(b) the consumer surplus at the equilibrium point, and (c) the producer surplus at the equilibrium point.
Answers
The option b. At the equilibrium point, the consumer surplus is equal to the area below the demand curve and above the equilibrium price. (c) At the equilibrium point, the producer surplus is equal to the area above the supply curve and below the equilibrium price.
In microeconomics, consumer surplus refers to the difference between what consumers are willing to pay for a product and what they actually pay. At the equilibrium point, consumer surplus is defined as the area under the demand curve and above the equilibrium price. The demand curve shows the quantity of a product that consumers are willing and able to purchase at different price levels. The equilibrium price is the price at which the quantity demanded of a product is equal to the quantity supplied, meaning that the market is in balance.
Producer surplus, on the other hand, is the difference between the price that producers receive for a product and the minimum price they are willing to accept. At the equilibrium point, producer surplus is defined as the area above the supply curve and below the equilibrium price. The supply curve shows the quantity of a product that producers are willing and able to offer for sale at different price levels. The equilibrium price is the price at which the quantity demanded of a product is equal to the quantity supplied, meaning that the market is in balance.
To know more about surplus visit:
https://brainly.com/question/14329098
#SPJ11
Related Questions
Select the correct answer. which type of property can be observed only by changing a substance into a different substance? a. intensive property b. extensive property c. physical property d. chemical property
Answers
The correct option is D: Chemical property
Chemical properties can be observed only by changing a substance into a different substance.
When a substance or a material undergoes a chemical change then it becomes a completely different kind of substance.
In chemistry, chemical properties are the kind of properties that can be observed or measured as a result of a chemical change.
Examples of chemical changes are rusting of iron, combustion of a compound or fossil fuels, flammability, reactivity, acidity, and toxicity.
To put it simply, a chemical property brings a complete change to the identity of a matter.
If you need to learn more about the Chemical properties click here:
https://brainly.com/question/28308645
#SPJ4
Hydrated sodium tetraborate is heated
to drive off the water. You find there are
0.01312 mol Na2B4O7 and 0.1311 mol
H2O in the sample. What is the formula
of the hydrate?
A. Na2B4O7 H₂O
B. Na2B4O7 2H₂O
C. Na2B4O7-10H₂O
D. Na2B4O7-13H₂O

Answers
Answer: C. Na2B4O7•10H2O
Explanation:
Hope this helps :)
The formula is tetrasodium borate-Na2B4O7.10H2O
Given- 0.0132 mol Na2B4O7 and 0.1311 mol H2O
Step 1Using the molar mass of the anhydrous Na2B4O7 and its mass percentage, we can calculate the molar mass of the hydrate (if we look at it as 100% of the mass) by stoichiometry.
Molar mass of Na = (22.990 g/mol)
Molar mass of B = (10.811 g/mol)
Molar mass of O = (15.999 g/mol)
Molar mass of Na2B4O7= 2⋅22.990 g/mol +4⋅10.811 g/mol +7⋅15.999 g/mol = 201.217 g/mol
201.217g/mol : 52.8%=x g/mol : 100%
x g/mol = 201.217 g/mol⋅100%÷52.8 %
x g/mol= 381.093 g/mol
Step 2In 381.093 g of hydrate, we have 201.217 g of anhydrous Na2B4O7 , the rest of the mass is water.
381.093g−201.217g= 179.876 g of water
Molar mass of H = 1.008 g/mol
Molar mass of O = 15.999 g/mol
Molar mass of H2O= 1.008 g/mol+ 15.999g/mol = 18.015 g/mol
179.876g ÷18.015 = 9.98= 10 moles of water per mole of hydrate.
write an equation that shows the formation of a rubidium ion from a neutral rubidium atom.
Answers
The formation of a rubidium ion from a neutral rubidium atom can be represented by the following equation:
Rb -> Rb+ + e-
In this equation, the symbol "Rb" represents a neutral rubidium atom, while "Rb+" represents a rubidium ion that has lost one electron. The "e-" represents the electron that has been removed from the rubidium atom during ionization.
When a rubidium atom is subjected to enough energy, it can lose one electron to become a positively charged rubidium ion. This process is called ionization. The resulting rubidium ion has a positive charge and is attracted to negatively charged species such as other ions or electrons.
Overall, the formation of a rubidium ion from a neutral rubidium atom involves the loss of an electron. This process is essential in many chemical reactions, particularly those involving ionic compounds. The ability to control the ionization of rubidium and other elements is critical in many applications, including the development of new materials and technologies.
To learn more about rubidium ion, refer:-
https://brainly.com/question/28972491
#SPJ11
A wave on a lake has a wavelength of 5.0 M and a Frequency of 4 Hz. What is the speed of the wave in m/s?
Answers
Answer:
The appropriate speed will be "20 m/s".
Explanation:
The given values are:
Wavelength,
λ = 5.0 M
Frequency,
f = 4 Hz
Now,
The speed of the wave will be:
⇒ \(V=f\times \lambda\)
On putting the values, we get
⇒ \(=4\times 5.0\)
⇒ \(=20 \ m/s\)
in a nucleic acid, adjacent nucleotides are bound to each other in what way?
Answers
The adjacent nucleotides are bound to each other through a phosphodiester bond in a nucleic acid.
What is nucleic acid?Nucleic acid is a biopolymer made up of nucleotide monomers that make up nucleic acid chains. The nucleotide's three components are a five-carbon sugar, a phosphate group, and a nitrogenous base. Nucleic acids are present in all living cells, including viruses and bacteria, and they play a critical role in storing, transmitting, and expressing genetic information. RNA and DNA are two types of nucleic acids.
The phosphate group in one nucleotide forms a phosphodiester bond with the hydroxyl group on the sugar molecule of the next nucleotide in line in nucleic acids. This reaction is carried out by removing a molecule of water, resulting in a strong covalent bond between two nucleotides. These bonds make up the sugar-phosphate backbone of a nucleic acid chain, which is fundamental to its structure.
Learn more about Nucleic acid: https://brainly.com/question/17701344
#SPJ11
difference between practical work inside and outside laboratory
Answers
Practical work refers to the art of conducting experiments in order to answer certain research questions.
What is practical work?In science, practical work refers to the art of conducting experiments in order to answer certain research questions. This could occur in a laboratory under controlled conditions or in the field.
In the physical sciences, most of the practical work is conducted in the laboratory under controlled conditions. However, some experiments in the biological sciences and most experiments in the social sciences are conducted outside the laboratory.
Learn more about experiments:https://brainly.com/question/11256472
#SPJ1
A gas with a vapor density greater than that of air, would be most effectively displaced out off a vessel by?
Answers
A gas with a vapor density greater than that of air would be most effectively displaced out of a vessel by ventilation.
The purposeful introduction of external air into a place is called ventilation. Ventilation can be used to regulate indoor temperature, humidity, and air motion to improve thermal comfort, contentment with other aspects of the indoor environment, or other goals.
The two following principles determine the type of ventilation: Considering the impact of the contaminant's vapor density and either positive or negative pressure is applied. Consider a vertical tank that is filled with methane gas. Methane would leak out if we opened the top hatch since its vapor density is far lower than that of air. A second opening could be built at the bottom to greatly increase the process efficiency.
A faster atmospheric turnover would follow from air being pulled in via the bottom while the methane was vented out the top. The rate of natural ventilation will increase with the difference in vapor density. Numerous gases that require ventilation are either present in fairly low concentrations or have vapor densities close to one.
To know more about ventilation refer to: https://brainly.com/question/1121893
#SPJ4
An example of a decomposer is a:
rabbit
mushroom
tulip
Answers
Answer:
mushroom
Explanation:
What is the bond angle of a tetrahedral?
Answers
The bond angle of a tetrahedral molecule is approximately 109.5 degrees. This is because a tetrahedron is a three-dimensional shape with four equivalent sp3 hybridized orbitals forming the corners of the tetrahedron.
Understanding Tetrahedral Bond AnglesThe bond angle of a tetrahedral molecule is one of the most fundamental and important concepts in chemistry. A tetrahedron is a three-dimensional shape with four equivalent sp3 hybridized orbitals forming the corners of the tetrahedron. These hybridized orbitals result from the combination of the s and p orbitals of the central atom, creating four orbitals that are directed towards the four corners of the tetrahedron.
Each orbital can form a bond with an adjacent atom or molecule, resulting in four identical bonds in a tetrahedral molecule. The ideal bond angle between adjacent bonds in a tetrahedral molecule is 109.5 degrees, which is due to the geometric arrangement of the orbitals around the central atom. The four orbitals are arranged such that they are as far apart from each other as possible, resulting in an angle of 109.5 degrees between adjacent bonds.
Examples of tetrahedral molecules include methane (CH4), which has four identical C-H bonds arranged around the central carbon atom, and carbon tetrachloride (CCl4), which has four identical C-Cl bonds arranged around the central carbon atom. The understanding of tetrahedral bond angles is important in various fields of chemistry, including organic chemistry, biochemistry, and materials science, and it provides a basis for understanding the properties and behavior of many molecules.
To knowmore about the bond angle in a tetrahedral molecule, visit;
https://brainly.com/question/3995950
#SPJ4
Which of the following correctly describes solid, liquid, and gas states of matter?
Answers
Explanation:
In solids, the particles are tightly packed together. In liquids, the particles have more movement, while in gases, they are spread out. Particles in chemistry can be atoms, ions or molecules. It is important to understand the particle nature of matter.
you are Ramesh of 150 Green Avenue Ajmer you are interesting in going to Canada for higher studies write a letter to the director study board Consultancy Service
Answers
I'm writing to ask if it is possible to continue my education in Canada with the help of your prestigious consultancy service. Regarding the guidelines and prerequisites for applying to Canadian universities, I'd want to know more information.
[Your Name]
[Your Address]
[City, State, ZIP]
[Date]
[Director's Name]
Study Board Consultancy Service
[Consultancy Service Address]
[City, State, ZIP]
Dear Director,
Subject: Inquiry for Higher Studies in Canada
I was making plans for study abroad when I, happily, stumbled onto your advertisement in yesterday's newspaper.
I want to study overseas to take the SAT (Scholastic Assessment Test) course following my board exams. I am a student in class XIl. Although the SAT is difficult to pass, I will put in a lot of effort to do so.
If you could provide me all the information, including the course offered, the length of the course, and the fee structure at the address provided above, I would be eternally grateful.
Yours sincerely,
[Your Name]
To learn more about letter link is here
brainly.com/question/29035775
#SPJ4
According to the spectrum shift in this image, the star we are looking at is __________________________ and moving ______________________ us.
options:
blue-shifting ; closer to
blue-shifting ; further from
red-shifting ; closer to
red-shifting ; further from
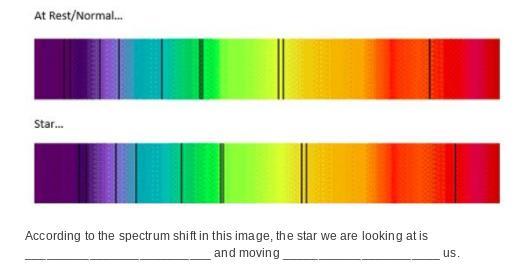
Answers
Answer: D
Explanation:
The lab frame is the same as the rest frame. In the lab, we see the first spectrum. But when we look at the star, we record the second spectrum. When stars move away from us, the light that they emit is red shifted. You can see this by comparing the lines, which are shifting towards the red part of the spectrum.
Magnesium fluoride can be formed by burning magnesium in fluorine gas. With reference to its bonding, explain why magnesium fluoride has a very high melting point.
Answers
Answer:
Magnesium Flouride is a ionic compound and thus has a giant lattice structure. Its ions are held together in this lattice by strong electrostatic forces of attraction. A large amount of energy is needed to overcome the strong electrostatic forces of attraction between the Mg2+ ions and the F- ions to separate the ions. Hence Magnesium fluoride has a very high melting point.
write a ground state electron configuration for each neutral atom
Pb
Sr
U
N
Ag
Ti
Ce
CI
Hg
please help me
Answers
Answer:
Pb[lead] [Xe]4f^145d^106s^26p^2
U[uranium] 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 5s^2 4d^10 5p^6 6s^2 4e^14 5d^10 6p^6
7s^2 5f^4
This notation can be written in core notation or noble gas notation by replacing the
1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 5s^2 4d^10 5p^6 6s^2 4e^14 5d^10 6p^6
7s^2 5f^4
with the noble gas [Rn].
[Rn]7s25f4
N[nitrogen] The full electron configuration for nitrogen is 1s^2 2s^2 2p^3.
Ti[titanium] Ti2+:[Ar]3d^2
Ti:1s^2 2s^2 2p^6 3s^2 3p^6 3d^2 4s^2
1s^2 2s^2 2p^6 3s^2 3p^5 = 17 electrons
(1) electron gain will result to a
negative charge (−), and
(2) electron loss will result to a positive charge (+),
1s^2 2s^2 2p^6 3s^2 3p^6 = 18 electrons
Hg[mercury] You should then find its atomic number is 80. It has a Xe core, so in shorthand notation, you can include [Xe]instead of
1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^2 5p^6,
for 54 electrons. For the 6th row of the periodic table, we introduce the 4f orbitals, and proceed to atoms having occupied 5d orbitals. We, as usual, have the ns orbitals, and n=? for the 6th period?
Mercury has a regular electron configuration. It becomes:
[Xe]4f145d106s2
Explanation:
socratic.org helped me! I'm really sorry if this is wrong!
The term hydrate can be applied to ionic compounds thatA. place hydrogen in the last formulaB. contains trapped water moleculesC. is not reduced down to its simplest formulaD. can be represented by both the stock system and prefix systems of nomenclature
Answers
ANSWER
The term hydrate contains trapped water molecules
STEP-BY-STEP EXPLANATION:
The term hydrate means a substance that contains water molecules
It also means that water is loosely bonded to the ionic compound
According to the options provided, The correct answer is option B ( contains trapped water molecules
Which statement identifies how the particles of gases affect one another’s motion?
Answers
The statement is They affect one another's motion only when they collide. The motion of the gas particles is unaffected by one another in the absence of collisions. One of the essential qualities of an ideal gas is this.
Which of the following best explains how particles move within a gas?Gas particles move quickly in all directions and regularly collide with one another and the container's side.
When particles are constantly moving, what is that condition of matter known as?According to scientists, all matter's subatomic particles are always in motion. To put it another way, matter is made up of kinetic energy. The kinetic theory of matter states that all matter is made up of particles that are constantly moving.
When two gas molecules collide, what occurs next?Collisions are fully elastic; although two molecules' orientations and kinetic energies change when they collide, the overall kinetic energy is conserved. Collisions do not become "sticky." The relationship between the average gas molecule kinetic energy and absolute temperature is direct.
To know more about the Collisions visit:
https://brainly.com/question/31270839
#SPJ1
Complete question;-
Which statement identifies how the particles of gases affect one another’s motion?
A) they affect one another's motion only when they collide.
B) they affect one another's motion only if there are forces of attraction between them.
C) they do not affect one another's motion.
A solution at 25°C is 1.0 x 10^-5 M H3O+. What is the concentration of OH- in this solution
Answers
Answer:
1.0 × 10–9 M OH–
Explanation:
pH = -Log[H+]
pOH = -Log[OH-]
But;
pH + pOH = 14
Therefore;
[H+] + [OH-] = 1.0 × 10^-14 M
Therefore;
[OH-] = 1.0 × 10^-14 M - (1.0 × 10^–5 M)
= 1.0 × 10^-9 M OH–
Electrons :____________.
a. comprise the majority of the mass of an atom.
b. are located in the nucleus of an atom.
c. have a positive charge of one.
d. are the subatomic particles most involved in bonding behavior of atoms.
e. do not participate in the bonding of atoms.
Answers
Electrons do not participate in the bonding of atoms. The correct option is e.
Electrons are subatomic particles that orbit the nucleus of an atom. They have a negative charge of -1 and are responsible for various properties of atoms, such as their reactivity and electrical conductivity. While electrons play a crucial role in chemical reactions and the formation of bonds between atoms, they do not directly participate in the bonding process.
Instead, it is the outermost electrons, known as valence electrons, that are involved in bonding behavior. Valence electrons are the electrons located in the outermost energy level of an atom and are responsible for forming chemical bonds with other atoms.
By sharing, gaining, or losing valence electrons, atoms can achieve a stable electron configuration and form bonds with other atoms to create compounds. Therefore, while electrons are essential for bonding to occur, they themselves do not directly participate in the bonding of atoms. Option e is the correct answer.
To know more about subatomic particles refer here:
https://brainly.com/question/29765133#
#SPJ11
Price increases on goods in the economy signal producers to __________ production.
A.
stop
B.
increase
C.
decrease
D.
maintain
Answers
Answer:
C. decrease
Explanation:
Price increases on goods in the economy signal producers to decrease production.
Producers benefits more when the price of goods and services increases in the market. This makes them have more profit.
To maintain this profit level, they often decrease their production output to create a form of artificial scarcity within the market to sustain the price at which their goods trades.
If the producer increases the amount of goods they produce, this flux of goods can cause a decrease in price as the consumer is favored with a good bargaining power.Answer:
C
Explanation:
edge 2021
Help with theses two different problems!
1.) 125mL of what is added to 45.3mL of 0.71m NaOH solution
2.) 550mL of water is added to 125mL of 3.01M KOH solution
Answers
1. the final concentration of NaOH after adding 125 mL of water to 45.3 mL of 0.71 M NaOH solution is approximately 0.189 M.
2. the final concentration of KOH after adding 550 mL of water to 125 mL of 3.01 M KOH solution is approximately 0.557 M.
1.) If 125 mL of water is added to 45.3 mL of a 0.71 M NaOH solution, the resulting solution will be a diluted NaOH solution. The addition of water will increase the total volume while reducing the concentration of NaOH. To determine the final concentration of NaOH, we need to consider the conservation of moles.
First, let's calculate the moles of NaOH in the initial solution:
moles of NaOH = volume (in L) × concentration (in M)
moles of NaOH = 0.0453 L × 0.71 M = 0.0321433 moles
After adding 125 mL (0.125 L) of water, the total volume of the solution becomes 0.0453 L + 0.125 L = 0.1703 L.
To find the final concentration, we divide the moles of NaOH by the total volume:
final concentration of NaOH = moles of NaOH / total volume
final concentration of NaOH = 0.0321433 moles / 0.1703 L ≈ 0.189 M
Therefore, the final concentration of NaOH after adding 125 mL of water to 45.3 mL of 0.71 M NaOH solution is approximately 0.189 M.
2.) If 550 mL of water is added to 125 mL of a 3.01 M KOH solution, the resulting solution will also be a diluted solution. Again, we will apply the conservation of moles to determine the final concentration of KOH.
First, calculate the moles of KOH in the initial solution:
moles of KOH = volume (in L) × concentration (in M)
moles of KOH = 0.125 L × 3.01 M = 0.37625 moles
After adding 550 mL (0.55 L) of water, the total volume of the solution becomes 0.125 L + 0.55 L = 0.675 L.
To find the final concentration, divide the moles of KOH by the total volume:
final concentration of KOH = moles of KOH / total volume
final concentration of KOH = 0.37625 moles / 0.675 L ≈ 0.557 M
For more such questions on concentration visit:
https://brainly.com/question/28564792
#SPJ8
In the following acid-base reaction,H3O+ is theHCl(g) + H2O(1)→H30+(aq) + CI-(aq)А.BСacidconjugateacidconjugatebase

Answers
When we have an acid-base reaction, the acid will be that substance that donates protons, in the form of H+ ions. The base will be the one that accepts the H+ ions. Now, the base that accepts the protons becomes a potential proton donor, thus becoming a conjugate acid. This is the case with H3O+.
H2O accepts H+ ions, so it is a base and when it becomes H3O+ it becomes a potential H+ proton donor, that is, a conjugate acid.
so, the answer will be B. Conjugate acid
Which amino acid is least likely to produce a hydrogen bond with water or polar molecules?
Answers
Is a substance pure or a ____________?
Answers
Answer:
Pure substance is which it consists only one type of Atom , molecule , or compound....
Explanation:
HOPE IT HELP MARK AS BRAINLIST
Dihydrogen monoxide is a(n) ____. A. covalent compound B. molecular formula C. empirical formula D. Ionic compound
Answers
Answer:
D. Ionic compound
Explanation:
Just based on my opinion
Correct me if I'm wrong tnx:<
Dihydrogen monoxide is a covalent compound. Option A is correct.
Covalent compounds are compounds that are held together by covalent bonds. Covalent bonds are formed when two atoms share electrons. In dihydrogen monoxide, the two hydrogen atoms share a pair of electrons with the oxygen atom. This forms a molecule of water, which is a covalent compound.
Ionic compounds are compounds that are held together by ionic bonds. Ionic bonds are formed when one atom donates electrons to another atom. In an ionic compound, the electrons are not shared, but are transferred from one atom to another. The molecular formula for dihydrogen monoxide is H₂O.
The difference between a molecular formula and an empirical formula is that the molecular formula shows the actual number of atoms of each element in a molecule, while the empirical formula shows the simplest ratio of the atoms of each element in a molecule. Option A is correct.
To know more about the Compound, here
https://brainly.com/question/29448163
#SPJ2
Plz help
A student determines that the mass of a metal rod
is 12.0 g. He places 10.0 mL of water in a 25 ml
graduated cylinder. When the metal rod is placed
in the water, the graduated cylinder then reads
15,0 mL. What is the density of the metal rod?
Answers
Answer:
Explanation:
There's a great many assumptions in this question. We have to assume that the rod was completely immersed. We also have to note that we can read the volumes to 3 places accurately. School graduated cylinders are not that accurate.
Leaving those objections aside, here is how the question should be done.
Final volume = 15.0 mL
Initial volume = 10.0 mL
Water displaced = 5.0 mL
The amount of water displaced is the same as the volume of the metal rod.
Finally the mass of the rod is 12.0 grams.
Density = mass / volume
Density = 12.0 grams / 5.0 mL
density= 2.4 grams / mL a mL and a cc^3 are the same thing.
density = 2.4 grams / cc^3
Vocabulary crossword puzzle properties of minerals
Answers
Answer:
? no image
Explanation:
Calculate the % composition of these compounds:A. Ethane (C2H6)B. Sodium hydrogen sulfate (NaHSO4)
Answers
The percentage composition of ethane is 39.99% carbon
60.01% hydrogen and that of sodium hydrogen sulfate is 19.15% sodium 0.84% hydrogen 26.71% sulfur 53.30% oxygen
A. Ethane (C2H6)
To calculate the percent composition of ethane, we need to determine the molar mass of the compound, which is the sum of the atomic masses of all the atoms in the molecule.
Molar mass of ethane = (2 × molar mass of carbon) + (6 × molar mass of hydrogen)
= (2 × 12.011 g/mol) + (6 × 1.008 g/mol)
= 30.07 g/mol
Now we can calculate the percent composition of each element in ethane:
% composition of carbon = (2 × molar mass of carbon ÷ molar mass of ethane) × 100%
= (2 × 12.011 g/mol ÷ 30.07 g/mol) × 100%
= 39.99%
% composition of hydrogen = (6 × molar mass of hydrogen ÷ molar mass of ethane) × 100%
= (6 × 1.008 g/mol ÷ 30.07 g/mol) × 100%
= 60.01%
Therefore, the percent composition of ethane is:
39.99% carbon
60.01% hydrogen
B. Sodium hydrogen sulfate (NaHSO4)
To calculate the percent composition of sodium hydrogen sulfate, we need to determine the molar mass of the compound.
Molar mass of NaHSO4 = molar mass of Na + molar mass of H + molar mass of S + 4 × molar mass of O
= 22.99 g/mol + 1.008 g/mol + 32.06 g/mol + 4 × 16.00 g/mol
= 120.06 g/mol
Now we can calculate the percent composition of each element in sodium hydrogen sulfate:
% composition of sodium = (molar mass of Na ÷ molar mass of NaHSO4) × 100%
= (22.99 g/mol ÷ 120.06 g/mol) × 100%
= 19.15%
% composition of hydrogen = (molar mass of H ÷ molar mass of NaHSO4) × 100%
= (1.008 g/mol ÷ 120.06 g/mol) × 100%
= 0.84%
% composition of sulfur = (molar mass of S ÷ molar mass of NaHSO4) × 100%
= (32.06 g/mol ÷ 120.06 g/mol) × 100%
= 26.71%
% composition of oxygen = (4 × molar mass of O ÷ molar mass of NaHSO4) × 100%
= (4 × 16.00 g/mol ÷ 120.06 g/mol) × 100%
= 53.30%
Therefore, the percent composition of sodium hydrogen sulfate is:
19.15% sodium
0.84% hydrogen
26.71% sulfur
53.30% oxygen
learn more about oxygen here:
https://brainly.com/question/2272415?utm_source=android&utm_medium=share&utm_campaign=question
#SPJ11
which of the following describes the reaction of molecules as snow melts
a) The ice absorbs heat energy and the molecules move further away
b) The ice releases heat energy and the molecules move further away
c) The ice absorbs heat energy and the molecules move closer together
d) The ice releases heat energy and the molecules move closer together
Answers
During the melting of ice, the ice absorbs heat energy and the molecules move further away; option A.
What is melting?Melting refers to the process by which a solid substance changes to liquid when heat is added to it.
The melting of pure substances occur at a definite temperature called the melting point of that substance.
The molecules of a substance move further apart when they melt as the attractive forces between them are weakened.
The melting of ice is an example of the process of melting.
During the melting of ice, the ice absorbs heat energy and the molecules move further away.
In conclusion, melting of solids occur when heat is added to the solid.
Learn more about melting at: https://brainly.com/question/40140
#SPJ1
Q4 This question relates the combustion reactions of acetylene, hydrogen and ethane. (a) Express the stoichiometric ecpigtions for the combustion reactions of acetylene, hydrogen and ethane with their respective standard heats of combustion obtained from physical property table. (b) Verify the standard heat of combustion of acetylene in Q4(a) by using heat of formation method. (c) The equation below shows the acerylene hydrogenation reaction: C2H2(g)+2H2(g)→C2H6(g) (i) Compute the standard heat of acetylcne hydrogenation reaction using tabulated heats of formation and heats of combustion. (ii) Verify the answer in Q4(e)(1) by using Hess's Law.
Answers
Stoichiometric equations for the combustion reactions ΔHf° (C2H2) = (2 x (-393.5)) + (-285.8) - (-1299.5) = +226.7 kJ mol-1(c) Acetylene hydrogenation reaction
Acetylene combustion reaction:C2H2 (g) + (5/2) O2 (g) → 2 CO2 (g) + H2O (l) ΔHc° = -1299.5 kJ mol-1 Hydrogen combustion reaction:2H2 (g) + O2 (g) → 2 H2O (l) ΔHc° = - 483.7 kJ mol-1Ethane combustion reaction:C2H6 (g) + (7/2) O2 (g) → 2 CO2 (g) + 3 H2O (l) ΔHc° = - 1560 kJ mol-1(b) Heat of formation method for verifying the standard heat of combustion of acetylene: The standard heat of combustion of acetylene from the heat of formation method is:ΔHc° (C2H2) = 2 ΔHf° (CO2) + ΔHf° (H2O) - 2 ΔHf° (C2H2) = -1299.5 kJ mol-1ΔHf° (CO2) = -393.5 kJ mol-1ΔHf° (H2O) = -285.8 kJ mol-1.
For verifying the answer in Q4(e)(1) using Hess's Law, we need to convert acetylene hydrogenation reaction into a combination of other reactions:Reaction 1:C2H2 (g) + (2.5) O2 (g) → 2 CO2 (g) + H2O (l) ΔH1 = -1299.5 kJ mol-1Reaction 2:2 CO2 (g) + 2.5 H2 (g) → C2H6 (g) + 5 O2 (g) ΔH2 = +1560 kJ mol-1After multiplying and adding the above equations, we get the required reaction as:C2H2 (g) + 2 H2 (g) → C2H6 (g) ΔH = -396.1 kJ mol-1.
To know more about reactions visit:
https://brainly.com/question/16737295
#SPJ11
Which one of the following statements explains why mass is lost when a student heats a sample of BaCl₂ • 2H₂O crystals?
Answers
Answer:
where are the statements??
Explanation: