An aqueous solution is prepared that is initially 0.100 M CdI4-2. After the equilibrium is established, the solution found to be 0.013 M in Cd2+. What are the equilibrium concentrations?
Answers
An aqueous solution is prepared that is initially 0.100 M CdI4-2. After the equilibrium is established, the solution found to be 0.013 M in Cd2+.
CdI4-2 ⇌ Cd2+ + 4 I- is the equilibrium constant expression is given as:
K = [Cd2+] [I-]^4 / [CdI4-2]
At equilibrium, the concentration of Cd2+ is 0.013 M. Let x be the concentration of CdI4-2 that reacts. Then the concentration of I- will be 4x.
Using the equilibrium constant expression, we can write:
K = (0.013) ([4x]^4) / x
Simplifying the equation,
K = 256 (0.013) x^15
Rearranging and solving for x, we get:
x = [CdI4-2] = 0.0417 M
[I-] = 4x = 0.167 M
[Cd2+] = 0.013 M
Therefore, the equilibrium concentrations are:
[CdI4-2] = 0.0417 M
[Cd2+] = 0.013 M
[I-] = 0.167 M
To know more about equilibrium click here, https://brainly.com/question/30807709
#SPJ11
Related Questions
Can You answer this Chemistry question?
Question in attached photo

Answers
Explanation:
i cant see the picture!!!
How many moles of PbCl2 are produced
if 14 moles of AlCl3 are consumed?
3Pb(NO3)2 + 2AlCl3 + 3PbCl2 + 2A1(NO3)3
Answers
Answer:21 moles of PbCl2 formed
Explanation: 3Pb(NO3)2(aq) + 2AlCl3(aq) --> 3PbCl2(s) + 2Al(NO3)3(aq)
14 moles AlCl3 x 3 moles PbCl2/2 moles AlCl3 =21 moles of PbCl2 formed
The number of moles of PbCl₂ produced when 14 moles of AlCl₃ are consumed in the reaction is 21 moles
Balanced equation3Pb(NO₃)₂ + 2AlCl₃ —> 3PbCl₂ + 2Al(NO₃)₃
From the balanced equation above,
2 moles of AlCl₃ reacted to produce 3 moles of PbCl₂.
How to determine the mole of PbCl₂ producedFrom the balanced equation above,
2 moles of AlCl₃ reacted to produce 3 moles of PbCl₂.
Therefore,
14 moles of AlCl₃ will react to produce = (14 × 3) / 2 = 21 moles of PbCl₂.
Thus, 21 moles of PbCl₂ were obtained from the reaction.
Learn more about stoichiometry:
https://brainly.com/question/14735801
A 5-column table with 2 rows. Column 1 is labeled number of protons, with entries A and D; column 2 is number of neutrons, with entries 7 and E; Column 3 is atomic number, with entries B and 26; Column 4 is Mass Number, with entries 15 and 56, and Column 5 is Element (symbol) with entries C and F. Using the periodic table, complete the table to describe each atom. Type in your answers.
Answers
Answer:
A = 8
B = 8
C = Oxygen (O)
D = 26
E = 30
F = Iron (Fe)
Explanation:
protons neutrons atomic number mass number element
A 7 B 15 C
D E 26 56 F
mass number = protons + neutrons
E = 56 - 26 = 30
A = 15 - 7 = 8
protons = atomic number
B = 8
D = 26
From atomic number:
C = Oxygen (O)
F = Iron (Fe)
Answer:
8
8
o
26
30
fe
Explanation:
edge 21
The period of a mechanical wave is 5 seconds. What is the frequency of the wave?
0.2 Hz
2.5 Hz
depends on the speed of the wave
10 Hz
Answers
Answer:
.2 Hz
Explanation:
1/period = frequency
1/5 = .2 Hz
Producers are generally found at the beginning of a food chain. Which
statement best explains why this is true? *
1. Producers are usually smaller in size than consumers.
2. Producers do not rely on other organisms for food.
3. There are always more consumers than producers in food chains.
Answers
Explanation:
Second answer is the correct one.
Diamond is one of the common crystalline forms of _____ in which each atom is bonded to 4 others by strong, _____ bonds to create a large 3-D array.
Answers
Diamond is one of the common crystalline forms of carbon in which each atom is bonded to 4 others by strong, covalent bonds to create a large 3-D array.
Diamond is one of the common crystalline forms of carbon. It is an allotrope of carbon, meaning it is a different form or structure of the same element. In diamond, each carbon atom is bonded to four neighboring carbon atoms, forming a three-dimensional lattice structure. These bonds are strong and covalent in nature, involving the sharing of electrons between atoms. The covalent bonds in diamond create a rigid and tightly-packed arrangement of carbon atoms, resulting in its exceptional hardness and durability. Due to the tetrahedral arrangement of carbon atoms, diamond possesses a high degree of symmetry and regularity in its crystal structure. This large, well-organized 3-D array of carbon atoms gives diamond its characteristic properties, such as its transparency, high thermal conductivity, and optical brilliance. Overall, diamond's unique structure and bonding make it one of the most prized and valuable gemstones in the world.
For more information on diamond visit https://brainly.com/question/32700996
#SPJ11
which represents a linear graph?


Answers
Answer:
The 2nd one
Explanation:
This is the picture btw because it wont let me say all the words.

Answers
Answer:
see image
D is the answer
Explanation:
see image
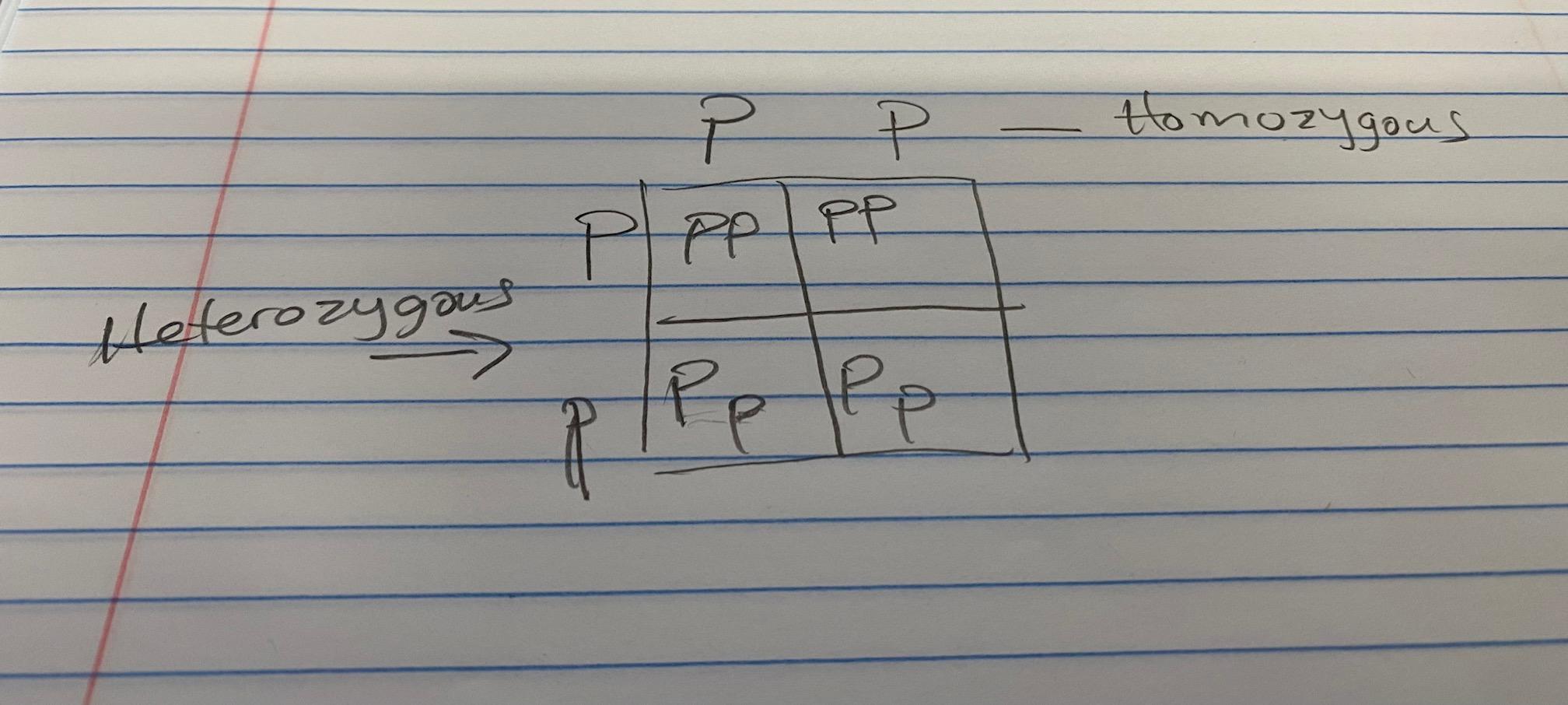
the box is like a mini multiplication table
What mass of iron should be produced if 11. 0g of aluminum react with 30. 0g of iron (III) oxide?
Answers
The mass of iron should be produced if 11. 0g of aluminum reacts with 30. 0g of iron (III) oxide is 10.50 g.
To determine the mass of iron produced, we need to use stoichiometry and the balanced chemical equation for the reaction between aluminum and iron(III) oxide.
The balanced chemical equation is:
2 Al + \(Fe_{2} O_{3}\) → + 2 Fe
From the equation, we can see that 2 moles of aluminum react with 1 mole of iron(III) oxide to produce 1 mole of iron.
First, we need to determine the limiting reactant by comparing the number of moles of aluminum and iron(III) oxide.
Moles of aluminum = mass of aluminum / molar mass of aluminum
= 11.0 g / 26.98 g/mol (molar mass of aluminum)
= 0.407 mol
Moles of iron(III) oxide = mass of iron(III) oxide / molar mass of iron(III) oxide
= 30.0 g / 159.69 g/mol (molar mass of iron(III) oxide)
= 0.188 mol
Since the stoichiometric ratio of aluminum to iron(III) oxide is 2:1, we can see that 0.188 mol of iron(III) oxide requires 0.376 mol of aluminum. However, we have only 0.407 mol of aluminum, which is in excess.
Therefore, the limiting reactant is iron(III) oxide. The amount of iron produced is determined by the moles of iron(III) oxide used. Moles of iron = 0.188 mol (same as moles of iron(III) oxide)
Now we can calculate the mass of iron produced using its molar mass (55.85 g/mol):
Mass of iron = Moles of iron × Molar mass of iron
= 0.188 mol × 55.85 g/mol
= 10.50 g
Therefore, the mass of iron produced is approximately 10.50 grams.
Know more about the Balanced chemical equation here:
https://brainly.com/question/13451900
#SPJ8
A truck was carrying a substance tank. The molecules of that substance were moving away from each other. The molecules truck parked overnight in a place where energy tranferred out of the substance. In the morning, the substance was a gas. How were the molecules moving in the morning? Explain why the molecules were moving that way after the energy was tranferred out of them.
Answers
Answer:
Molecules of matter such as liquid, gas and solid still move even if the energy is transferred because it isn't a hundred percent. There is still energy and the time it took to transfer that said energy would have changed its state of matter.
Explanation:
Solid -the molecules are tight together. (touching)
Liquid - the molecules are close but not touching about mid way(not to far not to close)
Gas- the molecules in gasses are far apart.
It almost always goes from solid to liquid to gas. but in extreme conditions it could be different.
If it helped please give a thanks on the thanks button
Hope you have a wonderful day :)
the taste of acid is sour
Answers
yes the taste of acid is sour
Explanation:
the acid increase the hydrogen concentration, which provides more ions for the tongue. Because the tongue gains ions, it registers the flavor as sour.
Answer will be MATlab code. Do not waste my time reposting the question, just answer the question with MATlab code and please explain so I understand what you do.
Ammonia (NH3) is a metabolite but is very toxic to aquatic life. NH3 and ammonium (NH4 + ) exist in equilibrium in an aqueous solution. The equilibrium constant K depends on temperature and pH. Nitrifying bacteria convert NH4 + to nitrite (NO2 - ). Nitrite can be further oxidized to nitrate (NO3 - ). Finally denitrification bacteria convert NO3 - to N2 completing the nitrogen cycle. Below are the reactions describing this part of the N cycle:
NH3(aq) + H202 NH(aq) 2 K} ; ks NH (aq) - N03(aq) NOz (aq) + NO3(aq) , ka ks NO3(aq) = N2(g)
Please write a MATLAB code to calculate and plot the concentration profiles of NH3, NH4 + , NO2 - and NO3 - as a function of time at T=298 K and neutral pH. The input for the code will include the rate constants k of the reactions and the initial concentrations [C] of the reactants. The output of the code will include the concentrations of both the reactants and products as a function of time.
Answers
Here is a MATLAB code that calculates and plots the concentration profiles of NH ₃, NH₄+, NO₂-, and NO₃- as a function of time at T=298 K and neutral pH, given the rate constants and initial concentrations:
```matlab
% Rate constants (k) and initial concentrations ([C])
k1 = 0.1; % Rate constant for NH₃ + H₂O₂ -> NH₂ + H₂O
k2 = 0.05; % Rate constant for NH₂ + NO₃- -> NO₂- + H₂O
k3 = 0.08; % Rate constant for NO₂- -> NO₃- + N₂
C_NH₃ = 1.0; % Initial concentration of NH₃
C_H2₂O₂ = 0.5; % Initial concentration of H₂O₂
C_NH₄ = 0.0; % Initial concentration of NH₄+
C_NO₂ = 0.0; % Initial concentration of NO₂-
C_NO₃ = 0.0; % Initial concentration of NO₃-
% Time vector
t = 0:0.1:10; % Time range from 0 to 10 with a step size of 0.1
% Calculation of concentrations at each time point
for i = 1:length(t)
NH₃(i) = C_NH₃ * exp(-k1*t(i));
NH₄(i) = C_NH₃ - NH₃(i);
NO₂(i) = C_NO₂ + k₂ * (NH₄(i) - C_NH₄) * t(i);
NO₃(i) = C_NO₃ + k₃ * NO₂(i) * t(i);
end
% Plotting concentration profiles
plot(t, NH₃, 'r-', t, NH₄, 'g-', t, NO₂, 'b-', t, NO₃, 'm-');
xlabel('Time');
ylabel('Concentration');
legend('NH₃', 'NH₄+', 'NO₂-', 'NO₃-');
```
The provided MATLAB code calculates and plots the concentration profiles of NH₃, NH₄+, NO₂-, and NO₃- as a function of time based on the given rate constants and initial concentrations. The code uses a time vector to define the time range for which the concentrations will be calculated.
Inside the for loop, the concentrations of NH₃, NH₄+, NO₂-, and NO₃- are calculated at each time point using the given rate constants and the previous concentrations. The concentration of NH₃ decreases exponentially over time due to the reaction NH₃ + H₂O₂ -> NH₂ + H₂O, where k1 is the rate constant. NH₄+ concentration is the difference between the initial NH₃ concentration and the current NH₃ concentration.
The concentration of NO₂- increases with time due to the reaction NH₂ + NO₃- -> NO₂- + H₂O, where k₂ is the rate constant. The change in NH₄+ concentration from its initial value is multiplied by k₂ and the time to calculate the increase in NO₂- concentration.
Finally, the concentration of NO₃- increases with time due to the reaction NO₂- -> NO₃- + N₂, where k₃ is the rate constant. The previous NO₂- concentration is multiplied by k₃ and the time to determine the increase in NO₃- concentration.
The resulting concentration profiles are then plotted using the plot function, with time on the x-axis and concentration on the y-axis. Each compound is represented by a different color line in the plot.
Learn more about the MATLAB code
brainly.com/question/31502933
#SPJ11
In your own words, define a fire.
What is a fire?
Answers
Answer:
Fire is something that's red and sorta orange is hot and can burn you but fire can be good and bad at some points for example fire can be used as a campfire,fireplace at home,and lighting candles. Fire is also very dangerous tho ,you can burn yourself if your not careful it can light your house on fire and that wouldn't be very safe. In simple words i would say fire is something that can have advantages and disadvantages.
Ahhh hope thats alright im on online classes but i wanted to help!!
Answer:
This would depend on the intensity or the size of a fire.
Explanation:
For instance, a small fire could be described as: kindled, flickering, dancing (in the case of a candle flame), smoldering, or crackling.
A medium size fire, such as a camp fire or a fireplace could be described as: crackling, blazing, burning, or roaring.
While a large or very intense fire (such as a forest fire or a house fire) could be described as: roaring, blazing, burning out of control, consuming, raging, or destroying.
If you were more looking for synonyms of ‘fire’, here are a few of my favorites: flame, blaze, incinerate, immolation, and inferno. (Edited to add a few I remembered: pyre, conflagration, and scorching.)
24. what is the most likely method of decay of the radioactive isotope technicium-99 (99tc)? a. alpha decay b. beta decay c. electron capture d. positron emission e. both electron capture and positron emission
Answers
The radioactive isotope Technium-99 decays most likely through alpha decay (99tc). An atomic nucleus emits an alpha particle during the radioactive decay process known as "alpha decay".
and then changes or "decays" into a separate atomic nucleus with a mass number that is decreased by four and an atomic number that is decreased by two. The nucleus of an atom of helium-4 is the same as an alpha particle. Radioisotopes are an element's radioactive isotopes. They are the atoms with unstable neutron-proton combinations or excess energy in their nuclei. During those processes, the radionuclide is said to experience radioactive decay, albeit the surplus energy may be put to use in any number of ways.
Learn more about radioactive isotope here
https://brainly.com/question/1907960
#SPJ4
12. Determine the mass in grams of each of the following.
a. 6.35 mol Al
b. 52.4 mol P
Please help❤️
Answers
Answer:
1 mole is equal to 1 moles Al, or 26.981538 grams.
Explanation:
171.3327663
1623.0250764
The mass in grams of the following is required.
The mass of Al is 171.323 g.
The mass of P is 1622.8 g.
M = Molar mass of Al = 26.98 g/mol
n = Number of moles = 6.35 mol
Mass is given by
\(m=nM\\\Rightarrow m=6.35\times 26.98\\\Rightarrow m=171.3\ \text{g}\)
The mass of Al is 171.323 g.
M = Molar mass of P = 30.97 g/mol
n = Number of moles = 52.4 mol
Mass is given by
\(m=nM\\\Rightarrow m=52.4\times 30.97\\\Rightarrow m=1622.8\ \text{g}\)
The mass of P is 1622.8 g.
Learn more:
https://brainly.com/question/7099389
https://brainly.com/question/13281749
what is cloud computing
Answers
Answer:
Cloud computing (English: cloud computing) is the general name of internet-based computing services for computers and other devices that provide computing resources that can be used at any time and shared among users. In this respect, cloud computing is not a product, but a service; By providing the sharing of software and information in the basic source, the existing information service; computers and other devices, similar to electrical distributors, over a computer network (typically the Internet).
Explanation:
One limitation of the linnaean classification system is that it.
Answers
One limitation of the linnaean classification system is that it is primarily based on physical attributes.
What is Linnean classification system?The classification system of Carolus Linnaeus is a system made up of hierarchical grouping of organisms into taxa.
The taxa he classified organisms into are as follows:
KingdomPhylumClassOrderFamilyGenus SpeciesHowever, one flaw of this system of classification is that it is based on solely physical qualities of organisms as there was no technical know-how of molecular biology.
Learn more about Linnean classification at: https://brainly.com/question/9880750
#SPJ2
What vale is represented by the symbol Mr
Answers
the answer for this question is Mister
The orbital of Venus is 0.72 AU what is this distance in kilometers
Answers
The orbital distance of Venus from the Sun is approximately 107,476,259.24 kilometers.
To find out the distance of an orbit, such as the orbital distance of Venus, in kilometers given its distance in Astronomical Units (AU), we will use the conversion factor where 1 AU is equivalent to 149,597,870.7 kilometers. This conversion factor helps us find out the value of any Astronomical Unit measurement in kilometers.
Therefore, the orbital distance of Venus in kilometers can be found by multiplying its distance in AU by the conversion factor of 149,597,870.7 km/AU. So, the long answer to this question is:Orbital distance of Venus = 0.72 AUConversion factor: 1 AU = 149,597,870.7 kmc Therefore, distance of Venus from the sun = 0.72 AU x 149,597,870.7 km/AU= 107,476,259.24 km
To know more about orbital visit:-
https://brainly.com/question/32355752
#SPJ11
2. If you put 156. 32g barium hydroxide into this reaction, how much aluminium hydroxide can be
produced?
Answers
When 156.32 g of barium hydroxide is reacted, approximately 142.34 g of aluminum hydroxide can be produced, based on the balanced chemical equation and stoichiometry.
To determine the amount of aluminum hydroxide that can be produced when 156.32 g of barium hydroxide is reacted, we need to consider the balanced chemical equation for the reaction and use stoichiometry.
The balanced chemical equation for the reaction is:
Ba(OH)2 + 2AlCl3 → 2Al(OH)3 + 3BaCl2
From the balanced equation, we can see that for every 1 mole of Ba(OH)2, 2 moles of Al(OH)3 are produced.
First, we need to calculate the number of moles of barium hydroxide (Ba(OH)2) in 156.32 g:
Molar mass of Ba(OH)2 = (137.33 g/mol + 2(16.00 g/mol + 1.01 g/mol)) = 171.34 g/mol
Moles of Ba(OH)2 = mass / molar mass = 156.32 g / 171.34 g/mol = 0.911 mol
Now, using the stoichiometry of the balanced equation, we can determine the moles of aluminum hydroxide (Al(OH)3) produced:
Moles of Al(OH)3 = 2 × Moles of Ba(OH)2 = 2 × 0.911 mol = 1.822 mol
Finally, to convert the moles of aluminum hydroxide to grams, we need to multiply by the molar mass of Al(OH)3:
Molar mass of Al(OH)3 = (26.98 g/mol + 3(16.00 g/mol + 1.01 g/mol)) = 78.00 g/mol
Mass of Al(OH)3 = Moles of Al(OH)3 × molar mass = 1.822 mol × 78.00 g/mol = 142.34 g
Therefore, when 156.32 g of barium hydroxide is reacted, approximately 142.34 g of aluminum hydroxide can be produced.
For more such questions on barium hydroxide visit;
https://brainly.com/question/29344018
#SPJ8
The vertical columns on the periodic table can be called families or?
Answers
The vertical columns may also be called "groups".
An -ate or -ite at the end of a compound name usually indicates that the compound contains
Answers
Answer:
Your answer would be "Polyatomic anions."
I ask that you search to ensure there is not a similar question as it would save you time.
https://brainly.com/question/14311288
I credit my answer to priyankatutor, his profile page is here:
https://brainly.com/app/profile/46866519/answers#:~:text=The%20First%20Sprout-,THANK,-YOU
IF I have 84.107 grams of Hydrogen
and 4.672 grams.
of water
can
of oxygen how many grams.
be formed
Answers
IF I have 84.107 grams of Hydrogen and 4.672 grams of water can of oxygen 4.153 gms be formed.
What is hydrogen?Methane, often known as natural gas, may be replaced with hydrogen, a clean fuel. It is the most prevalent chemical element and is thought to make about 75% of the universe's mass. Numerous hydrogen atoms may be found in water, plants, animals, and, of course, people here on earth.
Water is a liquid that facilitates the chemistry of life. Additionally, because it is a polar molecule, most other molecules may dissolve in it. As a result, we refer to water as a "solvent".
The decomposition of water is given as:
2H₂O → 2H₂ + O₂
36 gm 4 gm 32 gm
4.672 gm 84.107 gm
Thus, from 36 gm of water oxygen produced 32 gm.
So, from 4.672 gm of water oxygen produced 4.153 gm.
To know more about water refer to:
https://brainly.com/question/1313076
#SPJ1
How many kinds of chemically non-equivalent carbons are there in each of the following compounds? Br CH. CH3-CH=CH-CH, The number of chemically non-equivalent carbons is _____
OCH The number of chemically non-equivalent carbons is ______
Answers
BrCH has one kind of chemically non-equivalent carbon, while CH3-CH=CH-CH3 has two kinds of chemically non-equivalent carbons.OCH, there is only one kind of chemically non-equivalent carbon atom.
For BrCH, there is only one carbon atom, which is bonded to a bromine atom and three hydrogen atoms. Therefore, there is only one kind of chemically non-equivalent carbon atom.For CH3-CH=CH-CH3, there are two kinds of chemically non-equivalent carbon atoms. The two end carbons, which are bonded to three other carbon atoms, are chemically non-equivalent to the central carbon, which is bonded to two other carbon atoms.For OCH there is only one carbon atom, which is bonded to three hydrogen atoms and one oxygen atom. Therefore, there is only one kind of chemically non-equivalent carbon atom.
learn more about non-equivalent carbon, here:
https://brainly.com/question/30888690
#SPJ4
0.12g of rock salt was dissolved in water and titrated with 0.1moldm^-3 silver nitrate until the first permanent brown precipitate of silver chromate is seen. 19.70 cm^3 was required to titrate all the chloride ion. how many moles of chloride ion were titrated? what mass of sodium chloride was titrated? what was the % purity of the rock salt in terms of sodium chloride?
Answers
moles Cl⁻ : 0.00197
mass NaCl : 0.115
% purity : 95.83%
Further explanationMolarity shows the number of moles of solute in every 1 liter of solute or mmol in each ml of solution
\(\large \boxed {\bold {M ~ = ~ \dfrac {n} {V}}}\)
Where
M = Molarity
n = Number of moles of solute
V = Volume of solution
So to find the number of moles can be expressed as
n = V x M
mol Cl⁻Reaction
AgNO₃ + NaCl ⇒ AgCl + NaNO₃
Molarity(concentration) of AgNO₃ = 0.1 mol/dm³(L) = 0.1 M
Volume=V of AgNO₃ = 19.7 ml(cm³)
so mol of AgNO₃ :
\(\tt mol=M\times V\\\\mol=0.1~mol/L\times 0.0197~L\\\\mol=0.00197\).
From the equation, mol ratio AgNO₃ : NaCl = 1 ; 1, so mol NaCl= mol AgNO₃= 0.00197
NaCl⇒Na⁺+Cl⁻
mol Cl⁻ : mol NaCl = 1 : 1, mol Cl⁻ = 0.00197
mass NaCl (MW=58.5 g/mol) :
\(\tt mass=mol\times MW\\\\mass= 0.00197\times 58.5=0.115~g\)
% purity :\(\tt \%purity=\dfrac{mass~NaCl}{mass~rock}\times 100\%\\\\\%purity=\dfrac{0.115}{0.12}\times 100\%=95.83\%\)
What type of chemical bond is found between the atoms in molecular oxygen (O2)?
Answers
Answer:
The type of chemical bond found between the atoms in molecular oxygen (O2) is a covalent bond. Each oxygen atom has six valence electrons, and they share two electrons to each other to form a double bond between them, which stabilizes molecules with a total of 16 valence electrons.
The concentrations of Al and K in a sample of riverwater are 0. 0500 mg/kg and 1. 30 mg/kg, respectively. Express the concentration in molality
Answers
The concentration of Al in molality is approximately 0.0125 mol/kg, and the concentration of K in molality is approximately 0.0333 mol/kg.
To express the concentration in molality, we need to convert the given mass concentrations of Al and K into moles per kilogram (mol/kg).
First, we convert the mass concentrations from milligrams per kilogram (mg/kg) to grams per kilogram (g/kg) by dividing by 1000.
For Al:
Concentration of Al = 0.0500 mg/kg = 0.0500 g/kg
For K:
Concentration of K = 1.30 mg/kg = 1.30 g/kg
Next, we need to calculate the moles of Al and K using their respective molar masses. The molar mass of Al is approximately 26.98 g/mol, and the molar mass of K is approximately 39.10 g/mol.
For Al:
Moles of Al = Concentration of Al / Molar mass of Al
= 0.0500 g/kg / 26.98 g/mol
≈ 0.001853 mol/kg
≈ 0.0125 mol/kg (approximately)
For K:
Moles of K = Concentration of K / Molar mass of K
= 1.30 g/kg / 39.10 g/mol
≈ 0.0333 mol/kg (approximately)
Therefore, the concentration of Al in molality is approximately 0.0125 mol/kg, and the concentration of K in molality is approximately 0.0333 mol/kg.
To learn more about molality, here
https://brainly.com/question/14591804
#SPJ4
Please answer asap
Question 14 6 pts 4.6 kg/s of carbon dioxide undergoes a steady flow process. At the inlet state, the reduced pressure is 2 and the reduced temperature is 1.3. At the exit state, the reduced pressure is 3 and the reduced temperature is 1.7. Using the generalized compressibility and correction charts, what is the rate of change of total enthalpy for this process? Use cp 0.978 kJ/kg K. Express your answer in kW.
Answers
The rate of change of total enthalpy for the given steady flow process is 1.80032 kW.
The rate of change of total enthalpy for a steady flow process of carbon dioxide is to be determined using generalized compressibility and correction charts as given in the problem statement. The rate of change of total enthalpy can be given as: ΔH = ΔHs - ΔHf Where,
ΔHs = enthalpy change due to the change in specific heat at constant pressure
ΔHf = enthalpy change due to the change in specific volume at constant pressure. The given data can be plotted on generalized compressibility and correction charts as shown below: Generalized Compressibility Chart Solution: From the generalized compressibility chart, the value of Z1 can be obtained by using reduced pressure Pr1 = 2 and reduced temperature Tr1 = 1.3. The value of Z1 is found to be 0.9188. From the generalized compressibility chart, the value of Z2 can be obtained by using reduced pressure Pr2 = 3 and reduced temperature
Tr2 = 1.7.The value of Z2 is found to be 0.7976.The density of carbon dioxide at the inlet can be given as:
r1 = P1Z1 / RT1
= 2 x 0.9188 / (0.27 x 1.3)
= 1.6852 kg/m3. The density of carbon dioxide at the exit can be given as:
r2 = P2Z2 / RT2
= 3 x 0.7976 / (0.27 x 1.7)
= 2.3097 kg/m3. The specific volume of carbon dioxide at the inlet can be given as:
v1 = v1, r\ed x RT1 / P1
= 0.9978 x 0.27 x 1.3 / 2
= 0.1735 m3/kg.
The specific volume of carbon dioxide at the exit can be given as:v2 = v2, red x RT2 / P2
= 0.8769 x 0.27 x 1.7 / 3
= 0.1322 m3/kg. The enthalpy of carbon dioxide at the inlet can be given as:
H1 = cpT1
= 0.978 x 1.3 x 1000
= 1271.4 kJ/kg. The enthalpy of carbon dioxide at the exit can be given as:
H2 = cpT2
= 0.978 x 1.7 x 1000
= 1671.4 kJ/kg. The change in enthalpy due to the change in specific volume at constant pressure can be given as: ΔHf = (P2v2 - P1v1) / 1000
= (3 x 0.1322 - 2 x 0.1735) / 1000
= -0.002697 kJ/kg. The change in enthalpy due to the change in specific heat at constant pressure can be given as: ΔHs = cp (T2 - T1)
= 0.978 x (1.7 - 1.3) x 1000
= 391.2 kJ/kg. The rate of change of total enthalpy can be obtained by using the above-calculated values.
ΔH = ΔHs - ΔHf
= 391.2 - (-0.002697)
= 391.2 + 0.002697
= 391.202697 kJ/kg. The given mass flow rate is 4.6 kg/s. The power required for the steady flow process of carbon dioxide can be given as: P = mass flow rate x ΔH
= 4.6 x 391.202697
= 1800.32 W
= 1.80032 kW (Answer) Therefore, the rate of change of total enthalpy for the given steady flow process is 1.80032 kW.
To know more about enthalpy visit:-
https://brainly.com/question/32882904
#SPJ11
PLZ I NEED HELP I WILL GIVE 100 points for helping me
Silver nitrate reacts with barium chloride. If 39.02 grams of barium chloride are
reacted, how many grams of silver chloride are produced?
Answers
Answer:
4.2 g good luck with the answer
What is an expression of Dalton's law (k = constant)?

Answers
Answer:
I believe it's B: Ptotal=P1+P2+P3+...
Explanation: