A total of 607 226 candidates registered for the November 2020 NSC examination in SA, while 578 468 candidates wrote the examination calculatetthe number of candidates who did not write and provide a possible reason why
Answers
The number of candidates who did not write the November 2020 NSC examination in SA is 28,758.
To calculate the number of candidates who did not write the examination, we subtract the number of candidates who wrote the examination from the total number of registered candidates.
Total registered candidates = 607,226
Candidates who wrote the examination = 578,468
Number of candidates who did not write = Total registered candidates - Candidates who wrote the examination
= 607,226 - 578,468
= 28,758
Therefore, the number of candidates who did not write the November 2020 NSC examination in South Africa is 28,758.
Possible reasons why candidates did not write the examination could include:
Illness or health-related issues: Some candidates may have been unable to write the examination due to illness or other health-related reasons.Personal circumstances: Certain candidates may have faced personal circumstances such as family emergencies, personal commitments, or other unforeseen events that prevented them from taking the examination.Ineligibility: It's possible that some candidates who registered for the examination did not meet the eligibility criteria or were disqualified for various reasons.Lack of preparation: Some candidates may have chosen not to write the examination due to inadequate preparation or lack of confidence in their readiness for the exam.These are some possible reasons, but it's important to note that without specific data or information, the exact reasons for each candidate not writing the examination cannot be determined.
To learn more about number of candidates, here
https://brainly.com/question/32702803
#SPJ4
Related Questions
Discuss how chemical bonding explain the properties of chemical and biological polymers
Answers
Chemical bonding explains the properties of chemical and biological polymers by forming strong covalent bonds or flexible hydrogen bonds, which give the polymer its unique characteristics.
Exploring the Role of Chemical Bonding in the Properties of Chemical and Biological PolymersChemical bonding is a fundamental concept that explains the properties of chemical and biological polymers. Chemical bonds are formed when atoms interact with each other to form molecules or particles. In a polymer, the atoms are linked together in a repeating pattern, forming a long chain. These bonds give the polymer its unique properties, such as strength, flexibility, and the ability to interact with other molecules. The type of chemical bond formed between the atoms will determine the properties of the polymer.
Learn more about chemical bonds: https://brainly.com/question/20387565
#SPJ4
1.From the pictures above,which shows a higher temperature?why?
2.which has more heat?why?
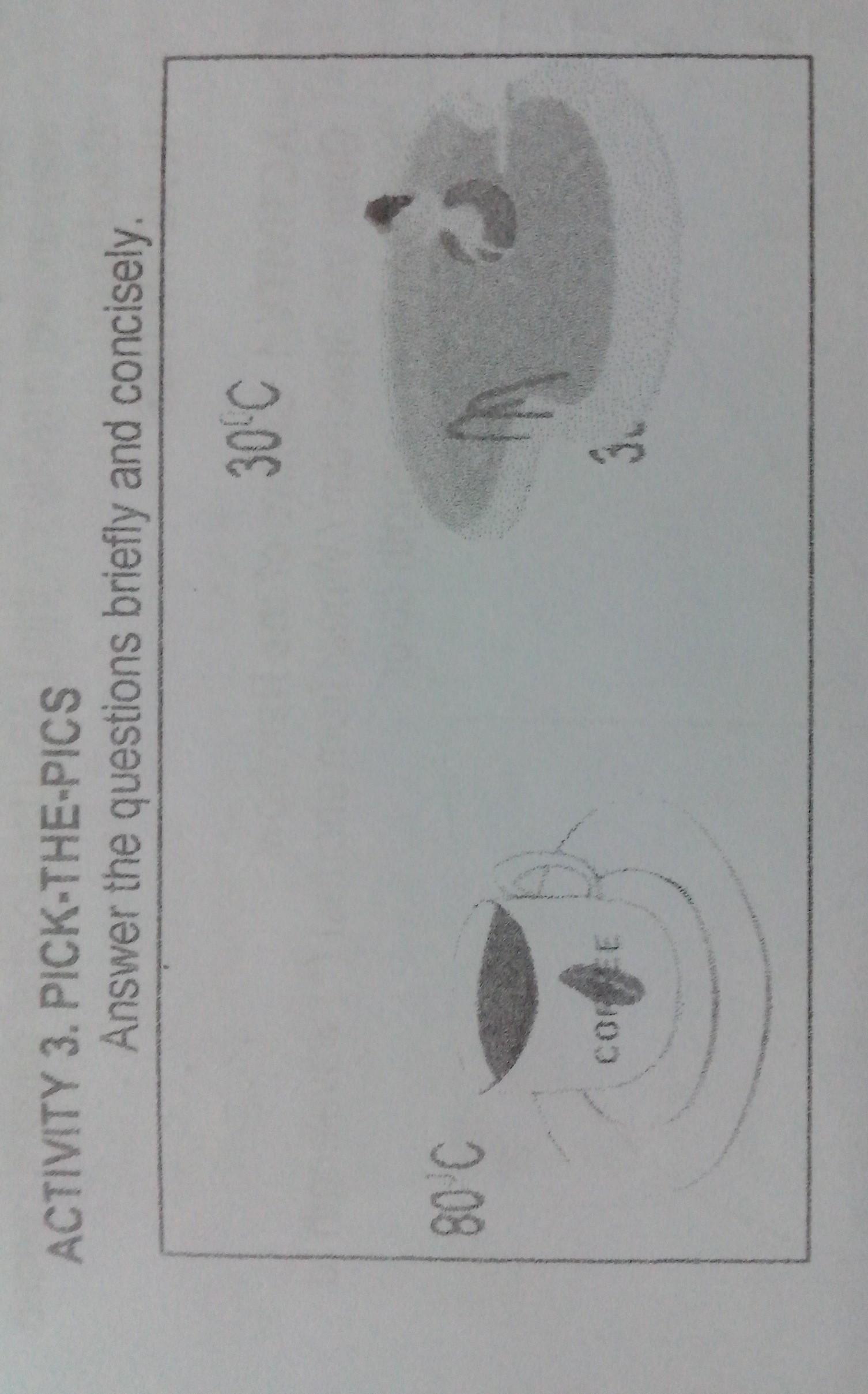
Answers
Answer:
The coffee has a higher temperature because a pool is so you can have fun and relax in while coffee can warm you up by drinking it.
Explanation:
Hope it helps :)
How many moles of Au are in 312 g of Au?
Answers
Answer:
1.583 moles
Explanation:
Rounded Atomic Mass of Au = 197 grams
\(\frac{312}{197} =1.5837, 1.584\)
Calculate the volume in liters of a 0.0026 mol/L copper(II) fluoride solution that contains 800. mg of copper(II) fluoride . Round your answer to significant digits.
Answers
The volume in liters of the given copper(II) fluoride solution is 30.3 L.To calculate the volume in liters of the given copper(II) fluoride solution.
We need to use the formula:
moles = concentration x volume
First, we need to convert the mass of copper(II) fluoride from milligrams to grams:
800. mg = 0.8 g
Next, we can calculate the number of moles of copper(II) fluoride:
moles = 0.0026 mol/L x V (where V is the volume in liters)
moles = (0.0026 mol/L) x V
We can now set up an equation to solve for V:
(0.0026 mol/L) x V = 0.8 g / 101.55 g/mol (the molar mass of copper(II) fluoride)
Simplifying the equation, we get:
V = (0.8 g / 101.55 g/mol) / 0.0026 mol/L
V = 30.29 L
Finally, we round our answer to three significant digits, giving us:
Volume = 30.3 L
To know more about concentration visit:
https://brainly.com/question/10725862
#SPJ11
what is the name of chewed food formed in the mouth?
Answers
How much energy is required to raise the temperature of 3 kg of lead from 15°C to 20°C? Use the table below and this equation: Q = MCAT.
The question is written right above the table given.
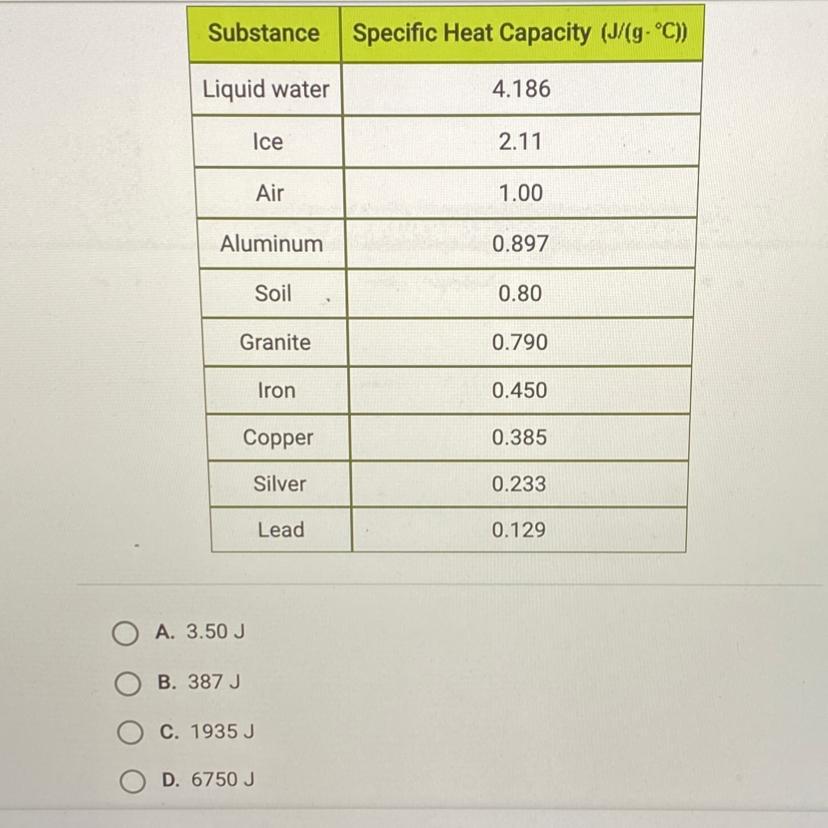
Answers
Answer:
1935J
Explanation:

Answer:
\(\boxed {\boxed {\sf C. \ 1935 \ J}}\)
Explanation:
The equation for this problem is:
\(q=mc\Delta T\)
where m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
The mass is 3 kilograms, but the specific heat capacity includes grams in the units. Convert kilograms to grams. There are 1000 grams in 1 kilogram.
\(\frac {1000 \ g}{1 \ kg}\)\(3 \ kg *\frac {1000 \ g}{1 \ kg}\)\(3 *1000 \ g = 3000 \ g\)The specific heat capacity for lead is found on the table. It is 0.129 J/g°C.
Let's find the change in temperature. It is raised from 15 °C to 20 °C.
\(\Delta T= final \ temperature - initial \ temperature \\\Delta T= 20 \textdegree C - 15 \textdegree C\\\Delta T= 5 \textdegree C\)Now we know every value.
m= 3000 g c= 0.129 J/g°CΔT= 5 °CSubstitute the values into the formula.
\(q= (3000 \ g)( 0.129 \ J/g \textdegree C)(5 \textdegree C)\)
Multiply the first 2 numbers together. The units of grams cancel.
\(q= (387 \ J/ \textdegree C )(5 \textdegree C)\)
Multiply again. This time the units of degrees Celsius cancel.
\(q= 1935 \ J\)
1935 Joules of energy are required and choice C is correct.
what general conclusions can you draw concerning the acidity or basicity of the hydroxides of the elements of the third period
Answers
The hydroxides of the elements in the third period of the periodic table can exhibit varying acidity or basicity. Here are some general conclusions that can be drawn:
1. Sodium (Na) and magnesium (Mg) hydroxides: Sodium hydroxide (NaOH) and magnesium hydroxide (Mg(OH)2) are both strong bases and highly alkaline in nature.
2. Aluminum (Al) hydroxide: Aluminum hydroxide (Al(OH)3) can act as both an acid and a base, depending on the conditions. It is amphoteric, meaning it can react with both acids and bases. In acidic solutions, aluminum hydroxide can accept protons and act as a base.
3. Silicon (Si), phosphorus (P), sulfur (S), chlorine (Cl), and argon (Ar) hydroxides: These elements in the third period do not readily form hydroxides under typical conditions. Instead, they form oxides.
Thus, the hydroxides of elements in the third period can exhibit a range of acidity or basicity. Some are strong bases, some are amphoteric, and others do not readily form hydroxides.
Know more about hydroxides:
https://brainly.com/question/30452297
#SPJ4
A strong covalent bond between amino acids that functions in maintaining a polypeptideʹs specific three-dimensional shape is a (an):________
Answers
A strong covalent bond between amino acids that functions in maintaining a polypeptide's specific three-dimensional shape is a disulfide bond.
Disulfide bonds are covalent bonds formed between the sulfur atoms of two cysteine residues in a polypeptide chain. These bonds play a crucial role in stabilizing the tertiary structure of proteins by holding specific regions together. Cysteine is an amino acid with a unique property; it contains a sulfur atom within its side chain. When two cysteine residues come close to each other in the protein's three-dimensional structure, the sulfur atoms can undergo oxidation, resulting in the formation of a covalent disulfide bond (S-S bond). This bond is relatively strong and can withstand the harsh conditions within the cell, contributing to the protein's stability.
Disulfide bonds are essential for maintaining the proper folding and structural integrity of many proteins. They help stabilize the protein's tertiary structure by forming bridges between distant parts of the polypeptide chain. These bonds are particularly important in proteins secreted outside the cell or proteins that are exposed to the extracellular environment, as they provide resistance against denaturation and proteolytic degradation.
Learn more about covalent bond
https://brainly.com/question/19382448
#SPJ11
Why is it dangerous to heat a liquid in a distilling apparatus that is closed tightly at every joint and has no vent to the atmosphere? Based on your understanding of the relationship between intermolecular interactions and boiling points, which liquid would you expect to have a lower boiling point, cyclohexanol or cyclohexene (below)? Draw a molecular-level picture of the types of intermolecular interactions you would expect in a solution of the pure liquid.
Answers
It is dangerous to heat a liquid in a closed distilling apparatus without a vent to the atmosphere because pressure builds up as the liquid vaporizes. This can lead to an explosion or equipment failure.
When a liquid is heated, its molecules gain energy and eventually transform into vapor. In a closed system, the vapor has nowhere to escape, causing an increase in pressure within the apparatus. As the pressure continues to rise, it can exceed the capacity of the equipment, leading to potential hazards such as an explosion or damage to the apparatus.
Regarding the boiling points of cyclohexanol and cyclohexene, cyclohexanol is expected to have a higher boiling point due to the presence of hydrogen bonding. Cyclohexanol has an OH group which can form hydrogen bonds, while cyclohexene lacks this functional group and can only form weaker London dispersion forces.
A molecular-level picture of cyclohexanol would show its molecules interconnected by hydrogen bonds between the oxygen atom of the OH group in one molecule and the hydrogen atom of the OH group in another molecule. In contrast, a molecular-level picture of cyclohexene would show its molecules interacting through weaker London dispersion forces, with no specific bond formation between them.
It is crucial to have a vent in a distilling apparatus to avoid dangerous pressure buildup. Based on intermolecular interactions, cyclohexanol has a higher boiling point due to hydrogen bonding, while cyclohexene has a lower boiling point due to weaker London dispersion forces.
To more about intermolecular interactions, click here
https://brainly.com/question/29690903?
#SPJ11
A measured value for the atomic radius of platinum
atoms was determined to be 143 picometers. What is the percent error of this measured
value?
A) 0.10%
B) 9.1%
C) 10.%
D) 13%
Answers
Since the actual value of the atomic radius of platinum atoms was not given in the problem, I chose 175 pm which gives an error of 18.28%.
The actual atomic radius of platinum atoms is 175 pm
We can see from the question that the measured value of the atomic radius of platinum atoms was determined to be 143 pm.
Recall that;
Percent error = Absolute value of (measured value - actual value)/actual value * 100
Since the actual value of the atomic radius was not given, let us take it to be 175 pm for the purpose of this problem
Percent error = Absolute value of (143 - 175)/175 * 100
Percent error = 18.28%
Learn more: https://brainly.com/question/4170313
The percent error of this measured value ; ( D ) approximately 13%
The percent error of a measured value
= [ ( actual value - measured value ) / ( actual value ) ] * 100
= [ ( 175 pm - 143 pm ) / ( 175 pm ) ] * 100
= 18.29%
From the options given the closest value to the calculated value is 13%
Hence we can conclude that the percent error of this measured value is approximately 13%.
Learn more : https://brainly.com/question/2138898
Although some data related to the question is missing the actual value of the atomic radius of platinum is 175 pm
What has a higher specific heat, water, or air? Why do you think so?
Answers
Water has a much higher specific heat, than air, because it takes more energy to heat water than it does to heat air.
What is Specific heat?This is a term which is referred to as the quantity of heat which is required to raise the temperature of one gram of a substance by one Celsius degree.
Water has a higher specific heat because it requires more energy to heat water than it does to heat air and an example is result of the specific heat of the two variables from various studies by scientists in various parts of the world.
Water has a specific heat of 4.186 J/g degrees celsius, versus air, which has a specific heat of 1.005 J/g degrees celsius which is therefore the reason why water was chosen as the correct choice and the one which has a higher specific heat.
Read more about Specific heat here https://brainly.com/question/27991746
#SPJ1
how much chemical is contained in 90% of all packages?
Answers
The amount of chemical contained in 90% of all packages can vary depending on the type of package and the specific type of chemical that is being used.
Generally speaking, when referring to chemicals, the majority of the time the concentration of the chemical in a package will be around 90%. This means that 90% of of the contents of the package will be the chemical itself.
However, it is important to remember that the exact amount of chemical contained in a package may not be 90%. This is because the type of chemical that is being used, the concentration of the chemical mixture, and the type of package itself can all have an effect on the exact amount of chemical contained in a package. In some cases, a package may contain as little as 75% of the chemical while in other cases, it may be as high as 99%.
Therefore, while it is generally safe to assume that 90% of a package will contain a chemical, it is important to take into consideration other factors in order to determine the exact amount of chemical contained in a package.
know more about concentration here
https://brainly.com/question/30862855#
#SPJ11
I need help w the moon phase for science
Answers
Answer:
where is the photo
Explanation:
where is it???
How do you know if your hypothesis is supported by your results or not?
PLEASE ANSWER!
(science)
Answers
Answer:
Your hypothesis is an educated guess of what the end results of an experiment will be, using what you already know about your experiment you are going to conduct. So when you receive your final results, if your hypothesis is correct, or even somewhat correct then you know that it is supported by your results. For example, if I were to conduct the Coca-Cola and Mentos experiment, I could make a hypothesis that the Coca-Cola will have a bigger eruption when I add more than one Mento to the bottle due to a higher amount of a chemical with the addition of each mento. When I receive my results that the eruption was bigger each time, I know that my results supported my hypothesis.
Explanation:
-Hope this helped
How can you manafacture nanowire batteries?
Answers
Answer:
Nanowires can be made from a wide variety of materials, including silicon, germanium, carbon, and various conductive metals, such as gold and copper. Their small size makes them good conductors, with electrons passing easily through them, a property that has allowed for important advances in computer science.
The temperature of 2.0 L of water is 42c which would cause the temperature of the water to increase to 50c
Answers
The temperature of 2.0 L of water is 42 °C removing thermal energy from the water cause the temperature of the water to increase to 50 °C.
What is thermal energy?Thermal energy is defined as the internal energy of a system that controls its temperature. Thermal energy is a type of kinetic energy, which is energy brought on by motion, and is brought on by the motion of particles. Solar power plants generate energy through the use of thermal energy.
Q = c × m × Δ T
ΔT = The system's temperature has changed.
The three different processes that lead to heat transmission are described here. When substances are heated, their volume rises, and when they are cooled, it falls.
Thus, the temperature of 2.0 L of water is 42 °C removing thermal energy from the water cause the temperature of the water to increase to 50 °C.
To learn more about thermal energy, refer to the link below:
https://brainly.com/question/11278589
#SPJ1
Matter is anything that takes up _______, has _______, and is made up of particles.
Answers
Answer:
space,mass
Explanation:
How is the weather in the two cities pictured most likely going to change in the next 24 hours
Answers
Answer:
theres no picture
Explanation:
A student is making tea on a cold day. When he tries to put honey in his tea, the honey will not flow out of the bottle. Describe how the student could make the honey flow out of the bottle more easily, and explain why your method would work.
Answers
Answer:
Warming it would make it come out. On a cold day the honey is frozen and atoms will not move as fast and are close toghether. When you warm it the atoms will move faster and the honey will come out of the bottle
Explanation:
How many of each type of atom would there be if these six water molecules underwent electrolysis?
Answers
Answer:Electrolysis is a process of passing electric current through water thereby splitting water molecules into hydrogen and oxygen.It is a process of breaking apart water molecules using electric current.In electrolysis of water, we use two copper wires, one twelve-volt battery, and some drinking water to do the tests.
Explanation:
Answer: The electrolysis of water will result in two hydrogen atoms and one oxygen atom.
Explanation: Hope this helps you :)
A closed cylinder is filled with CO₂ gas. The mass of CO₂ in cylinder is 4.4 g. Now express this amount of Carbon dioxide in following terms:
a) no. of moles of CO₂ molecules
b) Volume at NTP
c) No. of 'gram molecule
d) No. of CO₂ molecules
e) No. of carbon atoms
f) No. of mole of Oxygen atoms
g) No. of molecules of oxygen
Answers
#a
Molar mass of CO2=44g/mol
given mass=4.4g
\(\\ \sf\longmapsto No\:of\;moles=\dfrac{Given\:Mass}{Molar\;Mass}=\dfrac{4.4}{44}=0.1mol\)
#b
At NTP 1mol weigh 22.4L0.1mol weigh:-
\(\\ \sf\longmapsto 22.4(0.1)=2.24L\)
#c
No of molecules=Moles×Avagradro No
\(\\ \sf\longmapsto 0.1\times 6.023\times 10^{22}=6.023\times 10^{21}molecules\)
#d
CO_2 has 3atomsNo of molecules=
\(\\ \sf\longmapsto 3\times 6.023\times 10^{21}=18.069\times 10^{21}molecules\)
#e
CO2 has 1carbon\(\\ \sf\longmapsto No\:of\:molecules=6.023\times 10^{21}molecules\)
#f
Lets write balanced equation
\(\\ \sf\longmapsto C+O_2=CO_2\)
1mol of Oxygen exists#g
It has 2oxygen atoms
\(\\ \sf\longmapsto No\:of\:molecules=2(6.023\times 10^{21})=12.046\times 10^{21}\)
Describe how
electrical energy is used in a printer
pls help me ASAP
Answers
Answer:
The primary principle at work in a laser printer is static electricity, the same energy that makes clothes in the dryer stick together or a lightning bolt
Explanation:
FILL IN THE BLANK Dissolve a sugar cube in water and you still have sucrose, not ....................
This does not mean that sucrose or water cannot be broken down into simpler substances.
But methods must involve a ...........
Answers
Dissolve a sugar cube in water and you still have sucrose, not oxygen, carbon, and hydrogen. This does not mean that sucrose or water cannot be broken down into simpler substances.
But methods must involve a chemical change.
The change in which the molecular composition is completely altered and a new product is formed is called a chemical change.
Chemical changes create a new product.
The changes in chemical change are irreversible and permanent.
A chemical change occurs when the substance's composition is changed. When bonds are broken and new ones are formed a chemical change occurs.
Learn more about Chemical Change, here:
https://brainly.com/question/23693316
#SPJ4
1. what is hard water.
2. Mention four sources of water.
3. write down the chemical formula of water.
4. What is purification of water.
5. Mention two importance of purification of water.
6. Mention 4 method used ot purify water. 7. Mention 4 impurities found in water.
8. Explain the term contaminated of water.
9. State two physical properties of water. 10. mention the names of of the elements that causes hardness in water.
Answers
Explanation:
Hard water is a type of water that can not easily lather with soap. Springs, wells, pipe-borneH20. The 2 is in basePurification of water is the cleansing of water to avoid diseases, germs, bacteria. To prevent diseases, to make the water safe for drinkingBoiling,chlorination, distillationSorry about number seven! This is when water is unsafe or dirty, it cannot be used for the purpose for which it is intended Water is tasteless, water is colourlessCalcium tetraoxosulphate(vi), Magnesium tetraoxo sulphate (vi) and Calcium hydrogen trioxocarbonateexplain the following seeming contradiction: you have two gases, a and b, in two separate containers of equal volume and at equal pressure and temperature. there- fore, you must have the same number of moles of each gas. because the two temperatures are equal, the average kinetic energies of the two samples are equal. therefore, since the energy of such a system corresponds to
Answers
You have two gases, A and B, each in its own container of equal volume, pressure, and temperature. As a result, each gas must contain the same number of moles.
Because the two temperatures are the same, the two samples' average kinetic energies are also the same.
Kinetic energy is a type of energy that a moving object or particle possesses. When an item undergoes work—the transfer of energy—by being subjected to a net force, it accelerates and acquires kinetic energy. A moving object or particle's kinetic energy, which depends on both mass and speed, is one of its properties. The type of motion could be vibration, rotation on an axis, translation (or travel along a path from one place to another), or any combination of these.
A body's translational kinetic energy, or 1/2mv2, is equal to one-half the product of its mass, m, and the square of its velocity, v. In chemistry, the moles, sometimes spelled mol, is a common scientific measurement unit for significant amounts of very small objects like atoms, molecules, or other predetermined particles. The mole represents 6.02214076 1023 units, which is a very huge number.
Learn more about moles here:
https://brainly.com/question/14107397
#SPJ4
Students are to imagine that they are leading a one-week expedition to the Moon’s South Pole. In the essay, students will need to describe to NASA what (and who) they would bring to help make their expedition a success.
Answers
Answer:
On Sept. 15, 2020, NASA, in partnership with Future Engineers, launched the Artemis Moon Pod Essay Contest. The contest, which is open to all (public, private, and home school) students in grades K-12, asks participants to imagine they are leading a one-week expedition to the Moon’s South Pole. In the essay, students will need to describe to NASA what (and who) they would bring to help make their expedition a success.
The Moon Pod Essay Contest is presented in support of NASA’s Artemis program. The student challenge is part of NASA’s efforts to engage the public in its missions to the Moon and Mars. NASA is returning to the Moon for scientific discovery, economic benefits, and inspiration for a new generation. Working with its partners throughout the Artemis program, the agency will fine-tune precision landing technologies and develop new mobility capabilities that allow robots and crew to travel greater distances and explore new regions of the Moon.
Explanation:
Answer:
I would bring some warm clothes to keep me warm through the night because South Pole is cold. I would also bring blankets and sticks to make a fire. I would bring my bestfriend to keep me company.
Explanation:
What element has the electron configuration 1s22s22p63523p64523d4?
A.Chromium (Cr)
B.Magnesium (Mg)
C.Manganese (Mn)
D.Iron (Fe)
Answers
Explanation:
It's none of these.
Cr- 1s2 2s2 2p6 3s2 3p6 4s1 3d5
Mg- 1s2 2s2 2p6 3s2
Mn - 1s2 2s2 2p6 3s2 3p6 4s2 3d5
Fe- 1s2 2s2 2p6 3s2 3p6 4s2 3d6
I think there's a mistake in the question.
I really need your guys help, I need to pass.

Answers
Answer:
Question 4 is 291.0346
Explanation:
_4_NH3 +_3_02 ---> _2_N2 + __6_H20
How many moles of oxygen react with 0.69 moles of NH3?
Answers
Answer:
I hope this will help you and Please mark me as Brilliant
Explanation:
Consider this balanced chemical equation:
2 H2 + O2 → 2 H2O
We interpret this as “two molecules of hydrogen react with one molecule of oxygen to make two molecules of water.” The chemical equation is balanced as long as the coefficients are in the ratio 2:1:2. For instance, this chemical equation is also balanced:
100 H2 + 50 O2 → 100 H2O
This equation is not conventional—because convention says that we use the lowest ratio of coefficients—but it is balanced. So is this chemical equation:
5,000 H2 + 2,500 O2 → 5,000 H2O
Again, this is not conventional, but it is still balanced. Suppose we use a much larger number:
12.044 × 1023 H2 + 6.022 × 1023 O2 → 12.044 × 1023 H2O
These coefficients are also in the ratio of 2:1:2. But these numbers are related to the number of things in a mole: the first and last numbers are two times Avogadro’s number, while the second number is Avogadro’s number. That means that the first and last numbers represent 2 mol, while the middle number is just 1 mol. Well, why not just use the number of moles in balancing the chemical equation?
2 H2 + O2 → 2 H2O
is the same balanced chemical equation we started with! What this means is that chemical equations are not just balanced in terms of molecules; they are also balanced in terms of moles. We can just as easily read this chemical equation as “two moles of hydrogen react with one mole of oxygen to make two moles of water.” All balanced chemical reactions are balanced in terms of moles.
Example 8
Interpret this balanced chemical equation in terms of moles.
P4 + 5 O2 → P4O10
Solution
The coefficients represent the number of moles that react, not just molecules. We would speak of this equation as “one mole of molecular phosphorus reacts with five moles of elemental oxygen to make one mole of tetraphosphorus decoxide.”
Test Yourself
Interpret this balanced chemical equation in terms of moles.
N2 + 3 H2 → 2 NH3
Answer
One mole of elemental nitrogen reacts with three moles of elemental hydrogen to produce two moles of ammonia.
what produces energy from sugar through chemical reactions it can also called the PowerHouse of cell??
Answers
Answer:
Mitochondria are known as the powerhouses of the cell. They are organelles that act like a digestive system which takes in nutrients, breaks them down, and creates energy rich molecules for the cell. In cellular respiration sugar with the help of oxygen is broken down into ATP (energy molecule).