All stars evolve the same up through the red giant phase. What determines the life cycle path a star will take from there?
O The star's luminosity
O The star's composition
O The star's temperature
O The star's mass
Answers
Answer:
the star’s mass
Explanation:
the bigger the mass, the different lifestyle it will go through, our star will swell into a red giant then shed its outer layers until its a white dwarf then fade into a burned out cinder core.
Related Questions
What type of substance is gravel
Answers
Answer:
When substances do not mix thoroughly and evenly (like sand and gravel), the mixture is said to be heterogeneous. A heterogeneous mixture consists of visibly different substances. Another example of a mixture is salt dissolved in water.
Hope it helps!
How does the chemical formula for the sulfite ion differ from the chemical formula for the sulfate ion?
O sulfite contains 2 oxygens and sulfate contains 3 oxygens
O sulfite contains 3 oxygens and sulfate contains 2 oxygens
O sulfite contains 3 oxygens and sulfate contains 4 oxygens
O sulfite contains 4 oxygens and sulfate contains 3 oxygens
Answers
Answer:
Sulfite contains 3 oxygens and sulfate contains 4 oxygens
Explanation:
What would the corresponding
concentration values of H₂O be
for pH values: 1, 3, 5, 7, 9, 11?
Answers
Answer:
7,9,11
Explanation:
this is because water includes 0H, which would mean that it is more than 6
I need help ASAP, please.. Name two sodium salts that may be prepared by reacting sodium hydroxide with dilute sulphuric acid.
Answers
The two sodium salts that may be prepared by reacting sodium hydroxide and sulfuric acid are sodium sulfate and sodium bisulfate produced by neutralization .
What is a salt?
Salt is defined as a chemical compound which is ionic in nature and consists of cation and an anion.It has no net electrical charge as the charges are completely neutralized in case of salts.
They are classified as acidic, basic or neutral salts depending on the ions they produce on dissolution.They have wide variety of colors depending on the elements from which they are formed and have five basic tastes.Salts are usually odorless except a few exceptions . They are highly soluble in water and are insulators in the solid state, to make them conductive they need to be melted or dissolved.
Learn more about salts,here:
https://brainly.com/question/14618515
#SPJ2
Currently, nuclear energy provides only a small fraction (~10%) of energy used in the United States. In some other places, like France, it is close to 80%. Do you think nuclear energy will ever be a more widely used source of energy in the United States? Why or why not?
PLEASE HELP ASAP PLEASEEE
Answers
Nuclear energy is currently within almost every country becoming a growing source of energy use since it can provide a lot of energy within a short amount of time with little resources used to create it. It is quite possible that within the next few generations that nuclear energy could become a much larger source of energy within the United States. Although, many countries after the leak have resorted to other means of energy sources learning from the mistakes of Japan.
Hope this helps :)
Balance the following equation: H2O(aq) + Ca(NO3 )2(s) + (NH4)2HP04(s) + NH3(aq) → Calo(PO4)6 (OH)2(s) + NH4NO3(aq)
Answers
The balanced equation is \(H_2O(aq) + Ca(NO_3)_2(s) + (NH_4)2HPO_4(s) + NH_3(aq) - > Ca_{10}(PO_4)6(OH)_2(s) + 2NH_4NO_3(aq)\)
To balance the chemical equation:
\(H_2O(aq) + 5Ca(NO_3)_2(s) + 10(NH_4)2HPO_4(s) + NH_3(aq) - > Ca_10(PO_4)6(OH)_2(s) + 2NH_4NO_3(aq)\)
We need to ensure that the number of atoms of each element is equal on both sides of the equation.
First, let's balance the phosphorus atoms by multiplying (NH4)2HPO4 by 10:
\(H_2O(aq) + Ca(NO_3)2(s) + 10(NH_4)2HPO_4(s) + NH_3(aq) - > Ca_10(PO_4)6(OH)_2(s) + 20NH_4NO_3(aq)\)
Now we have 20 nitrogen atoms on the left and 40 on the right, so we can balance them by multiplying \(NH_4NO_3\)(aq) by 2:
\(H_2O(aq) + Ca(NO_3)_2(s) + 10(NH_4)2HPO_4(s) + NH_3(aq) - > Ca_{10}(PO_4)_6(OH)_2(s) + 2NH_4NO_3(aq)\)
Finally, we need to balance the calcium atoms by adding a coefficient of 5 to \(Ca_(NO_3)_2\)(s):
\(H_2O(aq) + Ca(NO_3)_2(s) + (NH_4)2HPO_4(s) + NH_3(aq) - > Ca_{10}(PO_4)6(OH)_2(s) + 2NH_4NO_3(aq)\)
Now we have balanced the chemical equation - there are 10 calcium atoms, 6 phosphorus atoms, 20 nitrogen atoms, and 62 oxygen atoms on both sides.
To know more about balanced equation click here
brainly.com/question/2976422
#SPJ11
In a calorimeter, 10.0 g of ice melts at 0oC. The enthalpy of fusion of the ice is 334 J/g.
How much heat was absorbed?
A.) 0.33 kJ
B.) .34 kJ
C.) 33.4 kJ
D.) 334 kJ
Answers
Answer:
D
Explanation:
In the problem above it is
q = mass x heat fusion
q = 10 g x 334 J/g = 3340 J. Since the ice is absorbing heat it is + 334 J.
Answer:
3.34 kJ
Explanation:
took the test

which of the following is a strong acid in aqueous solution? a. h3po4 b. hclo c. h3po3 d. hno2 e. hbr
Answers
The correct option is Hydrobromic acid (hbr), This is the strong Acid when compared to the other acids
Hydrobromic acid is the term for aqueous HBr.
Hydrogen bromide will be the name if there is no water, or if it is not aqueous, while hydrobromic acid will be the name if it is acidic and aqueous. Because it is acid, the suffix ic is used, and because it is aqueous, the suffix hydro is used.
To learn more about Hydrobromic acid please click on below link
https://brainly.com/question/11003770
#SPJ4
2. What is the maximum number of electrons that can occupy the n=3 shell of an atom, including all subshells within n=3? a) 2 b) 6 c) 10 d) 18 e) 32.
Answers
Answer:
18
Explanation:
use the equation 2n^2
The moothly loping valley formed when an ancient glacier lowly carved out the ide and bottom of the valley
Which proce directly led to the formation of thi valley
Answers
The majority of valleys are created by rivers or streams eroding the land's surface over a very lengthy period of time. Some valleys are created by glacial ice erosion.
Explain about the glacial ice erosion?Through erosion, or the removal of rock and sediment, glaciers can sculpt the terrain. Two alternative techniques can be used to erode bedrock: Abrasion: A glacier's bottom ice is not always pure; it frequently contains particles of rock, dirt, and debris. It is gritty, like to sandpaper.
Glaciers can alter the contour of the land when they spread out (grow) across its surface. They erode rock and sediment from the surface of the land, transport it from one location to another, and leave it in another. Thus, glaciers are responsible for both erosive and accumulative landforms.
Rock shards embedded in the ice abrade the underlying rocks, which is the main cause of glacial erosion.
To learn more about glacial ice erosion refer to:
https://brainly.com/question/8880387
#SPJ4
Calculate the volume of 1. 0M copper(II) sulfate solution required to completely react with 1. 0g of Fe(s) according to this equation: 2Fe(s)+3Cu2+(aq)→2Fe3+(aq)+3Cu(s)
Answers
The volume of 1.0 M copper(II) sulfate solution required to completely react with 1.0 g of Fe(s) is 26.9 mL.
To calculate the volume of 1.0 M copper(II) sulfate solution required to completely react with 1.0 g of Fe(s), we need to use stoichiometry. Here are the steps:
1. Calculate the number of moles of Fe(s) in 1.0 g:
1.0 g Fe(s) × (1 mol Fe/55.85 g) = 0.0179 mol Fe(s)
2. Use the balanced chemical equation to determine the number of moles of Cu2+(aq) needed to react with 0.0179 mol Fe(s):
0.0179 mol Fe(s) × (3 mol Cu2+/2 mol Fe) = 0.0269 mol Cu2+(aq)
3. Use the molarity of the copper(II) sulfate solution to calculate the volume of solution needed:
0.0269 mol Cu2+(aq) × (1 L/1.0 mol Cu2+) = 0.0269 L = 26.9 mL
Learn more about sulfate solution:
https://brainly.com/question/27101584
#SPJ11
H2SO4 + 2NaOH → Na2SO4 + 2H2O what volume of water vapor will be produced if you start with 71.5 g of sulfuric acid and excess of sodium hydroxide
Answers
Answer:
Explanation:
Find the molar mass of H2SO4
2H = 2 * 1 = 2
S = 1 * 32 =32
O4 = 4*16 = 64
total 98
Find the number of mols in 71.5 grams
mols = given mass / molar mass.
given mass = 71.5
molar mass = 98
mols = 71.5/98
mols = 0.7296 mols of H2SO4
Find the moles of H2O
From the Balanced equation, every mol of H2SO4 produces 2 moles of H2O
mols water = 2 * 0.7296
mols water = 1.4592
That's as far as I can take you. I have to know a great deal more to get the volume of H2O
dentify the type of reaction and balance the following equation
1. Br2 + KI ----> KBr + I2
2. P4 + O2 ----> P4O10
3. PBr3 + H2O ----> H3PO3 + HBr
4. C2H6 + O2 ----> CO2 + H2O
Answers
Answer:
its rthhhh
Explanation:
In Experiment 2 a gas is produced at the negative electrode.
Name the gas produced at the negative electrode.
Answers
In Experiment 2, the gas produced at the negative electrode is typically hydrogen (H2).
\(\huge{\mathfrak{\colorbox{black}{\textcolor{lime}{I\:hope\:this\:helps\:!\:\:}}}}\)
♥️ \(\large{\underline{\textcolor{red}{\mathcal{SUMIT\:\:ROY\:\:(:\:\:}}}}\)
an instant cold pack takes advantage of a dissolution that is:
Answers
An instant cold pack takes advantage of an endothermic dissolution process.
Instant cold packs typically consist of two compartments containing separate substances, usually water and ammonium nitrate or urea. When the pack is activated by breaking a barrier between the compartments, the substances mix, leading to dissolution. The dissolution of ammonium nitrate or urea in water is an endothermic process, meaning it absorbs heat from the surroundings, resulting in a decrease in temperature. This temperature decrease causes the pack to become cold, providing a cooling effect. By utilizing an endothermic dissolution process, the instant cold pack can rapidly lower the temperature for therapeutic or comfort purposes.
To learn more about Instant cold packs :brainly.com/question/31443568
#SPJ11
Please help!!
All fake answers get reported immediately. Yes, I am a girl -_-

Answers
Answer:
1.)C₃H₈O
2)OH
3.)1-propanol
1.) C4H8O
2.) C2H6O or CH3CH2OH
3.)Ethanol
Explanation:
Hope this help
please tell me if i did it right. did i put the right electric charge
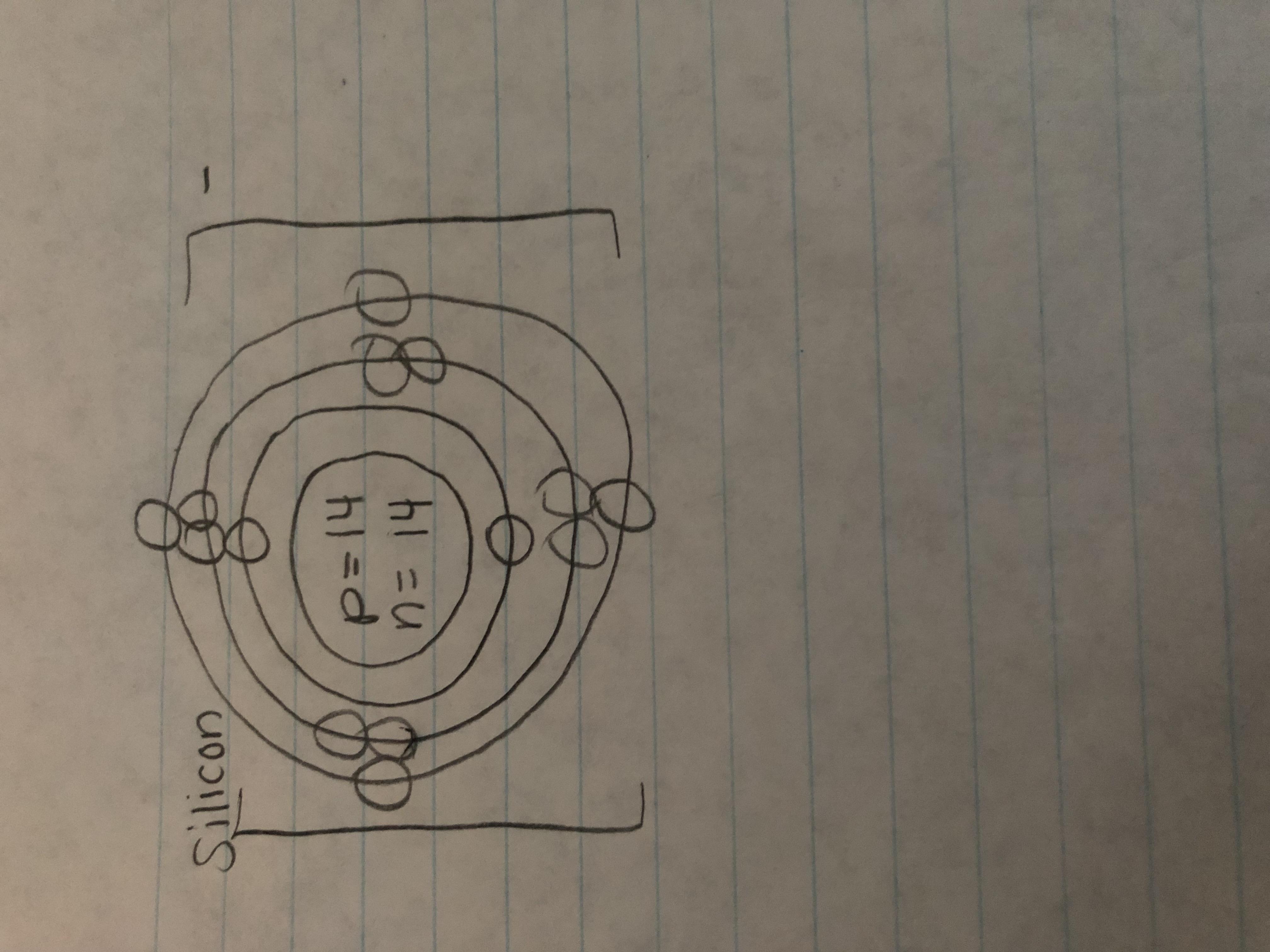

Answers
Answer:
its good but your answer
an original sample of the radioisotope fluorine-21 had a mass of 80.0 milligrams. only 20.0 milligrams of this original sample remain unchanged after 8.32 seconds. what is the half-life of fluorine-21? * (1) 1.04s
Answers
The half-life of fluorine-21 is approximately 15.8 seconds.
The half-life of a radioactive isotope is a measure of the time it takes for half of the original amount of the isotope to decay. In the case of fluorine-21, the original sample had a mass of 80.0 milligrams. After 8.32 seconds, only 20.0 milligrams of this original sample remained unchanged. To calculate the half-life of fluorine-21, we can use the equation:
Half-life = (8.32 seconds) × (ln(80.0 mg / 20.0 mg) / ln(2))
where ln(80.0 mg / 20.0 mg) is the natural logarithm of the ratio of the initial to remaining mass, and ln(2) is the natural logarithm of 2.
Half-life = 8.32 × (2.302 / 0.693) seconds
Half-life = approximately 15.8 seconds
So, the half-life of fluorine-21 is approximately 15.8 seconds. This means that, if we started with 80.0 milligrams of fluorine-21, after 15.8 seconds, only half of that original amount (or 40.0 milligrams) would remain unchanged.
to learn more about half-life visit: https://brainly.com/question/1160651
#SPJ4
determine the electron geometry (eg) and molecular geometry (mg) of pf5.
Answers
The electron geometry (eg) of PF5 is trigonal bipyramidal because there are five electron pairs around the central phosphorus atom. These five electron pairs include four fluoride atoms and one lone pair.
The molecular geometry (mg) of PF5 is also trigonal bipyramidal because the bond angles between the fluoride atoms and the lone pair are 90 degrees and 120 degrees. The fluoride atoms are positioned in the equatorial plane while the lone pair is located in the axial position, resulting in a molecular shape that resembles two pyramids attached at their bases. Overall, the PF5 molecule has a symmetrical structure and no dipole moment due to the arrangement of the atoms.
Hi! The electron geometry (EG) and molecular geometry (MG) of PF5 (phosphorus pentafluoride) can be determined using VSEPR theory. PF5 has 5 bonding electron pairs around the central phosphorus atom (5 F atoms). In PF5, there are no lone pairs of electrons on the central atom. This gives it an EG of trigonal bipyramidal, as the electron pairs are arranged symmetrically. Since all the electron pairs are bonding pairs, the MG is also trigonal bipyramidal. To summarize, PF5 has an electron geometry and molecular geometry of trigonal bipyramidal.
To know about geometry :
https://brainly.com/question/31408211
#SPJ11
How does pH affect cation exchange and mineral retention in the soil?
Answers
pH plays a crucial role in cation exchange and mineral retention in soil. Low pH (acidic conditions) increases the release of cations from soil particles, while high pH (alkaline conditions) promotes cation retention and reduces their availability for plants.
The pH of soil affects cation exchange and mineral retention through its influence on the soil's electrical charge and the solubility of minerals. In acidic conditions (low pH), the soil becomes positively charged, which leads to the release of cations from soil particles.
The high concentration of hydrogen ions (H+) displaces cations from the exchange sites on clay particles, allowing them to be leached away or become more available for plant uptake. This increased cation release can result in nutrient deficiencies for plants.
Conversely, in alkaline conditions (high pH), the soil becomes negatively charged. This facilitates the retention of cations on soil particles, reducing their availability for plant uptake.
The elevated concentration of hydroxide ions (OH-) can compete with cations for binding sites on clay minerals and organic matter, effectively immobilizing the cations and decreasing their mobility in the soil.
Therefore, maintaining an optimal pH range for specific crops is essential for promoting cation exchange and mineral availability in the soil. pH management through soil amendments and fertilization practices can help create favorable conditions for nutrient uptake and plant growth.
Learn more about cation here:
https://brainly.com/question/1626694
#SPJ11
Which of the following reactants can oxidize sodium?
lithium
barium
aluminum
potassium

Answers
Answer:
The answer is Aluminum
Hope this helps you
Help me get it right and no links

Answers
Answer:
i think the second answer " fish migration might be prevented" might possibly be your answer
25.00 mL of a barium chloride solution is titrated with 0.150 M silver nitrate solution. 18.55 mL of the silver nitrate solution is required to completely precipitate the chloride ion as silver chloride. What is the molarity of the barium chloride solution?
Answers
The molarity of the barium chloride solution is 7.
What is molar mass?
The molar mass is the substance; it helps to determine the mass of the sample substance to the atom of the sample or substance. The molar mass depends on the molecular formula and the isotopes of the atom. Molar mass is used for the inducement of electric charge. Molar mass is the measurement of the volume of the mass. The molar mass is expressed in the unit of dalton.
What is mole ?
A mole is the atom's elementary particle, an ion. The mole of the substance is always related to the Avogadro number. The mole is always associated with the weight or mass of the element or substance. The standard unit of a mole is mol. The mole is a significant factor of the reactant and products to form an equation. A mole calculates the atom, ion, and substance weighs.
Therefore, The molarity of the barium chloride solution is 7.
Learn more about molar mass from the given link.
https://brainly.com/question/837939
#SPJ1
BALANCE THE FOLLOWING REACTIONS:
(13,14,15,17,18,20, AND 21) IV’E ERASED MY INCORRECT ANSWERS
Also each number is a small number, it isn’t supposed to be big, i dont know how to type with smaller numbers
13)___AlBr3 + ___K2(SO4) ___KBr + Al2(SO4)3
14)___O2 + ___CH4 ___CO2 + H2O
15) ___Na3(PO4) + ___CaCl2 ___NaCl + ____Ca3(PO4)2
17) ___Al + ___HCl ___H2 + ___AlCl3
18) ___F2 + ___N2 ___NF3
20) ___NH3 + ___H2(SO4) ___(NH4)2(SO4)
21) ___C3H8 + ___O2 ____Co2 + ___H2O

Answers
The subsequent The following chemical equations are balanced:
What is an example of a chemical equation?Chemical equations serve to represent the transformation of reactants into products. For instance, iron sulfide is created when iron (Fe) and sulfur (S) combine (FeS). S(s) + Fe(s) = FeS (s) The + symbol denotes an iron-sulfur reaction.
A basic chemical equation is what?A chemical equation can be used to condense the details of a chemical process. A chemical equation's generic form is: Products Reactants. In a chemical equation, the reactants are the chemicals that are present before the action begins, and the product are the materials that are created during the reaction.
2 AlBr3 + 3 K2(SO4) → 6 KBr + Al2(SO4)3
2 O2 + CH4 → 2 CO2 + 2 H2O
2 Na3(PO4) + 3 CaCl2 → 6 NaCl + Ca3(PO4)2
2 Al + 6 HCl → 3 H2 + 2 AlCl3
3 F2 + N2 → 2 NF3
2 NH3 + H2SO4 → (NH4)2SO4
C3H8 + 5 O2 → 3 CO2 + 4 H2O
To know more about Chemical equation visit:
https://brainly.com/question/30087623
#SPJ1
A liquid solvent is added to a flask containing an insoluble solid. The total volume of the solid and liquid together is 91.0 mL. The liquid solvent has a mass of 48.4 g and a density of 0.865 g/mL. Determine the mass of the solid given its density is 2.75 g/mL
Answers
After finding the mass of the liquid solvent and subtract it from the total mass of the solid and liquid, the mass of the solid is 96.25 g.
To determine the mass of the solid, we need to find the mass of the liquid solvent and subtract it from the total mass of the solid and liquid.
Volume of solid + liquid = 91.0 mL
Mass of liquid solvent = 48.4 g
Density of liquid solvent = 0.865 g/mL
Density of solid = 2.75 g/mL
Let's calculate the volume of the liquid solvent using its mass and density:
Volume of liquid solvent = Mass of liquid solvent / Density of liquid solvent
Volume of liquid solvent = 48.4 g / 0.865 g/mL
Volume of liquid solvent ≈ 56.0 mL
Now, let's calculate the volume of the solid by subtracting the volume of the liquid solvent from the total volume:
Volume of solid = Total volume - Volume of liquid solvent
Volume of solid = 91.0 mL - 56.0 mL
Volume of solid = 35.0 mL
Next, we can use the density of the solid to find its mass:
Mass of solid = Density of solid * Volume of solid
Mass of solid = 2.75 g/mL * 35.0 mL
Mass of solid = 96.25 g
Therefore, the mass of the solid is 96.25 g.
To know more about mass, visit:
https://brainly.com/question/11954533#
#SPJ11
Levoxyl is a drug used to treat hypothyroidism. If a patient takes one 13 μ
g tablet per day, how many milligrams of Levoxyl are in their 1 month (30 day) supply?
Answers
A 30-day supply of Levoxyl at a dosage of one 13 μg tablet per day contains 0.39 mg of Levoxyl.
For a patient taking one 13 μg tablet of Levoxyl per day, the total amount of levothyroxine they are receiving is 13 micrograms. To calculate the total amount in a 30-day supply, we simply multiply this amount by 30.
13 μg/tablet x 1 tablet/day x 30 days = 390 μg
Note that the microgram (μg) is a unit of mass equal to one millionth of a gram, while the milligram (mg) is a unit of mass equal to one thousandth of a gram. To convert μg to mg, we divide by 1000:
390 μg / 1000 = 0.39 mg
Levoxyl is a medication used to treat hypothyroidism, a condition where the thyroid gland doesn't produce enough hormones to regulate the body's metabolism. The active ingredient in Levoxyl is levothyroxine sodium, which is a synthetic form of the hormone thyroxine (T4).
Levoxyl tablets are available in several strengths, ranging from 25 mcg to 300 mcg. The recommended dosage depends on the patient's age, weight, and the severity of their hypothyroidism. In general, adults with normal thyroid function usually require about 1.6 mcg of levothyroxine per kilogram of body weight per day.
For more question on Levoxyl click on
https://brainly.com/question/29924433
#SPJ11
why do competitors avoid using their compass overhead power lines
Answers
Answer:
Power lines produce a magnetic field
Explanation:
Power lines produce a type of magnet called an Electro-magnet which pulls metal magnetic objects towards it and the compass relies on magnetic feilds in order to point north.
Why must concentrated hydrochloric acid be added to manganese (IV) oxide slowly, a portion at a time?
Answers
Answer:
to produce hydrogen
Explanation:
dudugv. gueghhyrf. jjurdsrgjnbufurxjovezjidxhu. jhddf.
Hydrochloric acid reacts with manganese dioxide result in the evolution of chlorine gas. The HCl is added slowly to one portion at a time to collect the chlorine gas evolved with a pungent smell.
What is manganese oxide?Manganese oxide is an ionic compound between the transition metal Mn and atmospheric oxygen, where the oxidation state of Mn is 5. Transition metals shows variable oxidations states.
Manganese oxide reacts with hydrochloric acid to form manganese chloride, water and chlorine gas. This reaction is an example of displacement reaction.
Here we are using a concentrated HCl. As the concentration of reagents increases, the rate of reaction also increases. Thus if we use bulk HCl initially, more chlorine gas will be evolved with pungent smell and suffocating effects. That's why we have to add Con. HCl slowly with portion wise.
To find more on displacement reaction ,refer here:
https://brainly.com/question/29307794
#SPJ2
I need to learn how to balance this equation, but I am having trouble with it. Can you help me?__Zn + __HC2H3O2 → __Zn(CH3COO)2 + __H2
Answers
To balance a equation, we need to have the same number of atoms of each elements on both sides of the chemical reaction (reactant and product side).
I rewrote this substance(Zn(CH3COO)2) for better understanding.
__Zn + __HC2H3O2 → __Zn(C2H3O2)2 + __H2
Reactant side unbalanced:
Zn - 1
H - 4
C - 2
O - 2
Products side unbalanced:
Zn - 1
C - 4
H - 8
O - 4
Obs: when there is a part inside the parentheses, all the atoms are multiplied by the number outside the parentheses.
Now let's balance the equation changing the stoichiometric coefficient.
Zn + 2 HC2H3O2 → Zn(C2H3O2)2 + H2
Reactant side:
Zn - 1
H - 8
C - 4
O - 4
Product side:
Zn - 1
H - 8
C - 4
O - 4
Now the equation is balanced.
Answer: Zn + 2 HC2H3O2 → Zn(C2H3O2)2 + H2
The average atomic mass of silver is 107.8682 amu. the fractional abundance of the lighter of the two isotopes is ________.
Answers
The average atomic mass of silver is 107.8682 amu. The fractional abundance of the lighter of the two isotopes is 0.5185.
The metal has always been in perfect plenty. Both heavier and lighter particles have a combined abundance of 1.
Assume that a is the heavier particle's abundance.
The lighter particle will have an abundance of = 1-a.
Calculations for the element's atomic mass with isotopes go like this:
Average atomic mass equals the sum of the abundance of isotope 1 and isotope 2 masses.
107.8682 amu is the specified average atomic mass.
Lighter isotope 1 has a mass of 106.90509 amu.
The heavier isotope has a mass of 108.9047 amu.
putting the values :
107.8682 = 106.90509 (1 - a) + 108.9047a
107.8682 = 106.90509 - 106.90509a + 108.9047a
107.8682 - 106.90509 = 1.99961a
0.96311 = 1.99961a
a = 0.48
Thus, 0.48 is the heavier particle abundance.
Lighter particle abundance = 1 - a
The density of lighter particles is equal to 1 - 0.48.
Lighter particle abundance = 0.518
The lighter particle has a percent abundance of 51.8%.
The lighter of the two isotopes has a fractional abundance of 0.5185.
Question:
Silver has two naturally occurring isotopes with the following isotopic masses: 10747Ag 10947Ag 106.90509 108.9047 The average atomic mass of silver is 107.8682 amu. The fractional abundance of the lighter of the two isotopes is ________. Silver has two naturally occurring isotopes with the following isotopic masses: Ag Ag 106.90509 108.9047 The average atomic mass of silver is 107.8682 amu. The fractional abundance of the lighter of the two isotopes is ________. 0.24221 0.48168 0.75783 0.90474 0.51835
To learn more about isotopes click on the link below:
https://brainly.com/question/364529
#SPJ4