Are fossil fuels natural or synthetic?
Answers
Answer:
Fossil fuels are made naturally, however they are considered non-renewable since they take such a long time to form.
Related Questions
7) The mass of an electron is approximately equal to 1/1368 of the mass of
A) a positron
B) a proton
C) an alpha particle
D) a beta particle
Answers
Answer:B
Explanation:
The end of life for a group of organisms, usually a species, is called a
Answers
Answer:
Extinction
Explanation:
Which of the following is not a racquet sport?
A. Badminton
B. Volleyball
C. Table tennis
D. Squash
Answers
Answer:
Volleyball
Explanation:
A P E X :)
Volleyball is not a racquet sport. Thus, the correct option is B.
What are Sports?Sports may be defined as any physical activity that involves physical endeavor and skill in which an individual or team participates against another or others for entertainment. Sports are good for physical as well as mental health of an individual.
The dimensions of a badminton racquet have a maximum length of 680mm, while its overall width is permitted to be 230mm.
The dimensions of a table tennis racquet have an average of length 17 cm., a width of 15 cm., and an overall length ranging between 240 to 260mm.
The dimensions of a squash racquet have a maximum dimension of 686mm in length, while 215mm in width. It has a maximum stung area of 500 square centimeters.
Therefore, Volleyball is not a racquet sport. Thus, the correct option is B.
To learn more about Sports, refer to the link:
https://brainly.com/question/1744272
#SPJ2
A scientist has two substances that she is testing in her lab: a pink substance and a green substance. At room temperature, both substances are liquids. The scientist transferred the same amount of energy into both substances. She finds only the pink substance changed phase. How is the pink substance different from the green substance?.
Answers
The pink substance different from the green substance because the pink solution is thought to have a lesser intermolecular force than the green solution, which causes molecules to disperse as a result.
Consider the attraction between the molecules of the pink substance to be weaker than that of the molecules of the green substance. Each of its molecules separates from the others. It's important to take into account the following data:
In comparison to the green substance, the attraction between the molecules in the pink substance is thought to be less. It moves apart from one another and changes from liquid to gas.
So, we can say that the pink solution is thought to have a less intermolecular force than the green solution, which leads to the dispersion of molecules.
To learn more about Dispersion of molecules, here :
https://brainly.com/question/4301021?referrer=searchResults
#SPJ4
An ocean, a forest and a grassy meadow are each examples of a complete ecosystem. Complete ecosystems only contain:
a
animals
b
living and nonliving things
c
rocks and water
d
populations of plants and animals
Answers
Answer:
b living and non living things. If we look at the question the ocean is non living but the forest and grassy meadow are examples of living things
Nitrogen monoxide + oxygen → nitrogen dioxide.
How do.i write that in skeleton equation
Answers
NO + O2 —> NO2
if a solution containing 33.17 g of lead(ii) perchlorate is allowed to react completely with a solution containing 8.564 g of sodium sulfate, how many grams of solid precipitate will be formed?
Answers
18.3 g of solid precipitate formed during the reaction.
Formula of lead(ii) perchlorate = \(Pb(ClO4)_{2}\)
Formula of sodium sulfate = \(Na_2(SO_4)\)
Reaction of lead(ii) perchlorate and sodium sulfate
\(Pb(ClO4)_{2}\) + \(Na_2(SO_4)\) ----------> \(PbSO_4(s)\) + \(2Na(ClO4)_{2}\)
moles of \(Pb(ClO4)_{2}\) = \(\frac{given mass}{ Molar mass}\)
molar mass of \(Pb(ClO4)_{2}\) =207+(35.5+ 16*4)*2
molar mass of \(Pb(ClO4)_{2}\) =207+ 99.5*2
molar mass of \(Pb(ClO4)_{2}\) =406
given mass = 33.17g
moles of \(Pb(ClO4)_{2}\) = \(\frac{given mass}{ Molar mass}\)
moles of \(Pb(ClO4)_{2}\) = \(\frac{33.17}{406}\)
moles of \(Pb(ClO4)_{2}\) = 0.0817 moles
molar mass of \(Na_2(SO_4)\) = 2*23+ 32+ 4*16
molar mass of \(Na_2(SO_4)\) = 46+32+64
molar mass of \(Na_2(SO_4)\) = 142
moles of \(Na_2(SO_4)\) = \(\frac{given mass}{ Molar mass}\)
moles of \(Na_2(SO_4)\) = \(\frac{8.564}{142}\)
moles of \(Na_2(SO_4)\) = 0.0603 moles
since in the above reaction \(Pb(ClO4)_{2}\) is the solid precipitate so we have to find the weight of \(PbSO_4(s)\) formed after the reaction.
Given that the reaction is complete so one of the 2 reactant must completely ends since \(Na_2(SO_4)\) have less number of moles than \(Pb(ClO4)_{2}\) so , \(Na_2(SO_4)\) will disappear and hence according to Stoichiometry same number of moles of \(PbSO_4(s)\) will form
so number of moles of \(PbSO_4(s)\) formed = 0.0603
so, the weight of \(PbSO_4(s)\) formed = number of moles formed * molar mass of \(PbSO_4(s)\)
so weight of \(PbSO_4(s)\) formed =0.0603 * 303
weight of \(PbSO_4(s)\) formed= 18.27 g ≈ 18.3 g
To know more about solid precipitate click on link below:
https://brainly.com/question/11194650#
#SPJ4
Which features are created by groundwater erosion and deposition? Check all that apply. stalagmites sinkholes rills stalactites gullies rivers
Answers
Answer:
Stalagmites
Sinkholes
Stalacites
Explanation:
These features are created because the ground water dissolves solid rocks and move the solid rocks, dissolve solutions beneath the ground water gradually and therefore enlarging the cracks, which eventually form a cave. Ground water carries minerals which are then deposited and can form stalagmites or stalactites. If a stalactite and stalagmite combine together, they usually form a column.
Answer:
a,b,d
Explanation:
join monkee kind ditch humanity :D
determine the ph change when 0.085 mol hi is added to 1.00 l of a buffer solution that is 0.392 m in hclo and 0.356 m in clo-.
Answers
The pH change for the given acidic buffer solution is 0.22.
HClO and ClO- is an acidic buffer, and with addition of HI, the concentration of acid increases while the concentration of salt decreases.
pH=pKa +log (salt/acid)
=7.54+log (0.326/0.369)
=7.54-0.05
=7.49
On adding Hydrogen iodide,
pH=pKa+ log((0.326-0.085)/(0.369+0.085))
=7.54+log (0.241/0.454)
=7.54-0.27
=7.27
Thus, the value of pH change cab be calculated as follows:
=7.27-7.49
=0.22
To learn more about pH check the link below:
https://brainly.com/question/172153
#SPJ4
given 10g samples of licl, libr, lif, and lii put them in order from least to greatest numbers of atoms of lithium
Answers
The number of atoms of lithium in a compound depends on the chemical formula and the molar mass of each compound. To determine the number of atoms of lithium, we need to calculate the number of moles of each compound and then multiply it by Avogadro's number, which represents the number of atoms in one mole of substance.
Let's calculate the number of moles for each compound:
1. LiCl: The molar mass of LiCl is approximately 42.39 g/mol. Therefore, the number of moles can be calculated as 10 g / 42.39 g/mol = 0.236 moles.
Now, let's calculate the number of atoms of lithium for each compound by multiplying the number of moles by Avogadro's number (6.022 x 10^23 atoms/mol):
1. LiCl: 0.236 moles x (6.022 x 10^23 atoms/mol) = 1.42 x 10^23 atoms.
2. LiBr: 0.115 moles x (6.022 x 10^23 atoms/mol) = 6.93 x 10^22 atoms.
3. LiF: 0.385 moles x (6.022 x 10^23 atoms/mol) = 2.32 x 10^23 atoms.
4. LiI: 0.075 moles x (6.022 x 10^23 atoms/mol) = 4.52 x 10^22 atoms.
To know more about that atoms visit:
https://brainly.com/question/12500676
#SPJ11
A chemist titrates 190 ml of. 2412 nitrous acid solution with. 377 M KOH solution. Calculate the ph at equivalence. The pKa of nitrous acid is 3. 35
Answers
The equivalency solution has a pH of 2.624.
What is the procedure for making nitrous acid?Nitrous acid is frequently created by adding a mineral acid to aqueous sodium nitrite solutions. Typically, acidification is carried out at ice-cold temperatures, and HNO2 is consumed on-site. Nitrous acid in its free form is unstable and breaks down quickly.
In a neutralization process, weak nitrous acid (HNO2) reacts with strong basic KOH.
HNO2 + KOH → KNO2 + H2O
Then, we determine how many moles of KOH were used:
volume KOH x concentration equals moles KOH. KOH
moles KOH = 0.190 L x 0.377 mol/L
moles KOH = 0.07153 mol
Next, we calculate the initial concentration of HNO2:
concentration HNO2 = moles HNO2 / volume HNO2
concentration HNO2 = 0.07153 mol / 0.190 L
concentration HNO2 = 0.3765 M
[HNO2] = 0.5 x 0.3765 M
[HNO2] = 0.1883 M
The following equation can be used to model how nitrous acid dissociates in water:
HNO2 + H2O ⇌ H3O+ + NO2-
The following equation relates the pKa to the acid dissociation constant, Ka, for this reaction:
pKa = -log Ka
So we can find the Ka value from the given pKa:
pKa = -log Ka
3.35 = -log Ka
Ka = 10⁻³
Ka = 4.47 x 10⁻⁴
The relationship shown below is true for the concentrations of the species involved at equilibrium:
Ka = [H3O+][NO2-] / [HNO2]
Ka = [H3O+][NO2-] / [HNO2]
Ka = [H3O+] [HNO2]
Solving for [H3O+], we get:
[H3O+] = Ka / [HNO2]
[H3O+] = (4.47 x 10⁻⁴) / (0.1883 M)
[H3O+] = 0.002374 M
Finally, we can calculate the pH of the solution:
pH = -log[H3O+]
pH = -log(0.002374)
pH = 2.624
To know more about nitrous acid solution visit:-
https://brainly.com/question/17011556
#SPJ1
Which is a greater mass, 0.25 moles of carbon dioxide, CO2, or 1.5 x 1023 particles of carbon monoxide, CO?
Answers
0.25 moles of carbon dioxide has a greater mass which is equal to 11g.
There is a formula that reads,
"Mass = molecular mass × moles"
The molecular mass of carbon dioxide is 44 and remains constant.
Plugging in the equations we get,
Mass = 44 × 0.25
Mass of carbon dioxide = 11g.
Again, for mass of carbon monoxide, we get;
1 mole CO contains 6.022 × \(10^{23}\) molecules of CO.
So, mass of 6.022 × \(10^{23}\) molecules of CO = 28 g.
Therefore, mass of 1.5 × \(10^{23}\) molecules of CO,
(1.5×\(10^{23}\) ×28) / (6.022×\(10^{23}\)) = 6.97g ≈ 7g
Therefore, 0.25 moles of carbon dioxide has a greater mass.
Learn more about carbon dioxide here;
https://brainly.com/question/22530423
#SPJ9
HELP MEEE PLEASEEEEEEEEEEEEEE
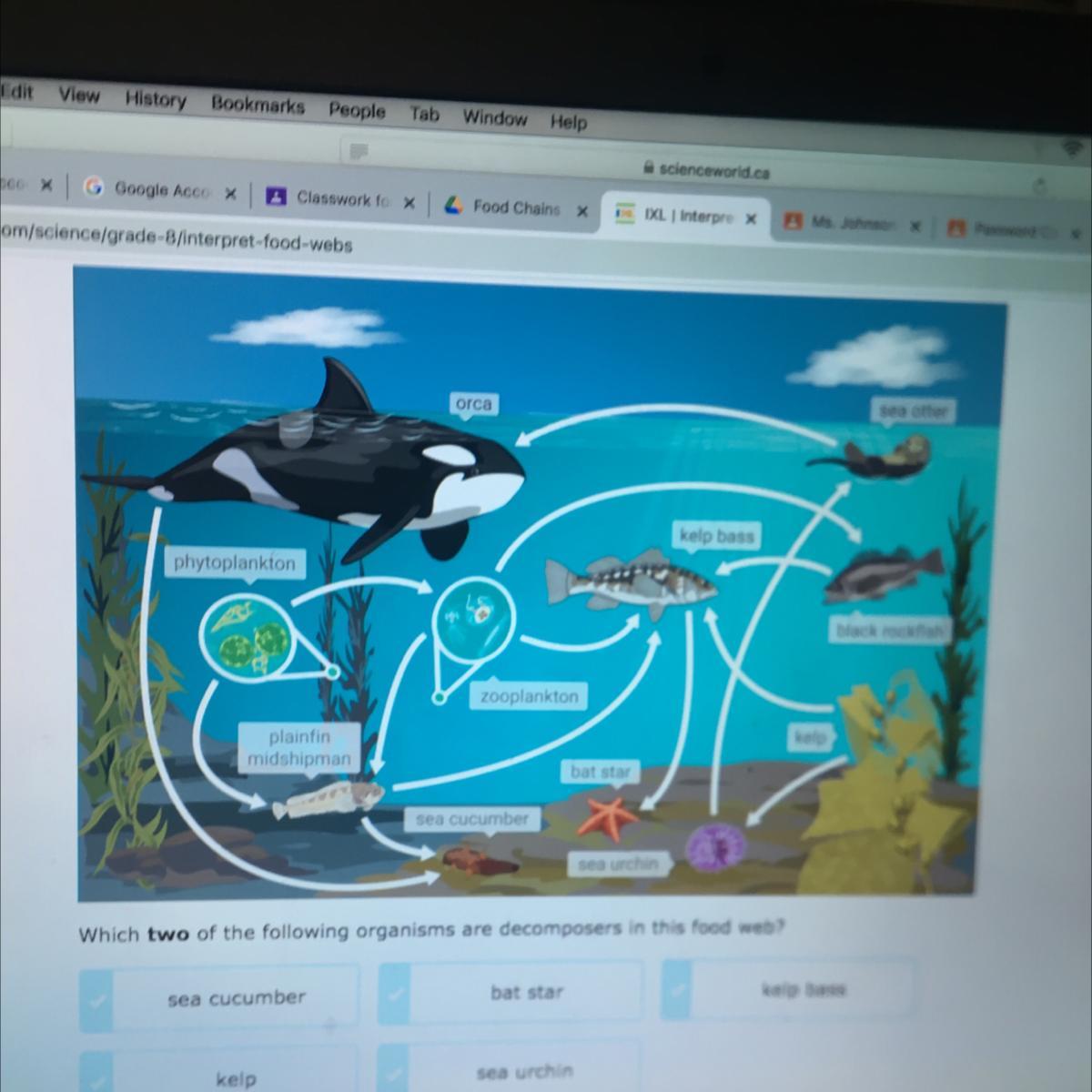
Answers
I think its the sea cucumber and sea urchin
but it could be the star too
The direct rays of the sun slowly move from 23.5° south to the
equator during what season?
Answers
Answer:
in December, so winter is the season
How many liters of cl2 gas at a pressure of 0.950 atm and a temperature of 298 k will
be collected from the reaction of 25.0 ml of a 0.100 m aqueous solution of kmno4 and
an excess of hcl?
show the steps please !
Answers
To calculate the volume of chlorine gas (Cl2) collected, we need to use the ideal gas law equation: PV = nRT.
Given:
Pressure (P) = 0.950 atm
Temperature (T) = 298 K
Volume (V) = ?
Concentration of KMnO4 solution = 0.100 M
Volume of KMnO4 solution = 25.0 mL
First, we need to find the moles of KMnO4 used in the reaction:
moles of KMnO4 = concentration × volume
moles of KMnO4 = 0.100 M × (25.0 mL / 1000 mL) [converting mL to L]
moles of KMnO4 = 0.0025 mol
The balanced chemical equation for the reaction between KMnO4 and HCl is:
2 KMnO4 + 16 HCl → 2 KCl + 2 MnCl2 + 8 H2O + 5 Cl2
From the balanced equation, we can see that 2 moles of KMnO4 react to produce 5 moles of Cl2. Therefore, the moles of Cl2 produced would be:
moles of Cl2 = (5/2) × moles of KMnO4
moles of Cl2 = (5/2) × 0.0025 mol
moles of Cl2 = 0.00625 mol
Now we can use the ideal gas law equation to find the volume of Cl2 gas:
PV = nRT
V = (nRT) / P
V = (0.00625 mol × 0.0821 L·atm/(mol·K) × 298 K) / 0.950 atm
V ≈ 0.150 L or 150 mL
Therefore, approximately 150 mL of Cl2 gas will be collected.
To calculate the volume of Cl2 gas, we first determine the moles of KMnO4 used by multiplying the concentration by the volume of the solution. Using the balanced equation, we then find the moles of Cl2 produced from the moles of KMnO4. Next, the ideal gas law equation (PV = nRT) is applied, rearranged to solve for volume (V). Plugging in the known values of moles (0.00625 mol), gas constant (0.0821 L·atm/(mol·K)), pressure (0.950 atm), and temperature (298 K), we can calculate the volume of Cl2 gas to be approximately 0.150 L or 150 mL.
Learn more about chlorine gas here:
https://brainly.com/question/18981083
#SPJ11
What structure controls the passage of substances into and out of a cell?
Answers
A mixture consists of substances that are physically blended but not chemically combined.
a. True
b. False
Answers
a. True A mixture consists of substances that are physically blended together but not chemically combined.
In a mixture, the individual substances retain their own chemical properties and can be separated by physical means such as filtration, distillation, or evaporation. Mixtures can be homogeneous (uniform composition throughout) or heterogeneous (non-uniform composition). Unlike in a chemical compound, where the elements are chemically bonded together in a fixed ratio, in a mixture, the substances retain their individual identities.
To learn more about Mixtures, visit:
https://brainly.com/question/12160179
#SPJ11
Se ard 25g de magneziu de puritate 90%. Ce volum de oxigen se consumă și câti moli dioxid de magneziu se formează
Answers
Answer:
10.5 dm3 O2
0.94 moles de MgO
Explanation:
La pregunta dice explícitamente que el magnesio es puro en un 90%.
Por lo tanto, masa de magnesio puro = 90/100 * 25G = 22,5 g
Número de moles de Mg = 22,5g / 24g/mol = 0,94 moles de Mg
La ecuación de reacción es;
2Mg (s) + O2 (g) ------> 2MgO (s)
Si 2 moles de Mg reaccionan con 1 moles de O2
0.94 moles de Mg reacciona con 0.94 * 1/2 = 0.47 moles de O2
Si 1 mol de O2 ocupa 22,4 dm3
0.47 moles de O2 ocupan 0.47 * 22.4 / 1 = 10.5 dm3
También;
2 moles de Mg producen 2 moles de MgO
Entonces, 0.94 moles de Mg producen 0.94 moles de MgO
liquidus line separates which of the following combinations of phase fields? a) alpha and alpha+beta b) Liquid and Liquid + alpha c) alpha and Liquid + alpha d) Liquid +alpha and alpha+beta
Answers
The liquidus line separates the following combinations of phase fields: Liquid and Liquid + alpha. The correct option is b.
What is a phase field? A phase field is a technique for representing the microstructure of materials. It is used in materials science, mathematics, and computer science to simulate and study the behavior of materials in the solid and liquid phases. It is a multi-component field that contains information on the concentration of various components, their phase, and the local temperature, as well as other relevant variables.
The liquidus line is defined as the boundary between the liquid phase field and the field that includes both the liquid and the alpha phase. As a result, the liquidus line separates the following combinations of phase fields: Liquid and Liquid + alpha.
So, the correct option is b) Liquid and Liquid + alpha.
Learn more about Liquidus at https://brainly.com/question/31486571
#SPJ11
calculate the mass of 25,000 molecules of nitrogen gas. (1 mole = 6.02 × 1023 molecules) group of answer choices 7.00 × 105 g 5.81 × 10−19 g 5.38 × 1026 g 1.16 × 10−18 g
Answers
The mass of the 25,000 molecules of Nitrogen gas is found to be 0.0000017 g (1.7 x 10⁻⁶ g).
We must use the molar mass of nitrogen and the Avogadro's number to get the mass of 25,000 molecules of nitrogen gas.
Nitrogen (N₂) has a molar mass of 28 g/mol.
The number of particles in one mole of any substance determined by Avogadro is 6.02 x 10²³.
One nitrogen gas molecule's mass can be determined as follows:
Nitrogen's (N₂) molecular weight is 28 g/mol.
Nitrogen gas molecules total 25,000.
Nitrogen gas moles equal (25,000 molecules) / (6.02 x 10²³ molecules/mol)
(Num. of moles) x = mass of nitrogen gas (molar mass)
[(25,000 molecules) / (6.02 x 10²³ molecules/mol)] is a formula for the mass of nitrogen gas. x (28 g/mol)
Nitrogen gas mass = 0.00000117 g
Therefore, 25,000 nitrogen gas molecules have a mass of about 0.00000117 g.
To know more about Avogadro number, visit,
https://brainly.com/question/859564
#SPJ4
i cant nderstand how t write an electron configuration
Answers
Answer:
Explanation:
Naming ionic compounds building a triangle so that each edge has the same answers facing each other
When 2-methyl-2-butanol undergoes dehydration in acid, one product is:.
Answers
When 2-methyl-2-butanol undergoes **dehydration in acid**, one product formed is **2-methyl-2-butene**.
Dehydration is a reaction that involves the removal of a water molecule from a compound. In this case, 2-methyl-2-butanol reacts with an acid catalyst, such as sulfuric acid, which promotes the elimination of a water molecule. The reaction results in the formation of a double bond between two carbon atoms, creating an alkene. The specific alkene produced in this reaction is 2-methyl-2-butene, which has a double bond between the second and third carbon atoms and a methyl group attached to the second carbon atom. This process is an example of an E1 elimination reaction, which involves the formation of a carbocation intermediate before the formation of the final product.
Know more about elimination reaction here:
https://brainly.com/question/14693649
#SPJ11
A solution of potassium hydroxide (KOH) is added to a solution of hydrochloric acid. How many moles of potassium hydroxide would react with 1 mole of hydrochloric acid? (Hint: It may help to write out a balanced symbol equation for the reaction.)
Answers
The number of moles of potassium hydroxide that would react with 1 mole of hydrochloric acid would be 1 mole.
Stoichiometric mole ratio
The stoichiometric moles of species of a reaction are the coefficients of each species in the balanced symbol equation of the reaction.
The balanced equation of the reaction between potassium hydroxide (KOH) and hydrochloric acid (HCl) is expressed as follows:
\(KOH + HCl --- > KCl + H_2O\)
In this case, the stoichiometric mole of KOH is 1, that of HCl is 1, that of KCl is 1, and that of \(H_2O\) is also 1.
In other words, the stoichiometric mole ratio of KOH and HCl is 1:1. For every 1 mole of KOH, 1 mole of HCl is required for a complete reaction.
Now, the question says what mole of potassium hydroxide will be required to react with 1 mole of hydrochloric acid?
Following the mole ratio, 1 mole of KOH will also be required.
More on mole ratios can be found here: https://brainly.com/question/15288923
#SPJ1
Group 1 period 3 on periodic table
Answers
Answer:
unable to compute
Explanation:
Which is the pathway of light as it travels through the eye?cornea → iris → retina → lenscornea → iris → lens → retinairis → cornea → lens → retinaretina → lens → iris → cornea
Answers
Answer:
Cornea >iris>lens >retina
Explanation:
Can't exactly figure out the answer but I think it should look like this
Answer: cornea → iris → lens → retina
Explanation: I have my ways ;>
Pls, answer asap!
When two objects of different temperatures are placed in contact with one another, eventually: (choose one option from below)
a) both their average kinetic energy and temperature will be the same
b) their average kinetic energy will be the same
c) neither their average kinetic energy and temperature will be the same
d) their temperature will be the same
Answers
Answer:
a) both their average kinetic energy and temperature will be the same
Explanation:
Temperature is the measure of the average kinetic energy of a system. As temperature increases, so will kinetic energy. As kinetic energy increases, speed increases. As one decreases, so does the other. When two objects are in contact with each other, their molecules will exchange energy and cause an eventual equilibrium of temperature and kinetic energy.
Count the total number of atoms in CH4:
Answers
Answer:
5 atoms
Explanation:
there is 1 carbon atom and 4 hydrogen atoms
The equation below shows hydrogen reacting with oxygen to produce water.
2H2 + O2 Right arrow. 2H2O
If 16 mol of oxygen were reacted with excess hydrogen gas, how many moles of water would be produced?
4.0 mol
8.0 mol
16 mol
32 mol
Answers
The equation below shows hydrogen reacting with oxygen to produce water 2H₂ + O₂ → 2H₂O If 16 mole of oxygen were reacted with excess hydrogen gas, then 32 mole of water would be produced
Mole is the amount of substance of a system which contains as many elementary entities
Here given data is
Hydrogen reacting with oxygen to produce water and the reaction is
2H₂ + O₂ → 2H₂O
16 mol of oxygen were reacted with excess hydrogen gas
Then we have to calculate moles of water would be produced = ?
Moles of oxygen = 16 moles
From the balanced equation
1 mole oxygen react to give 2 moles of water
16 moles oxygen react to give 2×16 = 32 moles
Therefore 32 moles of water would be produced in the reaction
Know more about mole
https://brainly.com/question/10811423
#SPJ1
d. 32 mol in case you dont want to read that
Please show how you solved :)
What is oxygen solubility at 10m depth below sea level, 25 deg
C, 30 g/L salinity?
Answers
The solubility of oxygen at 10m depth below sea level, 25 degrees Celsius, and 30 g/L salinity is approximately 6.59 mg/L.
To calculate the solubility of oxygen at a specific depth below sea level, temperature, and salinity, we can refer to the oxygen solubility tables. The solubility of oxygen can vary depending on these factors.
1. Begin by identifying the given parameters:
- Depth: 10m below sea level
- Temperature: 25 degrees Celsius
- Salinity: 30 g/L
2. Use the given parameters to locate the corresponding values in the oxygen solubility table.
3. The solubility of oxygen at a depth of 10m below sea level, 25 degrees Celsius, and 30 g/L salinity is typically around 6.59 mg/L.
Therefore, the solubility of oxygen at 10m depth below sea level, 25 degrees Celsius, and 30 g/L salinity is approximately 6.59 mg/L.
Learn more about solubility from this link:
https://brainly.com/question/9098308
#SPJ11
The oxygen solubility at 10m depth below sea level, 25°C, and 30 g/L salinity is approximately 1538 mol/L.
To calculate the oxygen solubility at a specific depth below sea level, temperature, and salinity, we can use the solubility formula.
The solubility of a gas decreases with increasing temperature and salinity, and increases with increasing pressure.
Here's how you can calculate the oxygen solubility at 10m depth below sea level, 25°C, and 30 g/L salinity:
1. Determine the pressure at 10m depth below sea level: -
The pressure at sea level is approximately 1 atmosphere (atm).
The pressure increases by approximately 1 atm for every 10 meters of depth.
Therefore, at 10m depth, the pressure is approximately 2 atm.
2. Convert the temperature to Kelvin: -
To convert from Celsius to Kelvin, add 273 to the temperature.
25°C + 273 = 298 K.
3. Use the solubility formula:
The solubility of oxygen in water can be calculated using Henry's law:
S = k * P * C.
S is the solubility of oxygen in moles per liter (mol/L).
k is the Henry's law constant for oxygen in water at a specific temperature and salinity.
P is the partial pressure of oxygen in atmospheres (atm).
C is the concentration of oxygen in moles per liter (mol/L).
4. Look up the Henry's law constant for oxygen at 25°C and 30 g/L salinity:
The Henry's law constant for oxygen at 25°C and 30 g/L salinity is approximately 769 L*atm/mol.
5. Calculate the solubility:
S = (769 L*atm/mol) * (2 atm) * (1 mol/L). - S ≈ 1538 mol/L.
Therefore, the oxygen solubility at 10m depth below sea level, 25°C, and 30 g/L salinity is approximately 1538 mol/L.
Learn more about solubility from this link:
brainly.com/question/9098308
#SPJ11
based on the balanced equation: 2 K + Cl2 --> 2KCl what is the mole ratio of K to KCl
Answers
Answer:
2 : 2
Explanation:
The balanced chemical equation is given as:
2K + Cl₂ → 2KCl
From the balanced reaction equation, we can conclude that;
2 moles of K will react with 1 mole of Cl₂ 2 moles of K will produce 2 moles of KCl 1 mole of Cl₂ will produce 2 moles of KClTherefore, the mole ratio is 2:2 or 1:1 in the simplest form.