_____ are bits and pieces of material that end up in a body of water.
Answers
Answer:
sediments
Explanation:
Related Questions
Which two solutions, when mixed together, will undergo a double replacement reaction and form a white, solid substance?
(1) NaCl(aq) and LINO3(aq)
(2) KCl(aq) and AgNO3 (29)
(3) KCl(aq) and LiCl(aq)
(4) NaNO3(aq) and AgNO3(aq)
Answers
\(AgNO_3 + KCl\) → \(AgCl + KNO_3\) undergo a double replacement reaction and form a white, solid substance. Hence, option B is correct.
What is a double replacement reaction?A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products.
Double-replacement reactions generally occur between substances in aqueous solution. In order for a reaction to occur, one of the products is usually a solid precipitate, a gas, or a molecular compound such as water.
\(AgNO_3 + KCl\) → \(AgCl + KNO_3\) undergo a double replacement reaction and form a white, solid substance.
Hence, option B is correct.
Learn more about the double replacement reaction here:
https://brainly.com/question/19267538
#SPJ1
The photograph shows a strip of land with no trees on the inside cl
river. Which action most directly caused this strip of treeless land to form?
A. Water weathered rock and carried the particles here.
B. Tree roots broke up rock into particles here.
C. Glacial ice lifted up the particles and carried them here.
D. Wind carried particles and deposited them here.
Answers
An action which most directly caused this strip of treeless land to form is that: A. water weathered rock and carried the particles here.
What is an erosion?An erosion can be defined as a geological process that involves the wearing out of rock layers, earthen materials (soil) and the transportation of these solid materials by agents of denudation such as:
WaterWindWhat is weathering?Weathering can be defined as process that involves both the physical and chemical breakdown of rock into smaller pieces or fragments called sediments.
Based on this photograph (see attachment) which shows a strip of land with no trees on the inside curve of a river, we can infer and logically deduce that an action which most directly caused this strip of treeless land to form is because water weathered rock and carried the particles here.
Read more on erosion here: brainly.com/question/15663829
#SPJ1

Answer:
A!!!
Explanation:
www 15. Using the idea of particles explain why: a. The smell of burnt food travels through the houses? b. When two solids are placed on top of each other, they do not mix? c. Pumping up your bike tyres gives a smooth ride? d. Smokers can cause lung damage in other people? e. Heating gas in a closed container will increase its pressure? f. Poisonous gasses from a factory chimney can affect a large area?explain it ?
Answers
a. The smell of burnt food travels through the houses- Because the particles are really heated up and have a lot of kinetic energy, which causes them to move quickly, the stench of burnt food permeates the entire house.
b. When two solids are placed on top of each other, they do not mix- Because they have clear boundaries and a definite shape, two solids stacked on top of one another do not mix.
c. Pumping up your bike tires gives a smooth ride- Because the tire's air pressure is what maintains it in place. Even though the tires appear to be firm when you push on them with your palm, they actually have a lot of give as the vehicle's weight shifts and it bounces around the road.
d. Smokers can cause lung damage in other people- The heart and blood arteries are impacted by secondhand smoke, which raises the risk of suffering a heart attack. The risk of developing and passing away from heart disease rises when people are exposed to secondhand smoke.
e. Heating gas in a closed container will increase its pressure- When gases in containers are heated, the average speed of their molecules increases. They consequently strike the container walls more frequently, more forcefully, and with greater force. Therefore, when the gas's temperature rises, it is under more pressure.
f. Poisonous gasses from a factory chimney can affect a large area- manufacturing chimney smoke, and power plant emissions all contribute to air pollution. Acids are created when poisonous gases, like sulfur dioxide, combine with rain and mist. A large area of plants are killed when acid rain occurs.
To learn more about characteristics of particles refer- https://brainly.com/question/16982523
#SPJ9
How many orbitals are described by each of the below combinations of quantum numbers? n = 3, ℓ =2 orbitals n = 4, ℓ = 2, mℓ = 2 orbitals
Answers
Orbitals that are described by each of the combinations quantum numbers ; n = 3, ℓ =2 orbitals n = 4, ℓ = 2 is 5. ml=2 here is referring to last(5th) orbital space 4d.
What is orbitals?In atomic theory and quantum mechanics, atomic orbital is a function describing the location and wave-like behavior of electron in an atom.
Quantum numbers (n, l, ml, ms )
n=3, refers to 3rd energy level
3rd energy level has 3 possible values of l and d has 5 different orientations.
s=0 p=1 d=2
--> -2,-1,0,1,2
When; n=3 l=2, then number of orbitals is 5.
n=4 refers to 4th energy level ;
4th level has 4 possible l values;
s=0 p=1 d=2 f=3
When, n=4 l=2 and ml=2
The number of orbitals is 5 and ml=2 is referring to last(5th) orbital space 4d.
To know more about orbitals, refer
https://brainly.com/question/20319149
#SPJ4
plese hurrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrrry
Use the food web below and your knowledge of science to answer the question below.
Group 4 Food Web
Hawks are predators to small organism such as snakes, rabbits, and mice. What is a possible result of adding a hawk to this ecosystem?
Question 2 options:
The rabbit population will increase.
The grass population will increase.
The cougar population will increase.
All of the above

Answers
Answer:
Pretty sure that’s correct
Explanation:
Answer: The answer is:
The grass population will increase.
Explanation: Didn't see anything that could be correct, so I chose this on the K12 test and it was correct.
Can someone please help me on this?

Answers
Answer:
c
Explanation:
please add me as brainliest
. Using the Bohr model, calculate the energy of a photon emitted when an electron in a Li2+ ion moves from an orbit with n=3 to the orbit with n=2.
Group of answer choices
A. 7.826×1038 J
B. 9.079×10-19 J
C. 3.2685×10-18 J
D. 2.724×10-18 J
Answers
The energy of the photon emitted when an electron in a \(Li^{2+\) ion moves from an orbit with n=3 to the orbit with n=2 is \(-1.93 * 10^{-18} J\) or \(2.724 * 10^{-18} J\). The correct option is D.
The energy of a photon emitted when an electron in a \(Li^{2+\) ion moves from an orbit with n=3 to the orbit with n=2 can be calculated using the Bohr model.
The equation used to calculate the energy of the emitted photon is given by
E = -13.6 eV (1/nf2 - 1/ni2)
Where E is the energy of the emitted photon in eV, nf is the final level of the electron, and ni is the initial level of the electron.
Substituting in the given values, we get
E = -13.6 eV (1/22 - 1/32)
Simplifying, this gives
E = -13.6 eV (9/4 - 1/9)
E = -13.6 eV (8/9)
E = -12.093 eV
Converting eV to joules, we get
E = -12.093 eV × \(1.602 * 10^{-19\) J/eV
E = \(-1.93 * 10^{-18} J\)
Therefore, the energy of the photon emitted when an electron in a \(Li^{2+\) ion moves from an orbit with n=3 to the orbit with n=2 is \(-1.93 * 10^{-18} J\) J or \(2.724 * 10^{-18} J\).
For more question on photon click on
https://brainly.com/question/15946945
#SPJ11
Bohr's model consists of a small nucleus (positively charged) surrounded by negative electrons moving around the nucleus in orbits. The correct answer is D. 2.724×10-18 J.
According to the Bohr model, the energy of a photon emitted or absorbed during a transition of an electron between two energy levels in an atom is given by:
ΔE = E_final - E_initial = - R_H (\(1/n_final^2\) - \(1/n_initial^2\))
where R_H is the Rydberg constant, n_final is the final energy level, and n_initial is the initial energy level.
For the given transition of an electron from n=3 to n=2 in a \(Li2+\) ion, the Rydberg constant for \(Li2\) + is 2.179 × \(10^{-18}\) J, so we have:
ΔE = - (2.179 × \(10^{-18}\)J) (\(1/2^2\) - \(1/3^2\))
ΔE = 2.724 × \(10^{-18}\) J
Therefore, the energy of the photon emitted during the transition is 2.724 × \(10^{-18}\) J.
Learn more about Bohr model here :
https://brainly.com/question/3964366
#SPJ11
THREE QUESTIONS ANSWER TWO Question 1 a) Determine the pulse duration of a periodic pulse train whose duty cycle is \( 15 \% \) and period is 115 nanoseconds.
Answers
The pulse duration of periodic pulse train with a duty cycle of 15% and a period of 115 nanoseconds is 17.25 nanoseconds.
Duty cycle = 15% or 0.15
Time period = 115 nanoseconds
The ratio of the amount of time the signal spends in the "on" state to its overall duration is known as the duty cycle. The signal is on for 15% of the entire period when the duty cycle is given as 15% in this instance. Duty cycles are a term used to represent the percentage of time that an electrical signal is active in a device, such as the power switch in a switching power supply, or when an organism, like a neuron, fires an action potential.
Calculating the duty cycle and the period of the pulse train -
Pulse duration = Duty cycle x Period
= 0.15 x 115
= 17.25
Read more about pulse train on:
https://brainly.com/question/30548054
#SPJ4
a rock is examined to determine its age, and the ratio of uranium-235 to lead-207 is found to be 125,000:875,000. the half-life of uranium-235 is 700 million years. how old is the rock being examined?
Answers
The old age of rock when examined is 21.4 million years if the ratio of uranium-235 to lead-207 is found to be 125,000:875,000. the half-life of uranium-235 is 700 million years.
Decaying EquationP = [P + D] (1/2) ^(t/t(1/2))
where,
P represent the present parent amount
D is the present daughter amount
t(1/2) is the half life time period
t is the actual age
We know that,
t = (log[(P+D) /P] / log2) × t(1/2) ----------(1)
Given,
P = 97.6
D = 2.1
Ratio of P and D can be calculated as
P/D = 97.6/2.1
= 46.476
By substituting all the values in eq(1), we get
t = [(log 46.476 +1)/log2] × t(1/2)
Given,
The half life of U —Pb decay is 700 million years.
So,
t = [(log 46.476 +1)/log2] × 700
t = 21.4 million years.
Thus, we calculated that the the old age of rock when examined is 21.4 million years.
learn more about Half life period:
https://brainly.com/question/20309144
#SPJ4
DISCLAIMER:
The given question is misprint on portal.
Here is the correct form of question:
A rock is examined to determine its age, and the ratio of uranium-235 to lead-207 is found to be 125,000:875,000. the half-life of uranium-235 is 700 million years.
How old is the rock if it contains Uranium-235/ lead-207 ratio of 97.6 to 2.1?
how to tell the difference between ionic and covalent bonds
Answers
Comparing the electronegativities of the two elements is one method of predicting the type of bond that will form between them.
Ionic bonds are produced between atoms of metals and non-metals where the metal loses an electron to complete its octet and the non-metal acquires that electron to complete its octet. Covalent bonds are formed when two atoms share electrons to complete their octets.
Ionic chemicals are bound together by ionic bonds, whereas covalent compounds are held together by strong covalent bonds. While covalent molecules are normally insoluble in water, ionic compounds are. Additionally, covalent molecules are typically more flammable than ionic ones.
If the electronegativity of the two atoms differs by enough to allow one to totally draw an electron away from the other, the connection is ionic.
To know more about covalent bond:
https://brainly.com/question/32676803
#SPJ4
What is the volume in liters of 423 grams of SO2
Answers
ANSWER
The volume of SO2 is 147.89L
STEP-BY-STEP EXPLANATION:
Given information
\(\text{The volume of SO}_2\text{ is 423 grams}\)Step 1: Find the mole of SO2 using the below formula
\(\text{mole = }\frac{\text{ mass}}{\text{ molar mass}}\)Recall, the molar mass of SO2 is 64.066 g/mol
\(\begin{gathered} \text{mole = }\frac{423}{64.066} \\ \text{mole = 6.603 moles} \end{gathered}\)Recall that, 1 mole of gas is equivalent to 22.4L at STP
Let x represents the volume of SO2
\(\begin{gathered} 1\rightarrow22.4L_{} \\ 6.603\rightarrow\text{ x} \\ \text{cross multiply} \\ 1\text{ }\times x\text{ = 6.603 }\times22.4 \\ x\text{ = 147.89L} \end{gathered}\)Hence, the volume of SO2 is 147.89L
balance this
C3H3N + O2 ⇒ C + H2O + N2
Answers
Answer:
4C₃H₃N + 3O₂ → 12C + 6H₂O + 2N₂
Explanation:
The given reaction equation is expressed as:
C₃H₃N + O₂ → C + H₂O + N₂
The problem here entails balancing of the chemical reaction.
So;
assign letters a,b,c,d and e as coefficients that will balance the expression:
aC₃H₃N + bO₂ → cC + dH₂O + eN₂
Conserving C : 3a = c
H : 3a = 2d
N : a = 2e
O: 2b = d
let a = 1, c = 3, e = \(\frac{1}{2}\) , d = \(\frac{3}{2}\) , b = \(\frac{3}{4}\)
Multiply through by 4;
a = 4, b = 3, c = 12 , d = 6 and e = 2
4C₃H₃N + 3O₂ → 12C + 6H₂O + 2N₂
What makes lava flow in the manner that it does
Answers
Answer:
Lava flows are streams of molten rock that pour or ooze from an erupting vent. Lava is erupted during either nonexplosive activity or explosive lava fountains. ... But when basalt lava flows are confined within a channel or lava tube on a steep slope, the main body of the flow can reach velocities >30 km/h (19 mph).
Explanation:
a 50 g sample of ice at 0 c is placed in a glass beaker that contains 200g of water at 20 c. a. how much heat is needed to melt the ice? b. by how much would the temperature of the water change if it gave up that much heat to the ice? c. what will be the final temperature of the mixture (assume that the glass is a closed system)?
Answers
a. To melt the ice 16,700 joules of heat is required at 20 °C.
b. The temperature changes by 20 °C.
c. If we assume that the glass is a closed system the final temperature of the mixture will be 16.7°C.
a. The heat required to melt the ice can be determined using the following formula:
Q = mL,
where m is the mass of ice and L is the latent heat of the fusion of ice.
The mass of the ice, as stated in the question, is 50g.
The latent heat of the fusion of ice is 334 J/g.
Q = 50g × 334 J/g = 16700 J
Therefore, it would take 16,700 joules of heat to melt the ice.
b. Let us determine the change in temperature of the water.
The quantity of heat that the water at 20°C would lose is 16,700 joules. Using the following formula, we can calculate the change in temperature:
Q = mcΔT,
where m is the mass of the water, c is the specific heat of water, and ΔT is the change in temperature of the water.
The mass of the water is 200 g.
The specific heat of water is 4.18 J/g.°C.
ΔT can be calculated as:
Q = mcΔT
ΔT = Q/mc
ΔT = 16700J/(200g × 4.18 J/g.°C)
ΔT = 20.0°C
Hence, the temperature of the water would drop by 20.0°C.
c. The final temperature of the mixture (assuming that the glass is a closed system)
Since the ice has been melted and the water temperature has dropped by 20.0°C, the heat that was used to melt the ice has now been distributed over a larger volume of water.
The heat lost by the water (200 g) is the same as the heat gained by the ice (50 g).
Therefore,200g × (20.0°C) = 50g × Lf + 50g × (final temperature)
Given that the latent heat of the fusion of ice is 334 J/g, we can solve for the final temperature of the mixture:
1000°C = 167 J/g + 50g × (final temperature)50g × (final temperature)
= 1000°C - 167 J/g50g × (final temperature)
= 833J/g
Therefore, the final temperature of the mixture is (833J/g) / 50g = 16.7°C.
To learn more about temperature change refer - https://brainly.com/question/14854725
#SPJ11
Helpp! 100 POINTS!
What is the hydroxide ion concentration and the pH of 0.035 M Sr(OH)2 at 25 °C?
Answers
Answer:
So the pH of a 0.035 M Sr(OH)2 solution at 25 °C is approximately 12.85.
Explanation:
The dissociation of Sr(OH)2 in water can be represented as:
Sr(OH)2 (s) → Sr2+ (aq) + 2OH- (aq)
Since each formula unit of Sr(OH)2 produces two hydroxide ions, the hydroxide ion concentration ([OH-]) can be calculated as follows:
[OH-] = 2 × 0.035 M = 0.07 M
To calculate the pH, we can use the following equation:
pH = 14 - pOH
where pOH is the negative logarithm of the hydroxide ion concentration:
pOH = -log[OH-] = -log(0.07) = 1.15
Therefore,
pH = 14 - pOH = 14 - 1.15 = 12.85
So the pH of a 0.035 M Sr(OH)2 solution at 25 °C is approximately 12.85.
Sr(OH)2 (s) → Sr2+ (aq) + 2 OH- (aq)
Thus, the concentration of hydroxide ions in a 0.035 M solution of Sr(OH)2 is:
[OH-] = 2 × 0.035 M = 0.070 M
To find the pH of this solution, we can use the relationship between pH and the concentration of hydroxide ions:
pH = -log[H+]
Since the solution is basic, we know that the concentration of hydrogen ions is very small compared to the concentration of hydroxide ions, and we can assume that the concentration of water is constant at 55.5 M:
Kw = [H+][OH-] = 1.0 × 10^-14
Solving for [H+] gives:
[H+] = Kw / [OH-] = 1.0 × 10^-14 / 0.070 = 1.43 × 10^-13 M
Taking the negative logarithm of this value gives the pH:
pH = -log[H+] = -log(1.43 × 10^-13) = 12.85
Therefore, the hydroxide ion concentration of the 0.035 M Sr(OH)2 solution is 0.070 M, and the pH of the solution is 12.85 at 25 °C.
____ is the amount of matter in an object. Question 1 options: Matter Gravitational Pull Mass Weight
Answers
Answer:
It's Mass
Explanation: I took the quiz
Have a good day!
Mass is the amount of matter in an object. Therefore, option C is correct.
What is mass?Mass can be defined as an intrinsic property of a body. mass can be described as the quantity of matter in a body. Different atoms and different particles with the same amount of matter have nonetheless different masses.
Mass can be experimentally measured by the body's inertia, meaning the resistance to acceleration when a net force is applied. The mass determines the strength of its gravitational attraction to other bodies.
The SI base unit of measurement of mass is the kilogram (kg) and it is not the same as weight. Mass is generally determined by measuring the weight using a spring scale, rather than a balance scale and comparing it to known masses.
An object on the Moon will weigh less than it does on Earth due to the lower gravity but still have the same mass. Weight can be defined as a force, while mass is the property that determines the strength of this force.
Learn more about mass, here:
https://brainly.com/question/13040516
#SPJ6
David noticed that when he ate chocolates they melted in his hands faster when his hands were slightly wet from sweat than his hands were dry. He was curious to see if other liquids would affect how fast they would melt? He decided to test out four different liquids, water, milk, lemon-lime soda, and orange juice. He then timed how long it took them to melt. He thought the chocolate would melt faster in orange juice that the other liquids because it is more acidic. The results are below.
QUESTIONS:
1) What was David's Question?
2) What was David Hypothesis?
3) What his independent Variable?
4) What his Dependent variable?
Answers
Answer:
1. What liquid would melt the chocolate faster?
2. He thought that the orange juice would melt the chocolate faster.
3. The independent variable is the liquids.
4. The dependent variable is the chocolate.
Explanation:
1. He was curious to see if other liquids would affect how fast they would melt? This is his question.
2. He thought the chocolate would melt faster in orange juice than the other liquids because it is more acidic.
He made a prediction or a hypothesis.
3. His independent and dependent variables are chocolate and liquid. Since he wants to know how fast the chocolate melts in different liquids, how fast the chocolate melts is dependent on the liquid that he uses.
Name the type of equation then balance. Sn,(PO2)2 + Fe(CIO): - Sn(CIO3)2 + FePO,
Answers
Answer:
ionic compond
Explanation:
Sn(ClO3)4
Directions: Answer the following questions in your own words using complete sentences. Do not copy and paste from the lesson or the internet.
1. How are the forest biomes classified?
2. Describe each of the forest biomes.
3. Name some environmental concerns about the forest biomes?
4. What is one main contribution forests make to the environment?
5. Conduct research into one forest biome and identify a particular forest. Identify any environmental issues connected to your forest biome. Are there ways to solve some of those environmental concerns?
Answers
The supporting initiatives that focus on reforestation and restoration can help restore damaged areas and promote the resilience of the forest ecosystem.
Forest biomes are classified based on their geographical location, climate, and dominant plant species. The main classifications of forest biomes include tropical rainforests, temperate forests, and boreal forests.
Tropical rainforests are found in regions near the equator and are characterized by high temperatures, abundant rainfall, and a dense canopy of tall trees. They are known for their incredible biodiversity and complex ecosystems.
Temperate forests are found in regions with moderate climates and distinct seasons. They have a mix of deciduous and evergreen trees, and their foliage changes color in autumn. These forests support a wide range of plant and animal species.
Boreal forests, also known as taiga, are found in subarctic regions and are characterized by long, cold winters and short summers. They consist mainly of coniferous trees like spruce, pine, and fir. Boreal forests are essential for regulating global climate and support unique wildlife adapted to harsh conditions.
Some environmental concerns about forest biomes include deforestation, habitat loss, biodiversity loss, climate change, and illegal logging. Deforestation, primarily driven by human activities such as agriculture, logging, and infrastructure development, leads to the destruction of forests and the loss of wildlife habitats. This loss of biodiversity can have far-reaching ecological consequences.
Climate change also poses a threat to forest biomes, as it can alter precipitation patterns, increase the frequency of wildfires, and disrupt ecosystems. Illegal logging exacerbates these issues by contributing to deforestation and forest degradation.
One main contribution forests make to the environment is their role in carbon sequestration. Forests absorb carbon dioxide from the atmosphere through photosynthesis and store it in their biomass and soil. This helps mitigate climate change by reducing the concentration of greenhouse gases in the atmosphere.
The Amazon Rainforest, located in South America, is a prime example of a forest biome. It is the largest tropical rainforest in the world and plays a crucial role in global climate regulation and biodiversity conservation. However, the Amazon Rainforest faces significant environmental issues, including deforestation, illegal logging, and land conversion for agriculture.
Deforestation in the Amazon is primarily driven by the expansion of agricultural activities, such as cattle ranching and soybean production. This results in habitat loss for countless plant and animal species, including endangered ones. The clearing of land also releases large amounts of stored carbon into the atmosphere, contributing to climate change.
Solving the environmental concerns in the Amazon Rainforest requires a multi-faceted approach. It involves implementing stricter regulations and enforcement against illegal logging and land encroachment, promoting sustainable agricultural practices, supporting local communities, and increasing international efforts to protect and conserve this vital ecosystem.
For more such questions on ecosystem
https://brainly.com/question/26046675
#SPJ11
would deviations from the ideal gas law be observed for gaseous nitrogen at 180 gpa and room temperature? a no, because both temperature and pressure must increase before such deviations are observed b no, because nitrogen molecules are symmetrical and do not interact with each other
Answers
Deviations from the ideal gas law be observed for gaseous nitrogen at 180 gpa and room temperatureA. No, because both temperature and pressure must increase before such deviations are observed.
Deviation from the ideal gas law can be observed at high pressure and low temperature, or low pressure and high temperature. Therefore, at 180 GPa and room temperature, deviations from the ideal gas law may be observed for gaseous nitrogen. The deviation is caused by the intermolecular interactions between gas molecules, which become more significant at high pressure. Therefore, option A is incorrect, and option B is also incorrect because it incorrectly suggests that nitrogen molecules do not interact with each other, which is not true.
Learn more about ideal gas here:
https://brainly.com/question/28257995
#SPJ11
Calculate the average atomic mass of an element if an isotope with a mass of 55 amu has a natural abundance of 15% and an isotope with a mass of 56 amu has a percentage abundance of 85%. Please show your work and include appropriate units with your answer.
Answers
Answer:
Average atomic mass = F
1
M
1
+F
2
M
2
+...+F
n
M
n
Where F
1
,F
2
,...F
n
is the fraction representing the natural abundance of the isotope and M is the weight of the isotope.
Avg. At. Mass = (0.85×11)+(0.10×12)+(0.05×13)
= 9.35+1.2+0.65
Therefore, the average atomic mass = 11.2
help me plsssssssssssss

Answers
Habitat loss due to the increasing human
population has caused
A. the number of extinctions to decrease
B. reductions in endangered species
C. increased biodiversity
D. decreased biodiversity
Answers
Answer:
D. decreased biodiversity
Explanation:
Habitat loss due to the increasing human
population has caused decreased biodiversity
Another way of writing 0.00000731 g
Answers
Answer:
nanograms - 7310
kilograms - 7.310000000000001e-9
What are two qualities of nonmetals? Describe each
Answers
Answer:
In the elemental form, non-metals can be gas, liquid or solid. They aren't shiny (lustrous) and they don't conduct heat or electricity well. Usually their melting points are lower than for metals, although there are exceptions. The solids usually break easily, and can't bend like metals
Explanation:
A substance with a definite volume and a definite shape is classified as a what?
Answers
Answer:
Solids
Explanation:
It can be found
Solid
solid: Has a definite shape and volume. liquid: Has a definite volume, but take the shape of the container. gas: Has no definite shape or volume.
Zelda noticed a puddle outside her front door. She saw that the puddle got smaller every day, until the 3rd day when it was completely gone. The next week, she noticed the puddle again. This time the puddle was gone the next day. Since the sun was out the second week but not the first week, Zelda hypothesized that the heat from the sun was the reason for the water evaporating at a faster rate. If she were to set up two containers with equal amounts of water, what would be the best way for Zeldato test her hypothesis\
Answers
Answer: Zelda should place one container of water in sunlight (by a window or outdoors) and the other container in a dark room (closet) away from the sun.
Explanation: This would allow Zelda to test two different settings (sun and no sun) so she can test her hypothesis.
An IV is infusing at 120 mL/h. The concentration in the IV bag is 20 mg in 200 mL NS. What is the dosage rate in mg/min?
Answers
Answer:
0.2 mg/min
Explanation:
The IV bag is 20 mg/ 200 ml or 0.1 mg/ml
The rate is 120 ml/hr or 120 ml/hr x 0.1 mg/ml = 12 mg/hr
which is 12 mg/ 60 min = 0.2 mg/min
Select all the names that could be used to describe the figure
A parallelogram
B quadrilateral
c rhombus
D square
1017 Answered
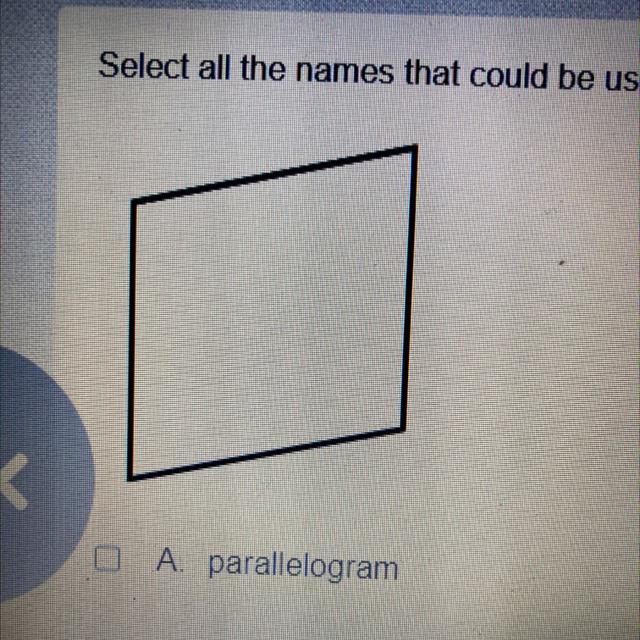
Answers
Answer:
Quadrilateral because there is no parallel and equal
Predicting Reaction Products
Balance the equations and predict the products for the following reactions:
Please help, my teacher never taught us this and it’s due today!
Will mark brainiest and give a lot of points!

Answers
The result of a chemical reaction or a process stage is a product (reaction product). a component of the reaction or mechanism stage that is not a substrate, reactant, reagent, beginning substance, or starting combination.
What is meant by reaction product?The organisms that result from chemical interactions are called products. Reactants undergo a high energy transition stage during a chemical process before becoming products. The reactants are consumed as a result of this process. It can occur spontaneously or be facilitated by catalysts, which reduce the energy of the transition state, and solvents, which provide the favorable molecular conditions for the reaction to occur.
Even in the case of reversible reactions, it is customary to draw products on the right side of chemical equations. Several characteristics of a chemical reaction, such as whether the reaction is exergonic or endergonic, are determined by the properties of products, such as their energies.
Learn more about reaction product
https://brainly.com/question/22616533
#SPJ1