Answers
Answer:
Although he was unaware ofit, Mendeleev had actually placed the elements inorder of increasing “atomic number,” a numberrepresenting the amount of positively charged protons in the atom (also thenumber of negatively charged electronsthat orbit the atom). Mendeleev went even further.
Related Questions
Why do silver and copper have similar properties? (science CER)
Answers
Answer:Copper is a chemical element with the atomic number 29, and the chemical symbol Cu. Silver is also found on the periodic chart of elements, and has a chemical symbol of Ag and an atomic number of 47.
Explanation:
Both silver and copper are transition metals of d-block in periodic table they are in the same group and thus shows similar chemical and physical properties.
What is group in periodic table?In periodic table, elements are classified into groups and periods. The horizontal rows are called periods and the vertical columns are called groups.
There are 18 groups and 7 periods in periodic table. Elements of the same group have same number of valence electrons and similar chemical and physical properties.
Silver, copper and gold are in 11th group of periodic table. Thus they have 9 valence electrons and exhibit similarity in properties. All these metals are good conductors and are used in electrical and optoelectronic devices.
To find more on silver, refer here:
https://brainly.com/question/6434391
#SPJ3
Question 2
Is oxygen a metal or a nonmetai? How many valence electrons does an oxygen atom have?
?
Answers
Answer:
Oxygen (O), nonmetallic chemical element of Group 16 (VIa, or the oxygen group) of the periodic table.
Six valence electrons
Explanation:
brainliest please
One of the hydrates of CoCl2 is cobalt(II) chloride dihydrate . A 56.2 gram sample of CoCl2 2 H2O was heated thoroughly in a porcelain crucible, until its weight remained constant. After heating, how many grams of the anhydrous compound remained?
Answers
The formula for cobalt(II) chloride dihydrate is CoCl2 · 2H2O, which means that each mole of this compound contains 1 mole of CoCl2 and 2 moles of H2O. To find the number of moles of CoCl2 in 56.2 grams of CoCl2 · 2H2O, we need to first find the molar mass of the compound:
Molar mass of CoCl2: 58.933 g/mol
Molar mass of 2H2O: 36.032 g/mol (2 × 18.016 g/mol)
Molar mass of CoCl2 · 2H2O: 58.933 g/mol + 36.032 g/mol = 94.965 g/mol
Now we can find the number of moles of CoCl2 in 56.2 grams of CoCl2 · 2H2O:
56.2 g / 94.965 g/mol = 0.591 moles
Since each mole of CoCl2 · 2H2O contains 1 mole of CoCl2, there are also 0.591 moles of CoCl2 in the sample.
We are told that the hydrate was heated until its weight remained constant, which means that all of the water was driven off and only the anhydrous CoCl2 remained. If the percent yield of the reaction was 100%, we would expect to recover the same amount of CoCl2 that was in the original sample (0.591 moles). However, we are told that the percent yield was only 65.5%, which means that the actual amount of CoCl2 recovered was:
0.655 × 0.591 moles = 0.387 moles
To find the mass of the anhydrous CoCl2 that remained, we can use the molar mass of CoCl2:
Molar mass of CoCl2: 58.933 g/mol
0.387 moles × 58.933 g/mol = 22.8 grams
Therefore, after heating, 22.8 grams of anhydrous cobalt(II) chloride remained.
The density of water is 1.0 mg
true or false ?
Answers
The density of water is 1.0 mg so it’s
Answer:
True. the density of water is 1.0 mg
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia.
N2(g)+3H2(g)⟶2NH3(g)
Assume 0.290 mol N2 and 0.954 mol H2 are present initially.
After complete reaction, how many moles of ammonia are produced?
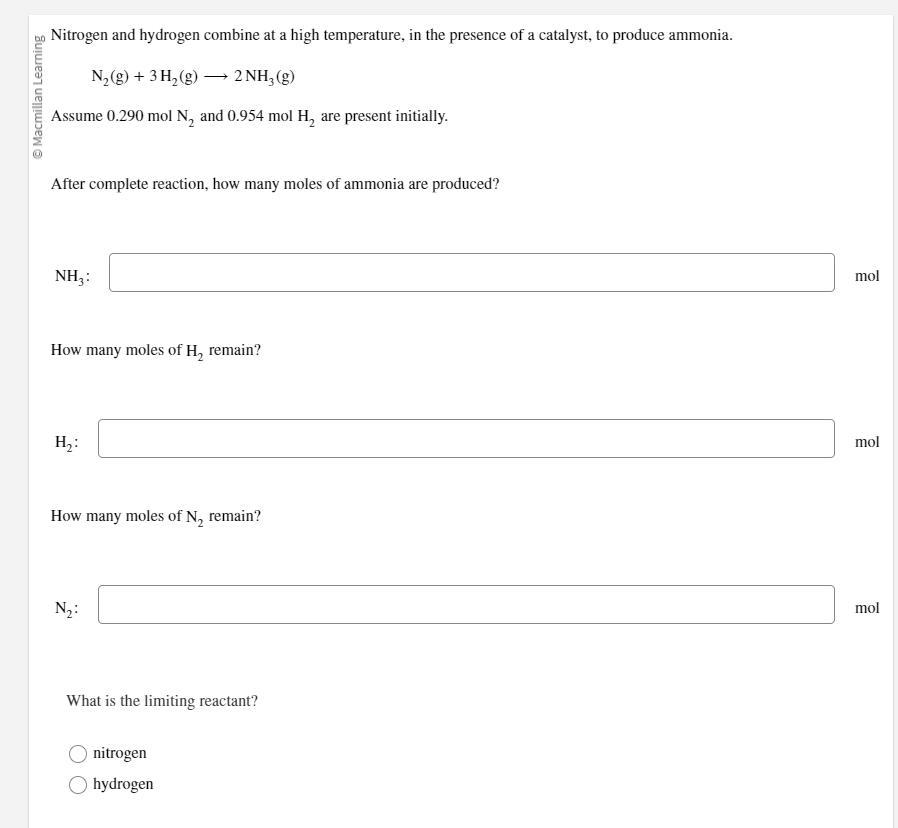
Answers
Answer:
17 reactions
Explanation:
Help me Guys please I need help with this My step sister is here and she's a demon and annoying Can I have good ideas what to do
Answers
Answer:
Lock her in the bathroom
Explanation:
Answer:
Ayoo
Explanation:
Which of the following do all food chains in an ecosystem depend on?
A.
consumers
B.
competition
C.
decomposers
D.
producers
Answers
why is it difficult for non metals to form positive ions
Answers
Answer:
Because the non-metal comes from the right hand side of the Periodic Table as we face it, its nuclear charge is very poorly shielded by its INCOMPLETE valence electronic shell, and this results in the well-known contraction of atomic radii across the Table from left to right.
Explanation:
Non metals usually gain electrons so they have more electron in the shells than protons in nucleus and hence they form negative ions. However, metals usually lose electron so they have more protons in nucleus than electrons in shells and hence they form positive ions.
help me i need it asap
Which example is a biotic factor of an aquarium ecosystem?
plastic plants placed in the gravel
algae growing on the glass
rock structure
gravel on the bottom of the aquarium
Which examples are an abiotic factor of an aquarium ecosystem? Select all that apply.
rock structure
temperature of the water
amount of algae on the glass
number of fish
Which example describes an abiotic factor interacting with a biotic factor?
The temperature of the air affecting the wind direction
Water eroding a rock
Cows eating nearby grass
The amount of sunlight affecting the growth of a plant.
Students in Ms. Brown's class are making observations in the garden outside of the school. They make a list of all the abiotic and biotic factors in their notebook. Part of the list is below:
Gravel
Grass
Butterfly
Sunflower
Bird feeder
Flowerpots
Which items on the list are a part of the garden ecosystem?
Grass, butterfly, sunflower
None of the listed items are a part of the ecosystem
All the listed items are part of the ecosystem
Gravel, bird feeder, flowerpots
Answers
The biotic factor of an aquarium ecosystem is algae growing on the glass.
The abiotic factors of an aquarium ecosystem are rock structure, temperature of the water, and amount of sunlight.
The example that describes an abiotic factor interacting with a biotic factor is the amount of sunlight affecting the growth of a plant.
All the listed items in Ms. Brown's class, including gravel, grass, butterfly, sunflower, bird feeder, and flowerpots, are a part of the garden ecosystem.
What is an ecosystem?An ecosystem is a community of living organisms (biotic factors) interacting with each other and with their physical environment (abiotic factors) in a specific area. It includes all the living and non-living components of the environment that interact with each other. Examples of ecosystems include a coral reef, a forest, a desert, and even an aquarium or a garden. Ecosystems can be large or small and can be found on land or in water. They are essential for the survival of living organisms as they provide food, shelter, and other resources necessary for life.
Read more on ecosystem here:https://brainly.com/question/842527
#SPJ1
Rank the following atoms in order of decreasing electronegativity.
a. Na, Li, K
b. K, Sc, Ca
c. As, Sn, S
Answers
In the first option , the order of decrease in electronegativity is Li, Na and K. In second option, the order is Ca, K, Sc The order in the third group is S, As, Sn.
What is electronegativity ?Electronegativity of an atom is the ability of its nucleus to attracts the share pair of electrons towards it. Elements like, fluorine, oxygen, nitrogen etc .are highly electronegative.
In periodic table, along a period electronegativity increases. Whereas down group, electronegativity decreases. The first groups elements are Li, Na , K and Cs and Fr. Hence, electronegativity decreases from Li to K.
Among, K, Sc and Ca, Ca is comparatively electronegative than K. Sc is a transition metal and not electronegative. In the last group the S is mot electronegative than As more than Sn.
Find more on electronegativity:
https://brainly.com/question/29203628
#SPJ1
10. Help, question is in picture below!


Answers
The set of reactions that best represents the diagram of a precipitation reaction is given below:
Li₂SO₄ (aq) + AgNO₃ (aq) ---->
The net ionic equation is given below:
SO₄²⁺ (aq) + 2 Ag⁺ (aq) ----> Ag₂SO₄ (s) (a precipitate)
What are precipitation reactions?Precipitation reactions are reactions in which two soluble solutions when they are mixed result in the formation of an insoluble precipitate.
Precipitation reactions rea usually double replacement reactions.
Double replacement reactions also known as double displacement reactions are reactions in which an exchange of radicals occurs between two metallic cations resulting in the formation of an insoluble compound that forms the precipitate observed.
Considering the given reactions and the products obtained:
NH₄Br (aq) + Pb(C₂H₃O₂)₂ (aq) ---> forms no precipitate
KNO₃ (aq) + CuCl₂(aq) ----> forms no precipitate
Li₂SO₄ (aq) + 2 AgNO₃ (aq) ----> 2 LiNO₃ (aq) + Ag₂SO₄ (s) (forms a precipitate)
NaClO₄ (aq) + CaCl₂ (aq) ----> forms no precipitate
Learn more about double replacement reactions at: https://brainly.com/question/23918356
#SPJ1
An empty flask's mass is 17.4916 g, its mass is 43.9616 g when filled with water at 20.0°C (density = 0.9982 g/mL). The density of "heavy water" at 20.0°C is 1.1053 g/mL. What is the mass of the flask when filled with heavy water at 20.0°C?
Answers
Answer:
0.24955Explanation:
17.4916g
43.9616g at 20.0°c
M= D÷V
M=V?
M=43.9616
-17.5916
007472
274700
The mass of the flask when filled with heavy water at 20.0°C is approximately 19.3525 grams.
To solve this problem, we can use the formula:
Mass = Volume × Density
Given that the density of water at 20.0°C is 0.9982 g/mL and the density of heavy water at 20.0°C is 1.1053 g/mL, we can calculate the volume of the flask by dividing its mass by the density of water:
Volume of flask = Mass of flask/Density of water
Volume of flask = 17.4916 g / 0.9982 g/mL
Volume of flask ≈ 17.5159 mL
Now that we know the volume of the flask, we can calculate the mass of the flask when filled with heavy water using the density of heavy water:
Mass of flask with heavy water = Volume of flask × Density of heavy water
Mass of flask with heavy water = 17.5159 mL × 1.1053 g/mL ≈ 19.3525 g
Learn more about mass calculation link is here:
brainly.com/question/33791791
#SPJ2
The following drawing is a representation of the exothermic reaction in which ozone forms dioxygen.
This reaction is likely to be nonspontaneous at all temperatures. nonspontaneous at low temperatures and spontaneous at high temperatures. spontaneous at low temperatures and nonspontaneous at high temperatures. spontaneous at all
Answers
The exothermic reaction is spontaneous at all temperature. That means at low temperature and also at high temperature the reaction is spontaneous.
In ozone layer when UV light passes, oxygen molecules are split up into their constituent oxygen atoms. These single atoms are then able to react with other oxygen molecules, forming ozone. It needs to absorb energy in order to occur. This also happens in reverse because ozone is so unstable and each ozone molecule soon splits into an oxygen molecule and an oxygen atom. This is an exothermic reaction. This means that ozone splitting results in heat which causes an increase in atmospheric temperature. Here solid is also getting converted to gas
So, spontaneity is increasing
ΔSo is positive
ΔHo is negative
ΔGo = ΔHo - T*ΔSo
Since ΔHo is negative and ΔSo is positive, ΔGo is always negative.
So, this reaction is spontaneous at all temperatures.
To learn more about Exothermic Reaction please visit:
https://brainly.com/question/2924714
#SPJ4
what is stastical thermodaynamics deal?
Answers
Explanation:
Statistical thermodynamics is a theory that uses molecular properties to predict the behavior of macroscopic quantities of compounds. ... From these energy-level data, a temperature-dependent quantity called the partition function can be calculated.
Answer:
the stastical thermodaynamics deal is essentially a theory that you can use the molecules and their properties in order to predict how the molecule will behave in a macroscopic quantities of compounds. Using this, you can also tell a temperature-dependent quantity called the partition function.
(I also didn't copy and paste this, so it's safe for you to use)
May I have brainliest please? :)
If the hydrogen ion concentration in the blood is above normal, which of the following might occur in order to reestablish homeostasis?
O Increase in tubular secretion of hydrogen ions (H).
O Decrease in ventilation rate.
O Plasma proteins ionize to release more H.
O Carbonic acid is broken down to bicarbonate and H.
Answers
If the hydrogen ion concentration in the blood is above normal, one of the ways to reestablish homeostasis is to Option A) increase the tubular secretion of hydrogen ions (H) in the kidneys.
This will help to remove excess H from the blood and bring the concentration back to normal levels. Another way to restore homeostasis is to increase the production of bicarbonate (HCO3-) in the body. This happens when carbonic acid is broken down to bicarbonate and H, which neutralizes the excess H in the blood and helps to restore the pH balance. However, decreasing the ventilation rate or having plasma proteins ionize to release more H would not be effective in bringing the hydrogen ion concentration back to normal levels.
Learn more about hydrogen ions here: brainly.com/question/20309096
#SPJ4
CHEMISTRY 100 POINTS MUST BE CORRECT



Answers
Under standard pressure, HOCH2CH2OH has the highest boiling point. This is due to the molecules' strong hydrogen bonds with one another.
Their boiling point is higher or is it CH3CH2OH?Because CH3CH2OH C H 3 C H 2 O H molecules may form hydrogen bonds with one another due to the presence of the hydroxyl (-OH) group, they will have a higher boiling point. With tiny molecules like the ones mentioned in this question, hydrogen bonds are the strongest intermolecular forces that can exist.
What types of forces exist between molecules in CH3CH2OCH2CH3?Similar in size to CH3CH2CH2CH3, CH3CH2OCH2CH3 exhibits dipole-dipole forces as well since it contains polar C-O bonds.
To know more about standard pressure visit:-
https://brainly.com/question/29129606
#SPJ1
Iron and Chlorine gas react according to the following balanced equation: 2 Fe(S) + 3 Cl2 (g) 2 FeCl3(s) a) Calculate the molar mass in grams of “one mole” of each of the following: Fe ________ Cl2 __________________ FeCl3 ______________
Answers
The molar mass in grams of "one mole" of each substance is:
Fe: 55.845 g/mol
\(Cl_2\): 70.906 g/mol
\(FeCl_3\): 162.204 g/mol
To calculate the molar mass in grams of "one mole" of each substance, we need to determine the atomic masses of the elements involved in the equation.
The atomic mass of iron (Fe) is 55.845 g/mol.
For chlorine (\(Cl_2\)), we need to consider that the molar mass of \(Cl_2\) is twice the atomic mass of chlorine because the formula shows that two chlorine atoms combine to form one molecule of \(Cl_2\). The atomic mass of chlorine is 35.453 g/mol, so the molar mass of \(Cl_2\) is 2 * 35.453 g/mol = 70.906 g/mol.
The formula for iron(III) chloride (\(FeCl_3\)) indicates that one mole of \(FeCl_3\)contains one mole of iron and three moles of chlorine. Therefore, we can calculate the molar mass of \(FeCl_3\)by summing the atomic masses of iron and chlorine:
Molar mass of \(FeCl_3\)= (1 * atomic mass of Fe) + (3 * atomic mass of Cl)
Substituting the values, we have:
Molar mass of \(FeCl_3\) = (1 * 55.845 g/mol) + (3 * 35.453 g/mol)
= 55.845 g/mol + 106.359 g/mol
= 162.204 g/mol
For more such questions on molar mass visit:
https://brainly.com/question/837939
#SPJ8
Calculate the molar it’s of a solution containing 29g of glucose dissolved in 24.0 g of water
Answers
Look at the diagram.
Which two structures are first to combine in translation?
1 and 4
2 and 3
3 and 4
1 and 2

Answers
The two structures are first to combine in translation are 3 and 4. Therefore, option C is correct.
What is translation ?The process in which the information encoded in the mRNA is used to direct the sequencing of amino acids, and therefore it finally synthesizes a protein is called as translation.
In translation the information passed from DNA to mRNA and turns it into a series of amino acids bound together with peptide bonds.
Translation is translation from one code of nucleotide sequence to another code of amino acid sequence.The two structures are first to combine in translation are 3 and 4.
Thus, option C is correct.
To learn more about translation, follow the link;
https://brainly.com/question/12463306
#SPJ1
sort the following numbers according to whether they should be rounded up or down when rounding to the nearest tenth (the first digit after the decimal). drag the appropriate items to their appropriate bins.
Answers
The numbers that will be rounded up are 124.77, 7.077, 19.45 and 3.179 and the numbers which will be rounded down are 4.318, 8878.2403, 66.9106 and 5.84.
To round off the decimal numbers to their nearest tenths, we look at the number on the hundredth place. If that particular number is greater than 5, we add 1 to the tenth value. If the number is less than 5, we leave the tenth place value as it is, and we also remove all the numbers which are present after the tenth’s place.
The numbers which will be rounded up are 124.77, 7.077, 19.45 and 3.179 since the digit on the hundredth place are greater than 5. The numbers which will be rounded down are 4.318, 8878.2403, 66.9106 and 5.84 since the digit on the hundredth place is lower than 5.
To know more about round off here
https://brainly.com/question/17214000
#SPJ4
Question 20 of 66
Which of the following species has the same number of protons as Fe3+?
A) Mn3+
B) Co2+
C) Fe2+
D) Nit
E) CO3+
Answers
If you have any questions please let me know.
Fe²⁺ has the same number of protons as Fe³⁺.
In order to know the number of protons of a species, we need to look for the atomic number (Z) in the Periodic Table.
The atomic number of Fe is 26, so it has 26 protons. Since protons are in the nucleus, they are not lost nor gained. Fe³⁺ results from Fe losing 3 electrons, but it has 26 protons as well.
Taking this into account, let's consider the number of protons of the following species:
A) Mn³⁺. It has 25 protons like Mn (Z = 25).
B) Co²⁺. It has 27 protons like Co (Z = 27).
C) Fe²⁺. It has 26 protons like Fe (Z = 26).
D) Ni. It has 28 protons (Z = 28).
E) Co³⁺. It has 27 protons like Co (Z = 27).
Fe²⁺ has the same number of protons as Fe³⁺.
You can learn more about atomic number here: https://brainly.com/question/17274608?referrer=searchResults

oxidation number of Ag in Ag2O
Answers
The oxidation number of Ag in Ag2O is +1.
In Ag2O, there are two silver atoms (Ag) and one oxygen atom (O). Oxygen is known to have an oxidation number of -2 in most compounds. Since the compound is neutral, the sum of the oxidation numbers of all the atoms must equal zero.
Therefore, the oxidation numbers of the two silver atoms must add up to +2 to balance out the -2 oxidation number of the oxygen atom. Since there are two silver atoms, each silver atom must have an oxidation number of +1 to yield a total oxidation number of +2 for the compound.
In Ag2O, the silver atoms lose one electron each to form Ag+ ions. This results in an oxidation number of +1 for each silver atom. The oxygen atom gains two electrons from the silver atoms to achieve a stable octet configuration, resulting in an oxidation number of -2 for the oxygen atom. The compound Ag2O is formed through the transfer of electrons, with each silver atom exhibiting an oxidation number of +1.
for such more questions on oxidation
https://brainly.com/question/13182308
#SPJ8
what is the PH scale of 0.02m of hydrochloric acid
Answers
Answer:
Explanation:
The pH of 0.02 M hydrochloric acid is approximately 1.7.
THANKS
IF THE ANSWER IS CORRECT , THEN MARK ME AS BRAINLIST
To determine the pH of a hydrochloric acid solution, we need to know its concentration. You mentioned a concentration of 0.02 M (molar), which refers to 0.02 moles of hydrochloric acid dissolved in 1 liter of solution.
Hydrochloric acid (HCl) is a strong acid that dissociates completely in water, meaning all HCl molecules release their hydrogen ions (H+) into the solution. Since the concentration is given as 0.02 M, it means there are 0.02 moles of H+ ions in 1 liter of the solution.
To calculate the pH, we can use the formula:
pH = -log[H+]
In this case, [H+] represents the concentration of hydrogen ions in moles per liter. Since hydrochloric acid is a strong acid and it dissociates completely, the concentration of hydrogen ions is equal to the concentration of HCl, which is 0.02 M.
pH = -log(0.02) ≈ 1.70
Therefore, a hydrochloric acid solution with a concentration of 0.02 M would have a pH of approximately 1.70, indicating it is strongly acidic.
Question 5 of 10Choose the correct statements.O A. Breaking bonds is exothermic.B. Making bonds is endothermic.C. Breaking bonds is endothermic.D. Making bonds is exothermic.

Answers
Explanation:
Breaking bonds
Energy is absorbed to break bonds.
Bond-breaking is an endothermic process.
--------------
Forming bonds
Energy is released when new bond forms.
Bond-making is an exothermic process.
Answer:
The correct statements are:
C. Breaking bonds is endothermic.
D. Making bonds is exothermic.
Dominic made the table below to organize his notes about mixtures. A 1-column table. The first column labeled properties of mixtures has entries has no set composition, must have more than one state of matter, must have more than one substance. What mistake did Dominic make? The title should read “Properties of Solutions” because some mixtures do not have all of the properties listed. There is a definite recipe to make each mixture, so the composition of a mixture is set. Although it is possible to have more than one state, it is also possible to have only one state. A single substance can be used to make a mixture if the substance is composed of more than one element.
Answers
Answer:
Exxplanation:
Answer:
Although it is possible to have more than one state, it is also possible to have only one state.
Explanation:
Question 1
Given the equation: Q = mcAT
Q = heat (in Joules)
m = mass (in grams)
C = 4.18 (specific heat capacity)
AT change in temperature (°C)
How many Joules of heat energy are absorbed when 200 grams of water are heated from 20 C to 60 C.

Answers
The amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C is 33,440 Joules.
To find the amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C, we can use the equation Q = mcAT.
First, we need to find the value of m, which is the mass of the water in grams. In this case, it is given as 200 grams.
Next, we need to find the value of AT, which is the change in temperature in degrees Celsius.
This can be calculated by subtracting the initial temperature from the final temperature, which gives us 60 C - 20 C = 40 C.
The specific heat capacity of water, C, is given as 4.18 Joules per gram per degree Celsius.
Now we can plug in the values into the equation:
Q = mcAT
Q = (200 g) x (4.18 J/g°C) x (40°C)
Q = 33,440 J
Therefore, the amount of heat energy absorbed when 200 grams of water are heated from 20 C to 60 C is 33,440 Joules.
for more such question on heat energy
https://brainly.com/question/25603269
#SPJ8
How does a dna molecule make a copy of itself
Answers
Answer:
See below
Explanation:
The process through which DNA replicates itself in the course of the cell division shall be DNA replication. There was a mistake. A 'Y' shape is generated by the separation of the two individual DNA strands called a 'field' replication. These two different strands act as models for the creation of new DNA strands
Hope this helps.
After extended stirring of a spatula sized portion of a solid drug in 100 mL of water, the drug appeared to be insoluble. Yet when the liquid was separated from the solid and heated, solid residue was observed forming as the liquid evaporated. This evidence suggests that the liquid portion of the mixture formed after stirring the drug with water was
Answers
Answer:
a saturated solution
Explanation:
A saturated solution is a solution that already contains just as much solute as it can normally hold at a given temperature.
When more solute is added to a saturated solution, the added solute does not dissolve.
The fact that when the liquid was separated and evaporated, some solid crystals were recovered means that the liquid has already dissolved the amount of solute that it can normally hold at that temperature. That is, the liquid is already a saturated solution hence more solute does not dissolve.
How many particles are in 67 grams of Oxygen?