Assuming that you have 0.4g of Y(OH)3, calculate the mass of of BaO2 required to react stoichiometrically(1Y:2Ba:3Cu) to produce YBa2Cu3O7.
Now calculate the mass of of CuO required to react stoichiometrically (1Y:2Ba:3Cu) to produce YBa2Cu3O7
Answers
1.232 g of BaO₂ is required to react stoichiometrically with 0.4g of Y(OH)₃ to produce YBa₂Cu₃O₇ and 0.867 g of CuO is required to react stoichiometrically with 0.4g of Y(OH)₃ to produce YBa₂Cu₃O₇.
What is the amount of BaO₂ and CuO required to react stoichiometrically with Y(OH)₃ to produce YBa₂Cu₃O₇?The equation for the reaction between Y(OH)₃, BaO₂, and CuO to produce YBa₂Cu₃O₇ is:
2BaO₂ + Y(OH)₃ + 3CuO -> YBa₂Cu₃O₇ + 3H₂O
From the stoichiometric ratio of 1:2:3 for Y, Ba, and Cu respectively, we can see that two moles of BaO₂, one mole of Y(OH)₃, and three moles of CuO are required to produce one mole of YBa₂Cu₃O₇.
To determine the amount of BaO₂ and CuO required to react stoichiometrically with 0.4g of Y(OH)₃, we can use the molar masses of the compounds to convert the mass of Y(OH)₃ to moles, and then use the stoichiometric ratio to calculate the amount of BaO₂ and CuO required.
Assuming the molar masses of Y(OH)₃, BaO₂, and CuO are 109.96 g/mol, 169.33 g/mol, and 79.55 g/mol respectively, we can calculate the number of moles of Y(OH)₃ in 0.4g as:
0.4g / 109.96 g/mol = 0.003636 mol Y(OH)₃
Using the stoichiometric ratio of 1:2:3, we can then calculate the number of moles of BaO₂ and CuO required as:
0.003636 mol Y(OH)₃ x (2 mol BaO₂ / 1 mol Y(OH)₃) = 0.007273 mol BaO₂
0.003636 mol Y(OH)₃ x (3 mol CuO / 1 mol Y(OH)₃) = 0.010908 mol CuO
Finally, we can convert the number of moles of BaO₂ and CuO to mass using their respective molar masses:
0.007273 mol BaO₂ x 169.33 g/mol = 1.232 g BaO₂
0.010908 mol CuO x 79.55 g/mol = 0.867 g CuO
Therefore, 1.232 g of BaO₂ and 0.867 g of CuO are required to react stoichiometrically with 0.4g of Y(OH)₃ to produce YBa₂Cu₃O₇.
Learn more about Stoichiometric ratio
brainly.com/question/6907332
#SPJ11
Related Questions
cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. balance the equation for the synthesis of cryolite. equation: al2o3 (s) naoh (l) hf (g) -> na3alf6 h2o (g) al2o3(s) naoh(l) hf(g)⟶na3alf6 h2o(g) if 13.0 kg of al2o3(s), 50.4 kg of naoh(l), and 50.4 kg of hf(g) react completely, how many kilograms of cryolite will be produced? mass of cryolite produced: kg na3alf6 which reactants will be in excess? naoh hf al2o3 what is the total mass of the excess reactants left over after the reaction is complete? total mass of excess reactants: kg qu
Answers
The kilograms of cryolite will be produced is 53.50 kg. the reactants will be in excess is NaOH and HF.
The balance equation is given as :
Al₂O₃ + 6NaOH + 12HF -----> 2Na₃AlF₆ + 9H₂O
molar mass of Al₂O₃ = 102 g/mol
molar mass of NaOH = 40 g/mol
molar mass of HF = 20 g/mol
moles of Al₂O₃ = mass / molar mass
= 13 × 1000 / 102
= 127.4 mol
moles of NaOH = mass / molar mass
= 50.4 × 1000 / 40
= 1260 mol
moles of HF = mass / molar mass
= 50.4 × 1000 / 20
= 2520 mol
dividing by the coefficient , we get :
Al₂O₃ = 127.4 / 1= 127.4 mol
NaOH = 1260 / 6 = 210 mol
HF = 2520 / 12 = 210 mol
mass of cryolite = moles × molar mass
= 2 × 127.4 × 210
= 53508 g = 53.50 kg
The reactant is in excess = NaOH and HF.
To learn more about cryolite here
https://brainly.com/question/13735947
#SPJ4
Which of the following is the most likely scientific explanation for why so many people report improvement after wearing this jewelry?
Answers
Some people may be experiencing the placebo effect, which is the best scientific explanation for why many people report improvement after wearing a particular piece of jewelry.
Option A is correct.
The placebo effect: what is it?A positive health outcome caused by a person's expectation that an intervention will help is known as the placebo effect. A positive response to a patient's treatment may also be triggered by the way a doctor or nurse interacts with them.
How does the brain respond to the placebo effect?As a result, "placebo effects" are positive brain-body responses to context information. Nocebo effects occur when brain responses to context information promote disease, distress, and pain instead.
Question incomplete:
Which of the following is the most likely scientific explanation for why so many people report improvement after wearing this jewelry
A. Some people may be experiencing the placebo effect
B. Some people will say whatever the company pays them to say
C. The ions in the jewelry change the electric field around the body, improving blood flow
D. The ions in the jewelry improve energy flow through the body
Learn more about placebo effect:
brainly.com/question/1173279
#SPJ1
Which mineral is hard enough to scratch calcite? *
1 talc
2 sulfur
3 halite
4 quartz
Answers
Answer:
Should be number 4, quartz.
Explanation:
Quartz is rougher than calcite, therefore it's able to scratch it.
What is the empirical formula for the compound that has 46 grams of sodium, 64 grams
of sulfur, and 48 grams of oxygen?
Answers
Answer:
Na2S2O3Explanation:
Divide each weight by their respective element weights.

Na\(_2\)S\(_2\)O\(_3\) is the empirical formula for the compound that has 46 grams of sodium, 64 grams of sulfur, and 48 grams of oxygen.
What is empirical formula?In chemistry, the equation of maybe a chemical compound is the simplest complete number percentage of atoms that make up a molecule. The empirical formulas for sulfur monoxide, SO, and disulfur dioxide, S2O2, are two simple instances of this concept.
They do not, nonetheless, share comparable molecular formulas, which indicate how many elements are contained in each chemical compound's molecules. An empirical formula does not specify the arrangement or number of atoms. Several polymers, including such silicon dioxide, nor ionic compounds, including such calcium chloride (CaCl2), have this property (SiO2).
moles of sodium = 46/23=2
moles of sulfur = 64 / 32=2
moles of oxygen =48 / 16 =3
Na\(_2\)S\(_2\)O\(_3\) is the empirical formula
Therefore, Na\(_2\)S\(_2\)O\(_3\) is the empirical formula.
To learn more about empirical formula, here:
https://brainly.com/question/14044066
#SPJ6
what is meant by the term, mass transfer? select one: mass transfer refers to the change in molar mass of a substance in the presence of an indicator. mass transfer refers to the bonding of an indicator to the dissolved species in solution. mass transfer is the net movement of mass from one location to another.
Answers
The answer to the above question is D. as mass transfer refers to the net transfer of mass from one place to another.
What does "mass transfer" mean?One of the fundamental concepts in the study of transport phenomena is mass transfer, which denotes the movement of mass from one location to another. In multiphase systems, mass transfer may occur within a single phase or across phase borders. Although it can also be characterised in terms of solid-phase materials, mass transfer often incorporates at least one fluid phase (gas or liquid) in engineering challenges.
The computation of mass flux in a system and the distribution of the mass of various species through time and space are both made possible by the theory of mass transfer.
To know more about solid-phase visit: https://brainly.com/question/17053072
#SPJ4
Give an example of a human activity that can alter chemical cycles and how.
Answers
Deforestation is an example of a human activity that can alter chemical cycles, specifically the carbon cycle. Deforestation refers to the permanent removal or clearing of forests or wooded areas, typically for human activities such as agriculture, logging, urban expansion, or the establishment of infrastructure.
When forests are cleared or destroyed through deforestation, the vegetation and organic matter in the forests are often burned or decomposed. This process releases a significant amount of carbon dioxide (CO2) into the atmosphere. Trees play a crucial role in the carbon cycle as they absorb CO2 through photosynthesis and store carbon in their biomass and soil. By removing forests, the natural carbon sink is diminished, and the balance of carbon dioxide in the atmosphere is disrupted.
Additionally, deforestation can lead to increased soil erosion. Without the tree roots and vegetation to hold the soil in place, erosion can occur more easily. This can result in the loss of valuable nutrients from the soil, such as nitrogen and phosphorus, which are essential for plant growth. The altered chemical composition of the soil affects nutrient cycling and availability for both plants and other organisms.
Overall, deforestation disrupts the natural balance of the carbon cycle and nutrient cycles, contributing to increased greenhouse gas emissions and nutrient depletion in ecosystems. It highlights how human activities can have significant impacts on chemical cycles and the functioning of ecosystems.
To know more about carbon cycle, click here, https://brainly.com/question/30508567
#SPJ11
A sealed 10. 0L flask at 400K contains equimolar amounts of ethane and propane in gaseous form
Answers
The partial pressure of ethane and propane in the flask are both 16.42 atm.
The given information tells us that the flask is sealed, which means that no gas can enter or leave the flask. It also tells us that the volume of the flask is 10.0L and the temperature is 400K. Finally, it tells us that there are equimolar amounts of ethane and propane in the flask.
From this information, we can assume that the total pressure inside the flask is the sum of the partial pressures of ethane and propane. This is because the ideal gas law tells us that PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the gas constant, and T is the temperature. Since the number of moles of each gas is the same, we can assume that their partial pressures are equal.
To find the partial pressures, we need to use the ideal gas law again. However, we need to know the total number of moles of gas in the flask. We can find this by using the fact that the amounts of ethane and propane are equimolar. Since the molar mass of ethane is 30 g/mol and the molar mass of propane is 44 g/mol, we know that the total mass of gas in the flask is 74 g. Dividing this by the sum of the molar masses (30+44=74 g/mol), we get the total number of moles, which is 1 mol.
Now we can use the ideal gas law to find the partial pressures. We'll use R = 0.08206 L·atm/(mol·K) as the gas constant. For ethane, we have:
PV = nRT
P_ethane * 10.0L = 0.5 mol * 0.08206 L·atm/(mol·K) * 400K
P_ethane = (0.5 mol * 0.08206 L·atm/(mol·K) * 400K) / 10.0L
P_ethane = 16.42 atm
For propane, we get the same result:
PV = nRT
P_propane * 10.0L = 0.5 mol * 0.08206 L·atm/(mol·K) * 400K
P_propane = (0.5 mol * 0.08206 L·atm/(mol·K) * 400K) / 10.0L
P_propane = 16.42 atm
Therefore, the partial pressure of ethane and propane in the flask are both 16.42 atm.
Know more about Partial Pressure here:
https://brainly.com/question/31214700
#SPJ11
What is the charge of a chromium ion that has lost 4 electrons?
Answers
Answer:
Explanation:
When an atom loses electron(s) it will lose some of its negative charge and so becomes positively charged. A positive ion is formed where an atom has more protons than electrons. In the opposite case when an atom gains electron(s) it becomes negatively charged (more electrons than protons)
and plzs do not hate
The charge of chromium after losing 4 electrons is +2.
What is the ionic charge?
Ions are two or more charged particles that make up ionic compounds. Electrostatic attraction keeps the ions together (in the solid phase) despite their opposing charges. Cations are positively charged ions, whereas anions are negatively charged ions. Many factors make ionic molecules significant.
They act as electrolytes for batteries and conduct electricity when dissolved in water. They could form a strong bond with other charged particles, such as those found in environmental samples or the human body. They find use in fireworks, flares, and lights because they are frequently multicolored when burned. The list is endless!
Studying the nomenclature used to give ionic compounds distinctive names is a good method to become familiar with and exposed to them. You will learn how to properly explain the identification of substances in language by converting from a chemical formula to a name—a highly essential problem as the diversity and complexity of known compounds keeps growing!
Therefore, the charge on chromium after losing 4 electrons is +2.
Read more about ions, here
https://brainly.com/question/1488567
#SPJ2
If 804000 j of energy are added to 8.70 l of
water at 287 k, what will the final temperature of the water be?
Answers
The final temperature of the water after adding 804,000 J of energy at an initial temperature of 287 K is 309.39 K.
The amount of energy added to the water is 804,000 J, and the amount of water is 8.70 L.
The formula q = mcΔT, where q is the heat added, m is the mass of the substance, c is the specific heat, and ΔT is the change in temperature.
The specific heat of water is given in J/gK. We know that 1 liter of water is equivalent to 1000 grams.
The heat added to the water (q) is 804,000 J.
The mass of water (8.70 L) by the density of water, which is 1000 g/L. Therefore, the mass of water is 8700 g.
The specific heat of water (c) is 4.184 J/gK.
The change in temperature (ΔT):
ΔT = q / (mc) = (804,000 J) / (8700 g × 4.184 J/gK) = 22.39 K
The initial temperature of the water is given as 287 K.
The change in temperature to the initial temperature:
Final temperature = Initial temperature + ΔT = 287 K + 22.39 K = 309.39 K
Temperature of the water after adding 804,000 J of energy at an initial temperature of 287 K is equal to 309.39 K.
know more about energy here: https://brainly.com/question/32814408
#SPJ11
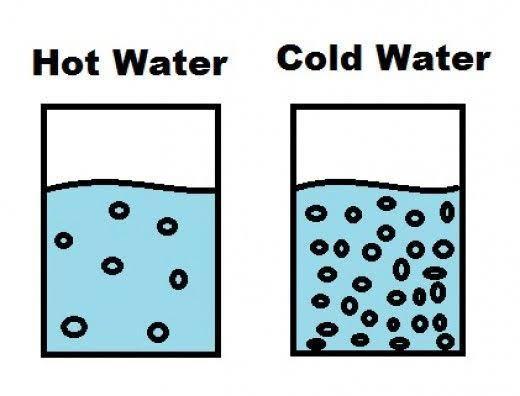
3- You saw a jar of hot water placed upside down over a jar of cold water. The hot
water stayed on top of the cold water without mixing. Why did the hot water stay on
top of the cold water?
Answers
Hot water stays on the top of cold water and doesn't mix. This is because of difference in DENSITY of water at different temperatures.
When we heat water, then the atoms gain energy from the heat and start moving away from each other. Now, when the atoms move away from each other than the (mass per unit volume) i.e. the density decreases. This makes water lighter.
When the temperature is less i.e. cold water then the atoms are more compactly packed and therefore the density of water is more at lower temperatures.
Since, hot water is lighter than cold water that is why it stays on top of the cold water.
If you had 2 oxygen atoms and 1 hydrogen atom, could you form a molecule?
Answers
Answer:
Yes!
Explanation:
You certainly could form something. It would be written as H02 and it is called Hydroperoxyl. This is formed by the transfer of a hydrogen atom to an oxygen atom.
40. 0% carbon, 6. 7% hydrogen, and 53. 3% oxygen with a molecular mass of 60. 0 g/mol. What is the molecular formula of the unknown compound?
Answers
The molecular formula of the unknown compound is C2H2O2.
To determine the molecular formula of the unknown compound, we need to calculate the empirical formula first and then find the multiple of its subscripts to obtain the molecular formula.
Given:
Percentage of carbon = 40.0%
Percentage of hydrogen = 6.7%
Percentage of oxygen = 53.3%
Molecular mass = 60.0 g/mol
Step 1: Convert the percentages to grams.
Assuming we have 100 grams of the compound:
Mass of carbon = 40.0 g
Mass of hydrogen = 6.7 g
Mass of oxygen = 53.3 g
Step 2: Convert the masses to moles using the molar masses of the elements.
Molar mass of carbon = 12.01 g/mol
Molar mass of hydrogen = 1.008 g/mol
Molar mass of oxygen = 16.00 g/mol
Number of moles of carbon = Mass of carbon / Molar mass of carbon
= 40.0 g / 12.01 g/mol
= 3.332 mol
Number of moles of hydrogen = Mass of hydrogen / Molar mass of hydrogen
= 6.7 g / 1.008 g/mol
= 6.648 mol
Number of moles of oxygen = Mass of oxygen / Molar mass of oxygen
= 53.3 g / 16.00 g/mol
= 3.331 mol
Step 3: Determine the empirical formula by dividing the moles by the smallest value.
Dividing the moles of carbon, hydrogen, and oxygen by 3.331 gives approximately 1 for each element.
So, the empirical formula of the compound is CHO.
Step 4: Determine the multiple of the subscripts to obtain the molecular formula.
To find the multiple, we divide the molecular mass by the empirical formula mass.
Molecular mass = 60.0 g/mol
Empirical formula mass = (12.01 g/mol) + (1.008 g/mol) + (16.00 g/mol) = 29.018 g/mol
Multiple = Molecular mass / Empirical formula mass
= 60.0 g/mol / 29.018 g/mol
= 2.07
Rounding to the nearest whole number, we get 2.
Therefore, the molecular formula of the unknown compound is C2H2O2.
learn more about molecular here
https://brainly.com/question/30640129
#SPJ11
Which of the amino acids could form a hydrogen bond with another amino acid in the chain to stablaize the secondary structure of a b-pleated sheet?
Answers
All of the amino acids could form a hydrogen bond with another amino acid in the chain to stabilize the secondary structure of a b-pleated sheet.
Protein is composed of amino acid linked with peptide bond.
Hydrogen bond is an electrostatic attraction between two polar groups that occurs when a hydrogen atom (H), covalently bound to a highly electronegative atom such as flourine (F), oxygen (O) and nitrogen (N) atoms.
In a beta-pleated sheet, hydrogen bonds are formed between carbonyl and amino groups of backbone (see the picture below).
Usually, amino acid proline is found in the edge strands in beta-sheets. Beta-branched amino acids valine, threonine and isoleucine and aromatic amino acid residues tryptophan, tyrosine and phenylalanine are found middle of beta-sheets.
More about beta-pleated sheet: brainly.com/question/5297569
#SPJ4

Given the speed of light as 3.0 ✕ 108 m/s, calculate the wavelength of the electromagnetic radiation whose frequency is 6.700 ✕ 1012 Hz.
Answers
Answer:
Wavelength = \(4.48*10^{-5}m\)
Explanation:
Given the following data;
Speed of light, V = 3 * 10^{8}m/s
Frequency, F = 6.700 * 10^{12}Hz.
To find the wavelength
We know that that the speed of light is given by the formula;
\( Speed of light, V = wavelength * frequency \)
Making wavelength the subject of formula, we have;
\( Wavelength = \frac {speed \; of \; light}{frequency} \)
Substituting into the equation, we have;
\( Wavelength = \frac {3 *10^{8}}{6.700 ✕ 10^{12}} \)
Simplifying the equation, we have;
Wavelength = 4.48*10^{-5} m
Therefore, the wavelength of the electromagnetic radiation is \(4.48*10^{-5} meters\)
Sarah wants to know where in her garden chamomile would grow the best. She thinks chamomile will grow best in the corner of the garden that gets the most sunlight. To test her hypothesis, she decides to plant several groups of chamomile in her garden as an experiment.
Answers
Answer:
This question is incomplete and lacks options, the complete part and the options are:
Which of the following variables should Sarah change from one group of chamomile to the next?
A. the location of the plants
B. the height of the plants
C. the type of plants
D. the amount of water she gives the plants
The answer is A
Explanation:
This question is asking for the INDEPENDENT VARIABLE in the experiment. The independent variable of an experiment is the variable that is changed or manipulated by the experimenter in order to bring about a response.
In this experiment where Sarah wants to know where in her garden chamomile plants would grow the best. She hypothesizes that chamomile will grow best in the corner of the garden that gets the most sunlight. However, to test this hypothesis, she decides to plant several groups of chamomile in her garden as an experiment. The variable that Sarah can change in the several groups of chamomile (independent variable) is the LOCATION OF THE PLANTS.
Note that, "where" is a question of location.
Answer:
The answer is C
Explanation:
this is my last 100 points so enchiladas or chilaquiles.
Answers
\(\begin{gathered}\begin{gathered} \\ \large{\pmb{\underline{\underline {\frak{AnSwer}}}}} \\ \end{gathered}\end{gathered} \)
Thank u soo much...
In the game of football sometimes we while looking at the football and trying to kick it with a greater force, collides with a player of opposite team. both feel hurt because each applies a force to other . When a gun is fired it exerts a forward force on the bullet .
Assuming complete dissociation of the solute, how many grams of KNO3 must be added to 275 mL of water to produce a solution that freezes at −14.5 ∘C? The freezing point for pure water is 0.0 ∘C and Kf is equal to 1.86 ∘C/m. If the 3.90 m solution from Part A boils at 103.45 ∘C, what is the actual value of the van't Hoff factor, i?
Answers
The actual value of the van't Hoff factor, i, is 2, as KNO₃ dissociates into two ions (K+ and NO₃-) in solution.
To calculate the grams of KNO₃ needed to produce a solution that freezes at -14.5 °C, we can use the formula:
ΔT = Kf * m * i
where ΔT is the change in freezing point, Kf is the molal freezing point depression constant, m is the molality of the solution, and i is the van't Hoff factor.
First, let's calculate the molality (m) of the solution:
m = moles of solute / mass of solvent (in kg)
Since we have 275 mL of water, which is equivalent to 0.275 L, and assuming water has a density of 1 g/mL, the mass of the solvent is 0.275 kg.
To determine the moles of solute, we need to use the equation:
ΔT = Kf * m * i
Substituting the given values:
-14.5 °C = (1.86 °C/m) * m * i
Solving for m * i:
m * i = -14.5 °C / (1.86 °C/m)
m * i = -7.80 m
Since KNO₃ dissociates into K+ and NO₃- ions, the van't Hoff factor (i) is 2.
Now we can solve for the molality of the solution (m):
m * i = -7.80 m
2m = -7.80 m
m = -7.80 m / 2
m = -3.90 m
Finally, we can calculate the moles of KNO₃:
moles of solute = m * mass of solvent (in kg)
moles of KNO₃ = (-3.90 m) * 0.275 kg
To convert moles to grams, we multiply by the molar mass of KNO₃, which is approximately 101.1 g/mol.
grams of KNO₃ = moles of KNO₃ * molar mass of KNO₃
Therefore, the number of grams of KNO₃ required to produce the solution is:
grams of KNO₃ = (-3.90 m) * 0.275 kg * 101.1 g/mol
The actual value of the van't Hoff factor, i, is 2, as KNO₃ dissociates into two ions (K+ and NO₃-) in solution.
Learn more about KNO₃ here ; brainly.com/question/14372543
#SPJ11
What is the approximate concentration of reaction product in a solution that has an absorbance of 0.7 at ph 6.0?
Answers
The approximate concentration of reaction product in a solution that has an absorbance of 0.7 at ph 6.0 is based on the equation A = εbc given in the passage, the concentration c is the absorbance A divided by the absorptivity ε in a 1 cm path length cell. 0.7 divided by approximately 1400 gives 500 μM.
A = εbc
0.7 = 1400 x c
7/14000 = 1/2000 = 0.0005 = 5x10^-4 = 500 μM
1 μM = 1 x10 ^-6
The concentration of a substance is the amount of solute present in a given volume of solution. Concentration is usually expressed in molarity, defined as the number of solutes in 1 L of solution. Solution concentration is a measure of the amount of solute dissolved in a given amount of solvent or solution.
Concentrated solutions are solutions that contain relatively large amounts of solutes. Dilute solutions are solutions that contain relatively small amounts of solutes. The definition of concentration means the amount of an ingredient or part relative to other ingredients or parts. An example of concentration is the amount of salt to water in a brine solution.
Learn more about concentration here
https://brainly.com/question/23437000
#SPJ4
What is the general form of a synthesis reaction?
O A. AB + CD → AC + BD
B. A + B → AB
O C. AB + C → AC + B
D. AB → A+B
Be
The answer is B
Answers
Answer:
B. A+ B → AB
Explanation:
general form of a synthesis reaction
PLEASE HELP ASAP!!!

Answers
As a result, the gas will be about 205 kelvin, or -68.5 degrees Celsius, in temperature.
What temperature is a gas at a 2 atm pressure and 2 l ?If a gas's temperature is increased to 927°C, so its pneumatic cylinder will be. A gas has a temperature of 127°C at 2 atm and 2 litres of volume. O 6 atm.
1 mole = 22.4 litres, correct?One mole ($6.023 times 1023 typical particles) of the any gas at STP takes up 22.4L of space. A mole of any gas takes up 22.4 litres at standard pressure and temperature (273K and 1atm).
To know more about kelvin visit:
https://brainly.com/question/12183328
#SPJ1
The reaction 2A + B → C + D is studied to determine the kinetics of the reaction at a certain temperature, which is held constant throughout the experiment.
a. Find the order with respect to A and B and explain your reasoning.
b. Write the rate law that is consistent with part (a).
c. Determine the value for k and specify its units.

Answers
The rate of reaction refers to how quickly or solwly a reaction occurs;
a) Both A and B are second order
b) The rate law of the reaction is R = k[A]^2 [B]^2
c)The rate constant is 5.21 * 10^5 mol-3L^3s-1.
What is the rate of reaction?The term rate of reaction refers to how quickly or slowly a reaction occurs.
a) To obtain the order with respect to A, we divide reaction (3) by (2) as follows;
5.01 * 10^-1/1.25 * 10^-1 = k[0.0070]^m [0.14]^n/k[0.0035]^m [0.14]^n
4 = 2^m
2^2 = 2^m
m =2
To obtain the order with respect to B, we divide reaction (2) by (1);
1.25 * 10^-1/3.13 * 10^-2 = k[0.0035]^m [0.014]^n/k[0.0035]^m [0.0070]^n
4 = 2^n
2^2 = 2^n
n = 2
b)The reaction is second order in both A and B hence the rate law is R = k[A]^2 [B]^2
c) We can obtain K using any of the reactions;
0.0313 molL-1s-1 = k[0.0035molL-1]^2 [0.0700molL-1]^2
k = 0.0313 molL-1s-1/[0.0035molL-1]^2 [0.0700molL-1]^2
k = 5.21 * 10^5 mol-3L^3s-1
Learn more about rate of reaction: https://brainly.com/question/8592296?
Please Help 30 Points + Brainlest Look At The Image Below And Answer The Flowing Question

Answers
Answer:
Between A and D
Explanation:
Polyelectrolytes are typically used to separate oil and water in industrial applications. The separation process is dependent on controlling the pH. Fifteen (15) pH readings of wastewater following these processes were recorded. Is it reasonable to model these data using a normal distribution? 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 1.0 10.0 10.5 7.6 11.4 11.4 10.0 Yes, it passes the "fat pencil" test. Therefore, a normal distribution is a reasonable model. No, it does not pass the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. O Yes, it passes the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. O No, it does not pass the "fat pencil" test. Therefore, a normal distribution is a reasonable model.
Answers
No, it does not pass the "fat pencil" test. Therefore, a normal distribution is not a reasonable model. Option B is the correct answer.
The "fat pencil" test is a quick visual check to determine if a dataset can be reasonably approximated by a normal distribution. In this case, the pH readings of wastewater show a significant deviation from a normal distribution. The presence of several low pH values (1.0) and a few high pH values (10.0, 10.5, 11.4) indicate a non-normal distribution with skewness and potential outliers. Therefore, it is not reasonable to model these data using a normal distribution.
Option B is the correct answer.
You can learn more about normal distribution at
https://brainly.com/question/4079902
#SPJ11
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
PLS HELP ME PLSS GUYS PLS HELP ME IN 1

Answers
Find the volume of oxygen with a density of 0.001331 g/ml that has a mass of 250.0 g.
Answers
Answer:
187,828.7 mLExplanation:
The volume of a substance when given the density and mass can be found by using the formula
\(volume = \frac{mass}{density} \\\)
From the question we have
\(volume = \frac{250}{0.001331} \\ = 187828.70025...\)
We have the final answer as
187,828.7 mLHope this helps you
Compare the diameter of the moon to the diameter of the Earth
Answers
Answer:
Ok Hold up. I will answer after I think of question
Explanation:
13. In the reaction: A + B = C, if 4.2 grams of A react to make 6.8 grams of C, then how many grams of B were reacted? Hint: Mass, like charge and energy, is conserved in all reactions. pls i need quick help
Answers
Answer:
Hit me with brainlist pls
Explanation:
A equals 6.8 and C is the final answer of the equation so you would subtract the answer from a which gives you 2.6 grams
A buffer solution contains ethanoic acid and its conjugate base; the pka of ethanoic acid is 4.74. Calculate the pH of the buffer solution?
Answers
At pH 5.0 the solution is buffer. A buffer solution contains ethanoic acid and its conjugate base; the pKa of ethanoic acid is 4.74.
A buffer solution will work between +/- 1 unit of pH around its pKa value of weak acid. A buffer solution is most effective for both for acid addition and base addition when the pH near to pKa value of weak acid. It is far essential to have answers whose pH does now no longer alternate even at the addition of sturdy alkalis or sturdy acids. Such solutions are known as buffer solution. Buffer capability is the capability of a buffer technique to withstand alternate in its pH. The equation is given by, pH = pKa + log [Salt] / [Acid]
Thus, the pH of the buffer solution is 5.0.
To learn more pH check the link below:
brainly.com/question/172153
#SPJ4
A perfume must easily _______ so that the particles can diffuse through the air and reach your nose . What is the missing word in this sentence?
Answers
Answer:
smell accordingly so that I can't diffuse the air and reach your nose
A perfume must easily evaporate so that the particles can diffuse through the air and reach your nose. The missing word in this sentence is evaporate.
What is evaporation ?Evaporation is also called as vaporization which is takes place on the surface of a liquid as it transitions into the gas phase. High concentrations of the chemical that is evaporating slow down evaporation by a significant amount in the surrounding gas.
Evaporation is a surface phenomenon because it occurs when molecules with higher kinetic energy from the top layer of the liquid escape into the air.
Diffusion is a process where particles travel from a highly concentrated location to a less concentrated area.
Thus, A perfume must easily evaporate so that the particles can diffuse through the air and reach your nose.
To learn more about the evaporation, follow the link;
https://brainly.com/question/5019199
#SPJ2