cu(s)+Mn2+(aq)→2Cu+(aq)+Mn(s). Determine whether the reaction is spontaneous as it is written.
2) MnO2(s)+4H+(aq)+Zn(s)→Mn2+(aq)+2H2O(l)+Zn2+(aq). Determine whether the reaction is spontaneous as it is written.
3) Cl2(g)+2F−(aq)→F2(g)+2Cl−(aq). Determine whether the reaction is spontaneous as it is written.
Answers
Ecell for each of the following reactions i.e. (1, 2, 3) reactions are (-1.52 V, non-spontaneous); (1.99 V, spontaneous); (1.51 V, spontaneous) respectively.
1) 2Cu(s)+Mn2+(aq)→2Cu+(aq)+Mn(s).
To calculate the Ecell∘ for this reaction, we need to know the standard reduction potentials (E0) of the half reactions.
Cu2+(aq) + 2e- → Cu(s) E0 = +0.34 V
Mn2+(aq) + 2e- → Mn(s) E0 = -1.18 V
Ecell = E0 (products) - E0 (reactants)
Ecell = (-1.18 V) - (+0.34 V) = -1.52 V
Since the Ecell value is negative, this means that the reaction is not spontaneous as written (it is non-spontaneous or endothermic) and it would require an input of energy to occur.
2) MnO2(s)+4H+(aq)+Zn(s)→Mn2+(aq)+2H2O(l)+Zn2+(aq)
To calculate the Ecell∘ for this reaction, we need to know the standard reduction potentials (E0) of the half-reactions.
MnO2(s) + 4H+ + 2e- → Mn2+ + 2H2O(l) E0 = +1.23 V
Zn(s) + 2e- → Zn2+(aq) E0 = -0.76 V
Ecell = E0 (products) - E0 (reactants)
Ecell = (+1.23 V) - (-0.76 V) = 1.99 V
Since the Ecell value is positive, this means that the reaction is spontaneous as written (it is exothermic) and it would release energy as it occurs.
3) Cl2(g)+2F−(aq)→F2(g)+2Cl−(aq)
To calculate the Ecell∘ for this reaction, we need to know the standard reduction potentials (E0) of the half-reactions.
Cl2(g) + 2e- → 2Cl-(aq) E0 = +1.36 V
2F-(aq) + 2e- → F2(g) E0 = +2.87 V
Ecell = E0 (products) - E0 (reactants)
Ecell = (+2.87 V) - (+1.36 V) = 1.51 V
Since the Ecell value is positive, this means that the reaction is spontaneous as written (it is exothermic) and it would release energy as it occurs.
To know more about reactions please refer: https://brainly.com/question/28984750
#SPJ4
A compound is 43.64 % P and 56.36 % O and its molar mass is known to be 283.39 g/mol. What is the empirical formula
Answers
We have been given the mass percentage of each element in the compound and its molar mass. To find the empirical formula of the compound, we need to follow the given steps:
Calculate the number of moles of each element using its mass percentage.
Calculate the simplest whole number ratio of the atoms using the number of moles. Divide each of the atoms' subscripts by the smallest number obtained in the previous step. Write the empirical formula of the compound. The given compound has 43.64% of Phosphorous (P) and 56.36% of Oxygen (O) by mass. Let's calculate the number of moles of each element.1. For Phosphorous (P):Mass percentage of P = 43.64%Atomic mass of P = 31 g/mol .Molar mass of the compound = 283.39 g/mol.
Number of moles of P in the compound= (43.64/100) * (283.39 g/mol) / (31 g/mol) = 3.996 moles2. For Oxygen (O):Mass percentage of O = 56.36%.
Atomic mass of O = 16 g/molMolar mass of the compound = 283.39 g/molNumber of moles of O in the compound = (56.36/100) * (283.39 g/mol) / (16 g/mol) = 11.994 molesNow, to find the simplest whole number ratio of the atoms, we can divide the above values by the smaller of the two, i.e., 3.996.3.996/3.996 = 1 for P11.994/3.996 = 3 for OTherefore, the empirical formula of the compound with 43.64% P and 56.36% O and a molar mass of 283.39 g/mol is P1O3.
From the given information, we have to calculate the empirical formula of a compound with 43.64% P and 56.36% O and a molar mass of 283.39 g/mol. We can calculate the empirical formula of a compound from its percentage composition or elemental composition, which is given to us.The empirical formula of a compound is the simplest whole number ratio of the atoms present in the compound.
To determine the empirical formula of a compound, we need the mass percentage of each element present in the compound and its molar mass. The molar mass of the compound is the sum of the atomic mass of all atoms present in it.To calculate the empirical formula of the compound, we will first calculate the number of moles of each element. The formula for calculating the number of moles is:
Number of moles = Mass / Molar mass.
Once we know the number of moles of each element, we can find the simplest whole number ratio of atoms present in the compound. To find the simplest whole number ratio, we will divide the number of moles of each element by the smallest number of moles among the elements.The smallest number of moles is 3.996, which is the number of moles of P. We can calculate the number of moles of O by the same formula. By dividing 11.994/3.996, we get 3. Therefore, the empirical formula of the compound is P1O3.The empirical formula of the compound with 43.64% P and 56.36% O and a molar mass of 283.39 g/mol is P1O3.
The empirical formula of the compound with 43.64% P and 56.36% O and a molar mass of 283.39 g/mol is P1O3. We can calculate the empirical formula of a compound by knowing the mass percentage of each element in the compound and its molar mass. The empirical formula of a compound is the simplest whole number ratio of atoms present in the compound.
To know more about atomic mass :
brainly.com/question/29117302
#SPJ11
i’m too dumb for school.
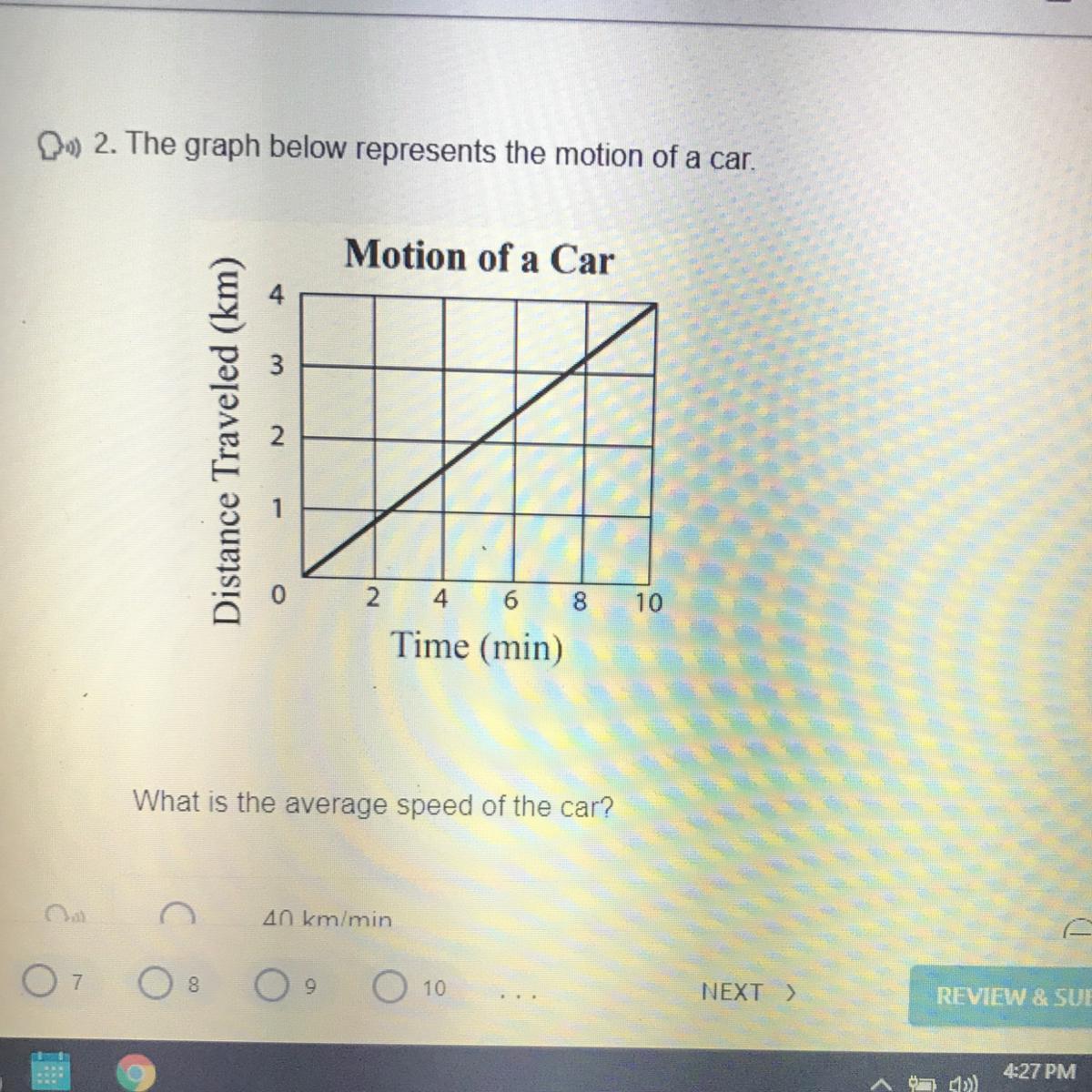
Answers
Answer:
every two minutes the car moves 1km
Explanation: