An analytical chemist is titrating 212.7 mL of a 0.6800 M solution of hydrazoic acid (HN3) with a 0.2900 M solution of KOH. The p Ka of hydrazoic acid is 4.72. Calculate the pH of the acid solution after the chemist has added 571.6 mL of the KOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of KOH solution added. Round your answer to 2 decimal places.
Answers
The pH of the acid solution after adding 571.6 mL of the KOH solution is approximately 7.71.
To calculate the pH of the acid solution after the addition of the KOH solution, we need to determine the number of moles of hydrazoic acid (HN₃) and potassium hydroxide (KOH) present in the solution.
First, let's calculate the number of moles of HN₃ in the initial solution:
moles of HN₃ = volume of HN₃ solution (L) × molarity of HN₃ solution (mol/L)
= 0.2127 L × 0.6800 mol/L
= 0.144696 mol
Next, let's calculate the number of moles of KOH added:
moles of KOH = volume of KOH solution added (L) × molarity of KOH solution (mol/L)
= 0.5716 L × 0.2900 mol/L
= 0.165964 mol
Since KOH is a strong base, it will react completely with HN₃ in a 1:1 ratio according to the balanced chemical equation:
HN₃ + KOH → KN₃ + H₂O
Since we have an excess of KOH (0.165964 mol), all the HN₃ (0.144696 mol) will react, and the remaining KOH (0.165964 - 0.144696 = 0.021268 mol) will determine the final concentration of hydroxide ions (OH-) in the solution.
Now, let's calculate the concentration of OH- ions in the final solution:
final volume of solution = initial volume of HN₃ solution + volume of KOH solution added
= 0.2127 L + 0.5716 L
= 0.7843 L
concentration of OH- ions = moles of KOH remaining / final volume of solution (mol/L)
= 0.021268 mol / 0.7843 L
= 0.02713 mol/L
Since we have the concentration of OH- ions, we can calculate the pOH:
pOH = -log10[OH-]
= -㏒₁₀(0.02713)
= 1.57
Finally, we can calculate the pH using the pOH and the pKa of HN₃:
pH = 14 - pOH - pKa
= 14 - 1.57 - 4.72
= 7.71
Learn more about KOH
brainly.com/question/29523756
#SPJ4
Related Questions
what is the total pressure of a gaseous mixture that contains three gases with partial pressures of 0.845 atm, 120 torr and 210 mm hg?
Answers
The total pressure of a gaseous mixture that contains three gases with partial pressures is 972 torr.
When more than one gas is present in a container, each gas exerts pressure, which is known as partial pressure. Its partial pressure refers to the pressure of any gas inside the container.
According to Dalton's Law, also known as the Law of Partial Pressures, the combined pressure of a mixture of gases is equal to the sum of their individual partial pressures.
1 - 0.845 atm - 0.854 atm x 760 Torr/atm = 642 torr
2 - 120 torr
3 - 210 mmHg - 1 mmhg is almost equivalent to 1 torr - therefore its 210 torr
total pressure - 642 + 120 + 210 = 972 torr
To know more about partial pressure visit the link:
https://brainly.com/question/13199169?referrer=searchResults
#SPJ4
According to the cup wall diagrams, why does the double wall vacuum
sealed cup outperform the other two cups
Because the particles are moving so fast in a vacuum that
they are able to pass through the walls and transfer more
kinetic energy to the liquid in the cup.
B
The vacuum allows for the all of the particles to pack in tightly
therefore nothing can pass through.
There is little to no transfer of kinetic energy between the
walls of the cups because there are no particles in a vacuum.
And since there is little to no transfer of kinetic energy there
is little to no transfer of temperature. Therefore the liquid
inside stays warmer/colder longer.
The particles in a vacuum do not move at all. Therefore there
is no kinetic energy and nothing can be transferred.
Answers
Answer:
i do not now
Explanation:
what is mass percent formula
Answers
The molar mass and mass of each element in a mole of the compound are both solved for in the Mass Percent formula. Mass Percentage = (Mass of Solute / Mass of Solution) × 100
What does "Mass Percent" mean?
A component in a particular combination or a concentration can be described using the mass percent symbol. The mass of solute contained in a specific mass of solution is indicated by the mass percentage used to characterize the solution composition. The concentration of the solute is expressed in terms of mass or moles. By multiplying the grammes of solute per gramme of solution by 100, the mass percent of a solution can be computed.
The molar mass and mass of each element in a mole of the compound are both solved for in the Mass Percent formula. The mass proportion of each element can be determined using these masses.
(Mass of Solute / Mass of Solution) × 100 Equals Mass Percentage
Example: The process of making an aqueous solution of sodium chloride (CaCl₂). Determine how much calcium chloride, in the form of a 5%(m/m) solution, can be generated from 100 g of the chemical.
To know about percent by mass, visit: https://brainly.com/question/14990953
#SPJ4
calculate the rf value for a spot in a chromatography experiment if the solvent moved 13.1 cm and the spot moved 9.5 cm from the origin.
Answers
The rf value for a spot in a chromatography experiment if the solvent moved 13.1 cm and the spot moved 9.5 cm from the origin is 0.73.
What is chromatography ?
Separating ingredients in a mixture using chromatography is a procedure. The mixture is dissolved in the mobile phase, which starts the process off, and then transports it through the stationary phase, which is the final phase.
What is experiment ?
A scientific experiment is a test carried out to ascertain what occurs to a subject under a given set of circumstances.
Therefore, rf value for a spot in a chromatography experiment if the solvent moved 13.1 cm and the spot moved 9.5 cm from the origin is 0.73.
Learn more about chromatography from the given link.
https://brainly.com/question/11960023
#SPJ4
ph before and after sulphuric acid is added
Answers
Answer:
added to what? base? if its added to base then it will increase in pH. H2S04(sulphuric acid is strong) so the pH is ard 3
If a titration net volume should be 20. 00 ml and a student accidentally goes one drop past the endpoint (~0. 05 ml). What is his percent error?
Answers
If a titration net volume should be 20. 00 ml and a student accidentally goes one drop past the endpoint (~0. 05 ml),then the percent error is 0.25%.
What is percent error?Percent error is defined as the difference of experimental value and theoretical value and measures the accuracy of the result found. The greater the error, lesser is the accuracy and vice versa.
Volume of a dropVd = 1mL/20 drops
where,
Vd is volume of one drop
Vd = 0.05mL
Calculation of errorError is defined as the ratio of change in volume divided by real volume.
e(%) = {(Vr-Vt) /Vt}×100
where,
Vr = real volume
Vd = theoretical volume
e(%) = {((20+0.05) -20) /20} × 100
e(%) = 0.25%
Thus, we find that the percent error on adding one drop is 0.25%.
learn more about percent error:
https://brainly.com/question/3105259
#SPJ4
Ethyl alcohol can be produced by fermentation of glucoe. If it take 5. 0h to produce 8. 0 kg of alcohol, how many day will it take to conume 1. 0 x 10^3 kg of glucoe
Answers
21.3 days will be taken to consume 1000kg glucose.
Fermentation is an alcoholic process where 1 molecule of glucose is converted to 2 molecules of CO2 and 2 molecule of ethanol/ethyl alcohol.
Fermentation takes place in absence of oxygen, which means in anaerobic conditions.
It takes place in presence of enzyme zymase.
The formula for alcoholic fermentation,
C6H12O6 -> 2C2H5OH + 2CO2
From the data given, we know
5 hr. = 8 kg alcohol.
Days to consume = 1000 kg glucose.
Mol ethanol:
5000/46 = 108.7 moles.
1/2 X 108.7 = 54.35
hence, 54.35 moles are produced in 5 hours.
moles of 1000kg of glucose:
10⁶g/180g/mol = 5555.5 moles.
so for 5555.5 moles the days we need is
5555.5/54.35 X 5 hours
= 511.085h
= 21.3 days.
To know more about alcoholic fermentation,
https://brainly.com/question/20662833
#SPJ4
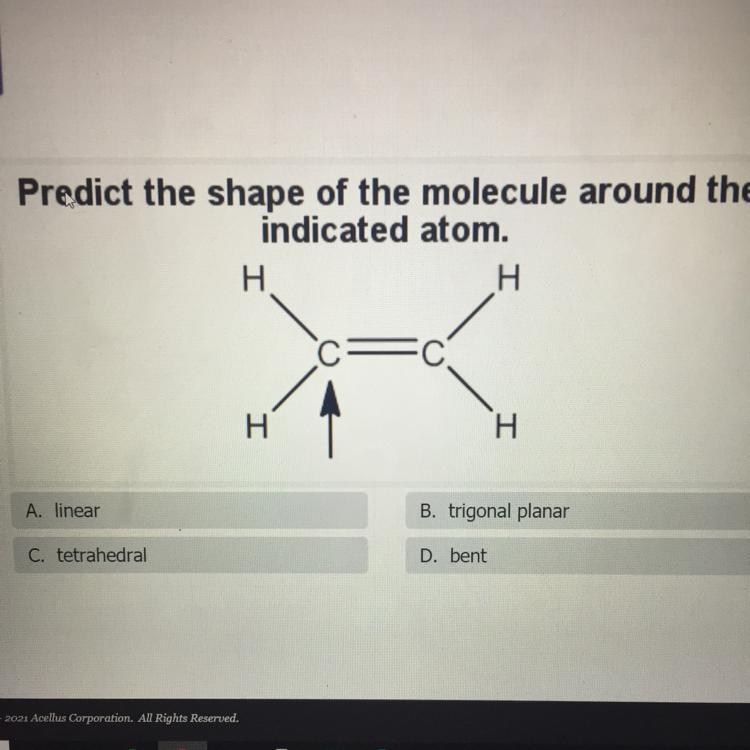
Predict the shape of the molecule around the
indicated atom.
A. linear
B. trigonal planar
C. tetrahedral
D. bent
Answers
2. If you put 156. 32g barium hydroxide into this reaction, how much aluminium hydroxide can be
produced?
Answers
When 156.32 g of barium hydroxide is reacted, approximately 142.34 g of aluminum hydroxide can be produced, based on the balanced chemical equation and stoichiometry.
To determine the amount of aluminum hydroxide that can be produced when 156.32 g of barium hydroxide is reacted, we need to consider the balanced chemical equation for the reaction and use stoichiometry.
The balanced chemical equation for the reaction is:
Ba(OH)2 + 2AlCl3 → 2Al(OH)3 + 3BaCl2
From the balanced equation, we can see that for every 1 mole of Ba(OH)2, 2 moles of Al(OH)3 are produced.
First, we need to calculate the number of moles of barium hydroxide (Ba(OH)2) in 156.32 g:
Molar mass of Ba(OH)2 = (137.33 g/mol + 2(16.00 g/mol + 1.01 g/mol)) = 171.34 g/mol
Moles of Ba(OH)2 = mass / molar mass = 156.32 g / 171.34 g/mol = 0.911 mol
Now, using the stoichiometry of the balanced equation, we can determine the moles of aluminum hydroxide (Al(OH)3) produced:
Moles of Al(OH)3 = 2 × Moles of Ba(OH)2 = 2 × 0.911 mol = 1.822 mol
Finally, to convert the moles of aluminum hydroxide to grams, we need to multiply by the molar mass of Al(OH)3:
Molar mass of Al(OH)3 = (26.98 g/mol + 3(16.00 g/mol + 1.01 g/mol)) = 78.00 g/mol
Mass of Al(OH)3 = Moles of Al(OH)3 × molar mass = 1.822 mol × 78.00 g/mol = 142.34 g
Therefore, when 156.32 g of barium hydroxide is reacted, approximately 142.34 g of aluminum hydroxide can be produced.
For more such questions on barium hydroxide visit;
https://brainly.com/question/29344018
#SPJ8
What is gluconeogenesis and where does it occur?
The three irreversible steps of glycolysis (name them) must be surpassed by what 3 enzymes/
Answers
The metabolic pathway by which glucose is synthesized from non-carbohydrate precursors such as amino acids, lactate, or glycerol is known as gluconeogenesis. This process occurs primarily in the liver.
What exactly is gluconeogenesis?When the intake of dietary is insufficient or absent, gluconeogenesis provides glucose. It is also required for the maintenance of acid-base balance, amino acid metabolism, and the synthesis of carbohydrate-derived structural components.
Glycolysis has three irreversible steps:
1. Hexokinase/Glucokinase: This enzyme catalyzes the first step of glycolysis, the phosphorylation of glucose to glucose-6-phosphate.
2. phosphofructokinase-1: This enzyme catalyzes the third step of glycolysis by converting fructose-6-phosphate to fructose-1,6-bisphosphate.
3. Pyruvate kinase: This enzyme catalyzes the final step of glycolysis, the conversion of phosphoenolpyruvate to pyruvate.
Which three enzymes are involved in glycogenolysis?Glycogen phosphorylase, phosphorylase kinase, and phosphoglucomutase are key enzymes in glycogenolysis.
To know more about the gluconeogenesis visit:
https://brainly.com/question/30908065
#SPJ1
the half life for the first order decomposition of h202 is 660 minutes. what is the rate constant for the reaction
Answers
The rate constant for the first-order decomposition of H2O2 with a half-life of 660 minutes is approximately \(0.00105 min^(-1).\)
To find the rate constant for the first-order decomposition of H2O2 with a half-life of 660 minutes, you can use the following formula:
rate constant (k) = ln(2) / half-life
Step 1: Plug in the given half-life value.
k = ln(2) / 660 minutes
Step 2: Calculate the rate constant.
\(k ≈ 0.00105 min^(-1)\)
So, the rate constant for the first-order decomposition of H2O2 with a half-life of 660 minutes is approximately 0.00105 min^(-1).
To know more about first-order decomposition :
https://brainly.com/question/4288256
#SPJ11
in an experiment, the molar mass of the compound was determined to be 180.15 g/mol. what is the molecular formula of the compound?
Answers
The molecular formula of the compound is C₆H₁₂O₆.
The molecular formula of a compound is determined by dividing the molar mass of the compound by the empirical formula mass. The empirical formula mass is the sum of the atomic masses of the elements in the empirical formula.
To determine the molecular formula of the compound, we first need to find the empirical formula mass. This can be done by using the atomic masses of the elements in the compound and multiplying them by the number of atoms of each element in the empirical formula.
For example, if the empirical formula of the compound is CH₂O, the empirical formula mass would be:
C: 12.01 g/mol x 1 = 12.01 g/mol
H: 1.01 g/mol x 2 = 2.02 g/mol
O: 16.00 g/mol x 1 = 16.00 g/mol
Empirical formula mass = 12.01 g/mol + 2.02 g/mol + 16.00 g/mol = 30.03 g/mol
Next, we can divide the molar mass of the compound by the empirical formula mass to find the molecular formula:
Molecular formula = Molar mass / Empirical formula mass
Molecular formula = 180.15 g/mol / 30.03 g/mol
Molecular formula = 6
Therefore, the molecular formula of the compound is 6 times the empirical formula, or C₆H₁₂O₆.
So, the molecular formula of the compound is C₆H₁₂O₆.
To know more about molecular formula refer here:
https://brainly.com/question/28647690#
#SPJ11
Answer:
First, I will convert from grams to moles. This will give me approximately 4.032 moles of carbon, 6.740 moles of hydrogen, and 3.344 moles of oxygen. Then, I will calculate the mole ratio, using whole numbers. I must also identify the least amount of moles in an element for this step, which is oxygen at 3.344 moles.
Oxygen = 3.344/3.344 = 1
Carbon = 4.032/3.344 = 1
Hydrogen = 6.740/3.344 = 2
The empirical formula will be COH2.
Now that we have the molar mass of the molecular formula, we can calculate for the molecular formula. First, we will begin by discovering the molar mass of the empirical formula, which will be C molar mass x 1 + O molar mass x 1 + H molar mass x 2. That will be equal to 60.949. After that, we can divide the molecular mass by the empirical formula molar mass, giving us approximately 3. Now we will multiply the empirical formula by 3, giving us our molecular formula, which will be equal to C3O3H6.
which of these would most likely affect ground water in a water washed
Answers
Answer: It supplies water to nearly half of US households.
Which of these solids are most likely amorphous solids? Select all that apply. Rubber sugar plastic candle wax graphite glass.
Answers
Answer:
Rubber, plastic,candle wax, graphite glass
Explanation:
they all lack internal structure
The solids are characterized as amorphous and crystalline solids based on the arrangement of atoms. The solids that are amorphous are rubber, plastic, candle wax, and glass.
What are amorphous solids?The solids have the arrangement of atoms in the lattice. The solids with an appropriate arrangement of atoms are crystalline solids. For example, sugar, graphite.
The solids with irregular arrangements of atoms in the lattice are amorphous solids. For example, glass, rubber.
Thus, the solids that are amorphous in nature are rubber, plastic, candle wax, and glass.
Learn more about amorphous solids, here:
https://brainly.com/question/4626187
________ limits the amount of food that can be consumed and the amount food that can be absorbed.4
Answers
The stomach's capacity and the efficiency of the digestive system are two factors that can limit the amount of food that can be consumed and absorbed.
One factor that limits the amount of food that can be consumed and absorbed is the capacity of the stomach. The stomach has a finite volume and can only expand to a certain extent. Once it reaches its capacity, it sends signals to the brain to indicate fullness, leading to a decrease in appetite and a natural limitation on food intake.Another factor is the efficiency of the digestive system. The small intestine is responsible for absorbing nutrients from food.
If there is a decrease in the surface area of the small intestine due to certain conditions or diseases, such as Crohn's disease or celiac disease, the absorption of nutrients may be impaired, resulting in limited nutrient uptake from food. It's important to note that there are other factors, such as individual metabolism, hormone regulation, and psychological factors, that can also influence food consumption and absorption.
To know more about stomach's visit:-
https://brainly.com/question/19098210
#SPJ11
Which term describes the information that a scientist gathers during an investigation? Data, hypothesis, observation, variable and why HURRY
Answers
During the research, the scientists collect the details and observations based on the information called data. Thus, option A is accurate.
What is data?A data is set of facts and observations including textual information, graphs, images, etc. It includes all the measurements and observations collected during the research work. The hypothesis is a thesis statement or a theory.
Observation in research is the gained information and facts during the research process that involves the recording of the data using some sort of tools. While variable is the set that can vary or change.
Therefore, the correct option is A. data.
Learn more about data here:
https://brainly.com/question/1362152
#SPJ1
Organisms typically have more than one form of each gene. If one form can mask the appearance of another form, that form is considered _______ the other form.
A.
better than
B.
dominant over
C.
recessive to
D.
worse than
Answers
If one form of a gene can mask the appearance of another form, that form is considered dominant over the other form. Option B.
What are dominant alleles?According to Mendel, genes are usually made up of 2 alleles. These alleles can be the same or different. When the alleles are the same, the gene is said to be homozygous. If the alleles are different, the gene is said to be heterozygous.
When the two alleles that make up a gene are different, one will be dominant and the other will be recessive. The dominant gene masks the effect of the recessive gene. In other words, the recessive gene cannot be expressed as long as it coexists with the dominant gene. In order for it to be expressed, it has to be in two copies or a homozygous recessive form.
For the dominant allele, however, only one copy is needed for it to be expressed.
In summary, if one form of a gene can mask the appearance of another form, that form is considered dominant over the other form.
More on genes can be found here: https://brainly.com/question/5519888
#SPJ1
What number need to go in front of ty HCI to balance the equation?
3H2 + 3Cl2 - ?HCI
Answers
As long as we have 3 in front of the compound so u have to multiple it by the number of the elements that are in the compound
We have 6 H
And 6Cl
So we need 6 HCl to balance the equation.
Hope that it helps
Indicators change color when an acid and a base are mixed together. The change in color most likely indicates that a chemical change has occured. a physical change has occured. a new acid has been produced. a new base has been produced.
Answers
Answer:
a chemical change
Explanation:
a physical change is mostly identified even without indicators i.e candle wax about forming a new acid or base I guess we have to include a ph scale to ascertain since the salt can be acidic but not necessarily an acid
Answer: The answer is A, a chemical change has occurred
Explanation:
I’m just a jenious
what are the formula molar mass and color of potassium permanganate
Answers
The molar mass of potassium permanganate (\(KMnO_4\)) is 158.03 g/mol, and it is a dark purple compound.
Potassium permanganate (\(KMnO_4\)) is a chemical compound that consists of one potassium (K) atom, one manganese (Mn) atom, and four oxygen (O) atoms. To determine the molar mass of potassium permanganate, we add up the atomic masses of these elements.
The atomic mass of potassium (K) is approximately 39.10 g/mol, the atomic mass of manganese (Mn) is approximately 54.94 g/mol, and the atomic mass of oxygen (O) is approximately 16.00 g/mol.
To calculate the molar mass of potassium permanganate, we multiply the number of each element by its atomic mass and then sum them up:
Molar mass of \(KMnO_4\)= (1 × 39.10 g/mol) + (1 × 54.94 g/mol) + (4 × 16.00 g/mol) = 39.10 g/mol + 54.94 g/mol + 64.00 g/mol = 158.03 g/mol.
Therefore, the molar mass of potassium permanganate is approximately 158.03 g/mol.
In terms of its color, potassium permanganate is a dark purple compound. This vibrant color is characteristic of its extended delocalized system of electrons, which absorbs light in the visible spectrum and reflects the purple wavelength. The intense purple color makes potassium permanganate easily recognizable in its solid or dissolved form.
To learn more about molar mass refer:
https://brainly.com/question/837939
#SPJ11
Potassium permanganate is a compound with the formula \(KMnO4.\) Its molar mass is approximately 158.03 g/mol. It is a dark purple crystalline solid used as an oxidizing agent and antiseptic.
Explanation:Potassium permanganate is a compound with the formula
\(KMnO4.\) Its molar mass is calculated by adding up the atomic masses of its constituent elements: potassium (K), manganese (Mn), and oxygen (O). The molar mass of potassium permanganate is approximately 158.03 g/mol.
Potassium permanganate is a dark purple crystalline solid. It is commonly used as a powerful oxidizing agent, antiseptic, and disinfectant. In terms of its color, potassium permanganate is a dark purple compound. This vibrant color is characteristic of its extended delocalized system of electrons, which absorbs light in the visible spectrum and reflects the purple wavelength. The color makes potassium permanganate easily recognizable in its solid or dissolved form.
Learn more about Potassium Permanganate here:https://brainly.com/question/35880995
#SPJ12
Which of the following is a synthesis reaction?
AgNO3 + NaCl → AgCl + NaNO3
CH4 + O2 → CO2 + H2O
SO3 + H2O → H2SO4
Cu + AgNO3 → Ag + CuNO3
Answers
Synthesis reaction
It is a reaction in which 2 or more reactants combine with each other to form one product .
Check option C
Sulphate and water are combining to form sulfuric acid .
Hence option C is correct
Answer:
SO3 + H2O => H2SO4
Explanation:
I took the test :)
what is periodic table
Answers
Answer:
The periodic table, in full periodic table of the elements, in chemistry, the organized array of all the chemical elements in order of increasing atomic number—i.e., the total number of protons in the atomic nucleus. When the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group) have similar properties. The initial discovery, which was made by Dmitry I. Mendeleyev in the mid-19th century, has been of inestimable value in the development of chemistry.
It was not actually recognized until the second decade of the 20th century that the order of elements in the periodic system is that of their atomic numbers, the integers of which are equal to the positive electrical charges of the atomic nuclei expressed in electronic units. In subsequent years great progress was made in explaining the periodic law in terms of the electronic structure of atoms and molecules. This clarification has increased the value of the law, which is used as much today as it was at the beginning of the 20th century, when it expressed the only known relationship among the elements.
Explanation:
is a table of the chemical elements arraged in order of atomic number
Calculate the volume of water which was produced when 1120 cm cubics of oxygen at s.t.p was liberated during the decomposition of hydrogen peroxide
Answers
Volume of H₂O produced = (1.8 g) × (1 cm³/g) = 1.8 cm³
Simplifying :
Molar volume of O₂ at STP = 22400 cm³/mol
No. of moles of O₂ liberated = (1120 cm³) / (22400 cm³/mol) = 0.05 mol
According to the given equation, mole ratio H₂O : O₂ = 2 : 1
No. of moles of H₂O produced = (0.05 mol) × 2 = 0.1 mol
Molar mass of H₂O = (1×2 + 16) g/mol = 18 g/mol
Mass of H₂O produced = (0.1 mol) × (18 g/mol) = 1.8 g
Volume of H₂O produced = (1.8 g) × (1 cm³/g) = 1.8 cm³
What happens in the decomposition of hydrogen peroxide?
The decomposition of hydrogen peroxide can be summarized by the chemical equation: which states that two molecules of hydrogen peroxide break down to form two molecules of water and one molecule of oxygen gas, along with heat energy.
Why does hydrogen peroxide decompose quickly?
In many living organisms hydrogen peroxide is a product of metabolism that must be broken down, since in appreciable concentrations it is toxic. The rate of decomposition is increased by the intra-cellular enzyme catalase.
At what temperature does hydrogen peroxide decompose?
Properties. The boiling point of H 2O 2 has been extrapolated as being 150.2 °C (302.4 °F), approximately 50 °C (90 °F) higher than water. In practice, hydrogen peroxide will undergo potentially explosive thermal decomposition if heated to this temperature
Learn more about decomposition of hydrogen peroxide:
brainly.com/question/20418092
#SPJ4
Please balance these chemical equations
Answers
The chemical equations given would be balanced below
How should a chemical equation be balanced?The balanced equation of the various given chemical equations can be determined only when the number of atoms on the product part is the same with the number of atoms on the reactant side.
For 1.)
\(N_{2} + 3H_{2} ----- > 2NH_{3}\)
For 2.)
\(2KCl_{3} ----- > 2KCl + 3O_{2}\)
For 3.)
\(2NaCl + F_{2} ----- > 2NaF + Cl_{2}\)
For 4.)
\(2H_{2} + O_{2} ----- > 2H_{2} O\)
For 5.)
\(Pb(OH)_{2} + 2HCl ----- > 2H_{2} O +PbCl_{2}\)
For 6.)
\(2AlBr_{3} + 3K_{2}SO{4} ---- > 6KBr + Al_{2}(SO_{4})_{3}\)
For 7.)
\(CH_{4} + O_{2} ----- > CO_{2} + 2H_{2} O\)
For 8.)
\(C_{3} H_{8} + 3O_{2} ----- > 3CO_{2} + 4H_{2} O\)
For 9.)
\(C_{8} H_{18} + 8O_{2} ----- > 8CO_{2} + 9H_{2} O\)
Learn more about chemical equation here:
https://brainly.com/question/30265799
#SPJ1
When an atom of a Radioactive element emits alpha radiation, an atom of a different element is formed. A different element is formed because the radioactive element has lost...
Answers
Explanation:
its its originality as an atom has to be pure
A different element is formed because the radioactive element has lost its originality as a pure atom when a radioactive element emits alpha radiation.
What is a radioactive element?Some elements of unstable atomic nuclei undergo radioactive decay to form stable nuclei because of the presence of excess nuclear charge inside them are known as radioactive elements.
The stability of nuclei of an element can be estimated by neutron to proton ratio. The atomic number up to Z= 20 is stable nuclei and contains an equal number of protons and neutrons. As the atomic number starts to increase, the repulsion forces between protons increase.
Thus, the neutron-to-proton ratios of stable nuclei increase with increasing atomic numbers. If elements with the atomic number, Z > 83 and n/p > 1.5 and they will be most unstable and radioactive.
For example, Pu-240 emits an alpha particle and gets converted into U-236 which is completely different from the original nuclei.
²⁴⁰Pu₉₄ → ⁴He₂ + ²³⁶U₉₂
Therefore, the radioactive element lost its identity after disintegration.
Learn more about the radioactive element, here:
https://brainly.com/question/23759636
#SPJ2
What is unique about carbons valence shell?
Answers
Answer: Carbon's valence shell is unique because it has 4 valence shell electrons, which means it is less likely to gain or lose electrons to other elements. Rather, it shares its electrons. In other words, it tends to form covalent bonds (4) rather than ionizing. This results in carbon being able to form long chains or rings.
NEED HELP ASAP!! FINAL EXAM
which of the following is an example of chemical change?
a. copper mixes with the air to form a greenish copper carbonate.
b. copper wire is a good conductor of electricity.
c. when heated to 100 degrees celsius, water boils.
d. after applying enough force, the board was broken in half
Answers
Answer:
An example of a chemical change is when copper mixes with the air to form a greenish copper carbonate. A chemical change is a type of change that involves a chemical reaction, in which the substances involved in the change are transformed into new substances with different chemical properties. In this case, when copper mixes with the air, it reacts with carbon dioxide and moisture to form a new substance called copper carbonate. This substance has a different color and chemical properties than the original copper, which indicates that a chemical change has occurred. In contrast, the other options you provided do not involve chemical reactions and are not examples of chemical changes.
Explanation:
Can someone help me with level 17 of Covalent Bonds o the Collision app? This is the last one and it's urgent.

Answers
The covalent compounds formed from the given elements are as follows:
Water; H₂OCarbo (iv) oxide; CO₂, and Nitrogen trifluoride; NF₃What are covalent compounds?Covalent compounds are compounds that are formed as a result of covalent bonds formed between atoms of different elements.
Covalent bonds are formed as a result of sharing of electrons between atoms of different elements usually non-metals.
Due to differences between the electronegativities of atoms of nom-metals, the electrons shared in covalent compounds are not always shared equally. Thus, several other intermolecular forces exist between molecules of covalent compounds.
Some of the intermolecular forces that exist in covalent compounds include:
London Dispersion forcesDipole Dipole forcesHydrogen bondingGiven the following elements: nitrogen, fluorine, oxygen, carbon, and hydrogen.
The covalent compounds that can be formed representing the given intermolecular forces are as follows:
Water; H₂O - hydrogen bonding exists between water moleculesCarbo (iv) oxide; CO₂ - London dispersion forces are the major intermolecular forces present in carbo (iv) oxide given moleculesNitrogen trifluoride; NF₃ - dipole dipole forces predominate in nitrogen trifluoride molecules.
Learn more about covalent compounds at: https://brainly.com/question/3447218
#SPJ1
A normal distribution has a mean of 98 and a standard deviation of 6. What is the probability that a randomly selected x-value from the distribution is at least 80
Answers
The probability that a randomly selected x-value from the distribution is at least 80 is approximately 0.9938.
What is the likelihood of selecting an x-value from the distribution that is greater than or equal to 80?In a normal distribution with a mean of 98 and a standard deviation of 6, we can calculate the probability of a randomly selected x-value being at least 80.
To do this, we need to find the area under the normal curve to the right of 80. By standardizing the value using the z-score formula, we can convert 80 to a z-score of (80 - 98) / 6 = -3.
The area to the right of this z-score can be obtained from a standard normal distribution table or using statistical software, which is approximately 0.9938.
A normal distribution is a common probability distribution that has a symmetric bell-shaped curve. It is characterized by its mean and standard deviation.
In this case, the mean is 98, indicating the center of the distribution, and the standard deviation is 6, representing the spread of the data around the mean.
The probability calculation involved converting the x-value of interest (80) to a z-score, which measures the number of standard deviations an x-value is from the mean.
By finding the area under the curve to the right of the z-score, we determine the probability of selecting an x-value that is at least 80.
Learn more about distribution
brainly.com/question/29664127
#SPJ11
predict the empirical formula of the ionic compound that forms from magnesium and fluorine.
Answers
MgF2 is the empirical formula of the ionic compound that forms from magnesium and fluorine.
The simplest whole number ratio of atoms in a compound is the empirical formula of a chemical compound in chemistry. [1] Sulfur monoxide's empirical formula, SO, and disulfur dioxide's empirical formula, S2O2, are two straightforward examples of this idea. As a result, both the sulfur and oxygen compounds sulfur monoxide and disulfur dioxide have the same empirical formula. They do not, however, have the same molecular formulae, which specify how many atoms are present in each molecule of a chemical compound.
To know more about empirical formula, click here,
brainly.com/question/1603500
#SPJ4