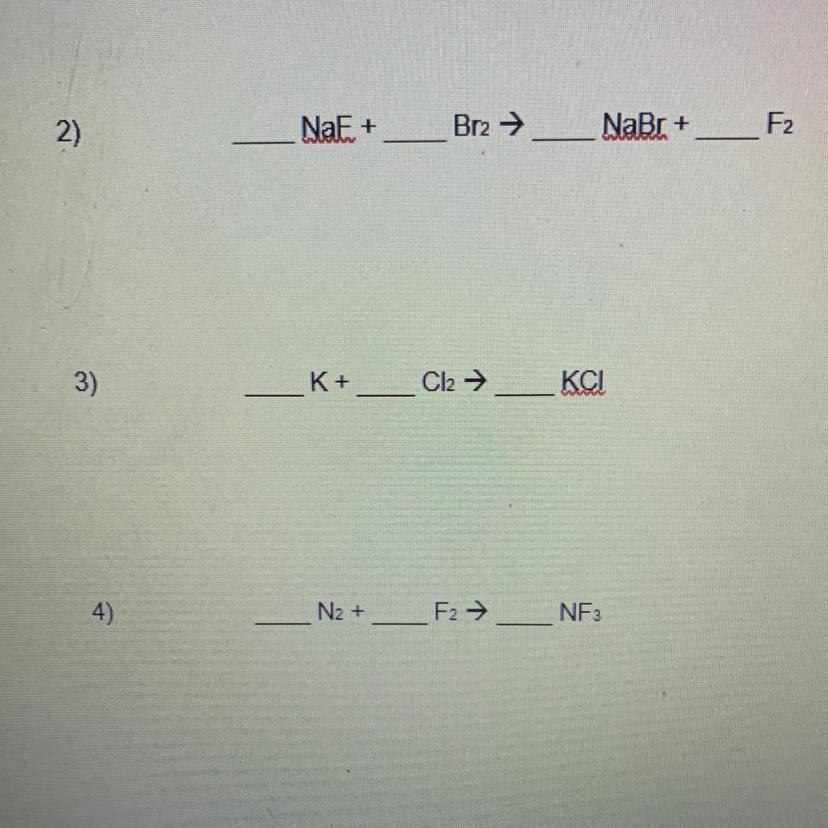
Answers
Answer:2NaF + Br2 —> 2NaBr + F2
2K + Cl2 —> 2KCl
N2+ 3F2 —> 2NF3
Explanation:
Related Questions
Find the number of moles of the following substances 210 grams of NaHCO3
Answers
The number of moles of 210 grams of NaHCO₃ is 2.5 moles.
How to find the Number of moles ?To calculate the number of moles use the formula
Number of moles = \(\frac{\text{Given Mass}}{\text{Molar Mass}}\)
Mass of NaHCO₃ = 210 g
Now we have to find the Molar mass of NaHCO₃
= Atomic mass of Na + Atomic mass of H + Atomic mass of C + 3 (Atomic mass of O)
= 23 + 1 + 12 + 3 (16)
= 36 + 48
= 84 g/mol
Now put the value in above formula we get
Number of moles = \(\frac{\text{Given Mass}}{\text{Molar Mass}}\)
= \(\frac{210\ g}{84\ g/mol}\)
= 2.5 moles
Thus from the above conclusion we can say that The number of moles of 210 grams of NaHCO₃ is 2.5 moles.
Learn more about the Moles here: https://brainly.com/question/15356425
#SPJ1
How is proper science validated?
*assumptions and quantitative data
*opinions and beliefs
*tests and personal claims
*experimentation and testing
Answers
Answer:
uestion
Answers 27
Similar questions
Related publications
Question
I think we make a mistake when we consider that data are qualitative if they come from applying qualitative methods and techniques or if they are collected in qualitative researches. I think we make the same mistake when we consider quantitative all data collected through, for example, questionnaires.
I think we have to consider the qualitative or quantitative character of data looking exclusively at data. Are they numeric? Are they textual or visual?
I think (as Traian Rotariu argues) that quantitative data are numeric and that qualitative data are textual or visual and that we could gather quantitative and qualitative data with each and every method and technique being it qualitative or quantitative.
For example most of the data gathered with questionnaires are qualitative in their primary form: opinions, gender, preferences etc. and just a few are quantitative in their primary form: income, age, children's number And more
Answer the questions below:
1.Give an example of something that has matter.
2.Give an example of something that doesn't have matter.
3.What is the difference between an chemical change and a physical change?
4.What 2 main kind of changes can you observe involve chemical reactions?
5.What is a precipitate?
6.What is the difference between an endothermic reaction and an exothermic reaction?
Answers
Answer:
1. some thing that has matter is an apple, a person,a table. things that does 2.not Light.,Sound.,Heat.Energy.Gravity.Time.A Rainbow.Love.
3. Physical changes only change the appearance of a substance, not its chemical composition. Chemical changes cause a substance to change into an entirely substance with a new chemical formula. Chemical changes are also known as chemical reactions.
4.A chemical reaction is usually accompanied by easily observed physical effects, such as the emission of heat and light, the formation of a precipitate, the evolution of gas, or a color change. Absolute confirmation of a chemical change can only be validated by chemical analysis of the products!
5.Precipitate: In chemistry, a solid formed by a change in a solution, often due to a chemical reaction or change in temperature that decreases solubility of a solid. In meteorology a precipitate is liquid or solid water (rain, snow, etc.) falling from the sky.
6.n simple terms, the endothermic reactions absorb energy from the surrounding that is in the form of heat. On the other hand, an exothermic reaction releases energy into the surrounding of the system.
hope this help plz give me a thxs and brainlyest and a five star thx and you welcome let me know if this helped
Explanation:
3. A Wilkinson’s catalyst is widely used in the hydrogenation of alkenes. Show a catalytic cycle, including: i. chemical structure of the catalyst, with complete stereochemistry ii. molecular geometry of catalyst iii. type of reactions involved iv. the appropriate starting material, reagent and solvent v. major and minor end-products vi. all intermediates, for each reaction stated in (iii)
Answers
We can see here that the catalytic cycle for the hydrogenation of alkenes using Wilkinson's catalyst involves several steps.
What are the steps involved?Here's an overview of the catalytic cycle, including the necessary details:
i. Chemical structure of the catalyst:
Wilkinson's catalyst, also known as chloridotris(triphenylphosphine)rhodium(I), has the following chemical structure: [RhCl(PPh3)3]
ii. Molecular geometry of the catalyst:
The Wilkinson's catalyst has a trigonal bipyramidal geometry around the rhodium center. The three triphenylphosphine (PPh3) ligands occupy equatorial positions, while the chloride (Cl) ligand occupies an axial position.
iii. Type of reactions involved:
The catalytic cycle involves several reactions, including:
Oxidative addition: The rhodium center undergoes oxidative addition, reacting with molecular hydrogen (H2) to form a dihydride intermediate.Alkene coordination: The alkene substrate coordinates to the rhodium center, forming a π-complex.Hydrogenation: The coordinated alkene undergoes hydrogenation, resulting in the addition of hydrogen atoms to the double bond and formation of a metal-alkyl intermediate.Reoxidation: The metal-alkyl intermediate reacts with a hydrogen molecule to regenerate the rhodium dihydride species.iv. Starting material, reagent, and solvent:
The starting material is an alkene, and the reagent is Wilkinson's catalyst ([RhCl(PPh3)3]). The reaction is typically carried out in a suitable solvent, such as dichloromethane (CH2Cl2) or tetrahydrofuran (THF).
v. Major and minor end-products:
The major end-product of the hydrogenation reaction is the fully saturated alkane, resulting from the addition of hydrogen across the double bond. The minor end-product may include cis- or trans-configured alkanes if the original alkene substrate possesses geometric isomers.
vi. Intermediates:
The intermediates in the catalytic cycle include:
Rhodium dihydride complex: [RhH2(PPh3)3]Alkene-Rhodium π-complex: [Rh(η2-alkene)(PPh3)3]Metal-alkyl intermediate: [Rh(alkyl)(PPh3)3]These intermediates play a crucial role in facilitating the hydrogenation reaction and enabling the catalytic cycle to proceed.
Learn more about Wilkinson’s catalyst on https://brainly.com/question/31972308
#SPJ1
how is the Bohr atomic model different from the plum-pudding model ?
Answers
The plum-pudding model suggested a uniform distribution of electrons within a positively charged atom, whereas the Bohr atomic model introduced the concept of quantized energy levels and specific orbits for electrons.
The Bohr atomic model and the plum-pudding model are two distinct models that were proposed to explain the structure of atoms, and they differ in their fundamental concepts.
The plum-pudding model, also known as the Thomson model, was proposed by J.J. Thomson in 1904. According to this model, an atom consists of a positively charged sphere (the "pudding") with embedded negatively charged electrons (the "plums").
In other words, the electrons were thought to be uniformly distributed throughout the positively charged atom. This model suggested that the atom was overall neutral and did not contain any distinct substructures.
On the other hand, the Bohr atomic model, proposed by Niels Bohr in 1913, introduced the concept of quantized energy levels within an atom. According to this model, electrons orbit the nucleus in specific, discrete energy levels or shells.
These energy levels are represented by fixed orbits or paths, with electrons occupying only certain allowed orbits. The model also introduced the idea that electrons can transition between energy levels by emitting or absorbing energy in discrete packets called photons. This model explained phenomena like atomic spectra and the stability of atoms.
For mopre such questions on Bohr atomic model visit:
https://brainly.com/question/18002213
#SPJ8
Fran finds a dark-colored rock that she identifies as basalt. Which property gives the rock its dark color?
O its shape
O its banding
its grain size
O its mineral content
Answers
Answer:
Mineral Content.
Explanation:
shape has nothing to do with the color displayed.
Nor does the grain size.
I don't know what banding is. Whatever it is, it is not the answer.
D: mineral content.
The diagram below shows a stack of rock layers. These layers have not changed position since they formed.
The fossils in Layer 2 are younger than those in Layer __ and older than the fossils in Layer __
Answers
Answer: The fossils in Layer 2 are younger than those in Layer 1, and older than the fossils in layer 3. (assuming layer 1 is at the bottom, and layer 3 is at the top.)
Explanation: Think of how this stack of rocks formed. Layer 1 would have deposited first along with it's fossils, followed by 2, 3, etc. Layer 2's fossils would be younger than Layer 1, but older than Layer 3.
Oxygen is:
a compound.
an element.
a solution.
a suspension.
Answers
A 13.0 ml sample of an acid requires 37,3 ml of 0.303N NaOH for neutralization. Calculate the normality of the acid.
Answers
The amount of 0.303N NaOH needed to neutralize a 13.0 ml sample of acid is 37,3 ml. Acid is 0.823N normal, according to the standard.
Explain about the neutralization.The idea of neutralization, according to which an acid reacts with a base to create salt and water. By comparing the molarity of the base to the amount of base needed for neutralization, it is possible to calculate the molarity of the acid. Once the molarity and the acid's valence (or charge) have been multiplied, the acid's normalcy can be determined.
By deducting the volume of NaOH (37.3 ml) from the volume of the acid sample in Step 1, you can calculate the amount of acid that was utilized in the neutralization procedure (13.0 ml).
Consequently, 13.0 ml to 37.3 ml
= -24.3 ml
Use the equation moles = normality x volume to determine the number of moles of acid that were used in the process.
Consequently, moles equal 0.303N x -24.3 ml.
= -7.33 moles
Determine the acid's normality by multiplying the volume by the formula normalcy = moles.
As a result: normalcy = -7.33 moles / 13.0 ml
= -0.823N
To learn more about neutralization, visit
brainly.com/question/15347368
#SPJ1
Calculate the standard cell potential for each reaction below, and note whether the reaction is spontaneous under standard state conditions.
(a) Mg(s) + Ni2+(aq)⟶ Mg2+(aq) + Ni(s)
(b) 2Ag+(aq) + Cu(s)⟶ Cu2+(aq) + 2Ag(s)
(c) Mn(s) + Sn(NO3)2(aq)⟶ Mn(NO3)2(aq) + Sn(s)
(d) 3Fe(NO3)2(aq) + Au(NO3)3(aq)⟶ 3Fe(NO3)3(aq) + Au(s)
Answers
The standard cell potential (also known as the standard electrode potential) is a measure of the potential difference between two electrodes in an electrochemical cell under standard state conditions.
(a) Standard Cell Potential for Mg(s) + Ni2+(aq)⟶ Mg2+(aq) + Ni(s):
E0cell = (0.79 - (-0.25))V = 1.04 V.
This reaction is spontaneous under standard state conditions.
(b) Standard Cell Potential for 2Ag+(aq) + Cu(s)⟶ Cu2+(aq) + 2Ag(s):
E0cell = (0.34 - (-0.52))V = 0.86 V.
This reaction is spontaneous under standard state conditions.
(c) Standard Cell Potential for Mn(s) + Sn(NO3)2(aq)⟶ Mn(NO3)2(aq) + Sn(s):
E0cell = (0.15 - (-0.14))V = 0.29 V.
This reaction is spontaneous under standard state conditions.
(d) Standard Cell Potential for 3Fe(NO3)2(aq) + Au(NO3)3(aq)⟶ 3Fe(NO3)3(aq) + Au(s):
E0cell = (1.23 - (-1.50))V = 2.73 V.
This reaction is spontaneous under standard state conditions.
Learn more about standard cell potential at : https://brainly.com/question/29653954
#SPJ4
chlorine gas occupies a volume of 25 mL at 27°C. To what volume will it occupy at 600 k
Answers
Answer: 50mL
Explanation: This is solved just like you'd solve a proportion in mathematics. But first, you need to make your temperature values have the same units. It is ideal to have both units in K almost 90% of the time. So to convert the values, you'll add 273 to your Celsius temperature: 27. This gives you 300. Then, you set 300K/25mL = 600K/?. We see that to get from 300 to 600, we just double our values. Therefore, we'll do the same to the 25mL. This gives us 50mL.
I hope this helps!
How many mL of 6.00 M HCl are needed to prepare 1500 mL of 0.200 M HCl solution?
Answers
Answer:
50mL
Explanation:
First find out how many moles will be needed in your final solution.
concentration (c) = Moles / Volume of solution (liters)
or rearrange the formula to find your Moles
Moles = concentration x volume of solution
convert your mL to L. 1500 mL is the same as 1.5 L
Moles = 0.200 moles/liter x 1.5 liter (the liters cancel each other out)
Moles = 0.3 moles
Now use the same formula to determine how much of the original solution you need to end up with what you want.
Rearrange the formula again to solve for the volume.
Volume of solution = Moles / concentration
Volume = 0.3 moles / 6.00 (moles/liter) here the moles cancel out
Volume = 0.05 L
convert your L into mL
Volume = 50 mL
50 mL of 6.00 M HCl are needed to prepare 1500 mL of 0.200 M HCl solution.
HOW TO CALCULATE VOLUME:
The volume of solution needed to prepare a diluted solution can be calculated using the following formula:C1V1 = C2V2
Where;
C1 = initial concentrationC2 = final concentrationV1 = initial volumeV2 = final volumeAccording to this question, C1 = 6.00 M, C2 = 0.200M, V1 = ?, V2 = 1500mL6 × V1 = 0.200 × 15006V1 = 300V1 = 300/6V1 = 50mLTherefore, 50 mL of 6.00 M HCl are needed to prepare 1500 mL of 0.200 M HCl solution.Learn more about how to find volume of solution: https://brainly.com/question/15832344?referrer=searchResults
Which bond occurs between two metal atoms?
Answers
Answer:
An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. A covalent bond involves a pair of electrons being shared between atoms.
Answer:
Hi! An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually a nonmetal. A covalent bond involves a pair of electrons being shared between atoms.
If this helped drop a brainley plz ;)
Explanation:
Consider the reaction 4FeS2 + 11O2 → 2Fe2O3 + 8SO2. If 8 moles of FeS2 react with 15 moles of O2, what is the limiting reactant? (3 points)
SO2
O2
Fe2O3
FeS2
Answers
Answer:
O2
Explanation:
for find the limiting reactant you must calculate the moles of the reactants from the amount that you have and from the MM:
MM FeS2 = 120n = 26.2g / 120g/mol = 0,218 mol
MM O2 = 32n = 5,44g/32g/mol = 0,17 mol
The limiting reactant is
O2
What are constraints
Answers
Answer:
ndcuffaStuff that restrains you from something or someone basicially handcuffs or shackles
Explanation:
Answer:
a limitation or restriction.
OR
stiffness of manner and inhibition in relations between people.
Help me out
On another planet, the isotopes of titanium have the given natural abundances.

Answers
The average atomic mass of titanium on the given planet is approximately 46.68164 atomic mass units (u). The average atomic mass may vary depending on the specific isotopic composition of titanium found on different celestial bodies or regions.
To calculate the average atomic mass of titanium on the given planet, we need to consider the natural abundances and masses of each isotope of titanium.
The average atomic mass is calculated by multiplying the natural abundance of each isotope by its respective mass and summing them up.
Let's perform the calculation step by step:
Step 1: Multiply the abundance of each isotope by its mass:
(73.700% * 45.95263 u) + (15.000% * 47.94795 u) + (11.300% * 49.94479 u)
Step 2: Calculate the individual contributions from each isotope:
= (0.737 * 45.95263) + (0.150 * 47.94795) + (0.113 * 49.94479)
Step 3: Add up the individual contributions:
= 33.84765431 + 7.1921925 + 5.64179347
Step 4: Sum up the contributions:
= 46.68164 u
Therefore, the average atomic mass of titanium on the given planet is approximately 46.68164 atomic mass units (u).
It's important to note that the calculation assumes the provided natural abundances are accurate and representative of the titanium isotopes on that planet.
for more questions on atomic mass
https://brainly.com/question/30390726
#SPJ8
4. The volume of a liquid sample is measured as 15.43 L. We need to know the volume in
mL.
3.
b.
What conversion factor would be used in the calculation?
Calculate the volume in mL.
Answers
Conversion factor used to convert volume is 1L = 1000 mL.
15.43L = 15430mL
The same attribute is expressed using a unit conversion but in a different unit of measurement. For instance, time can be expressed in minutes rather than hours, and distance can be expressed in kilometers, feet, or any other measurement unit instead of miles. Measurements are frequently offered in one set of units, like feet, but are required in another set, like chains. A conversion factor is a mathematical equation that facilitates an equal exchange of feet for chains.
A conversion factor is a number that is used to multiply or divide one set of units into another. If a conversion is required, it must be done using the correct conversion factor to get an identical value.
To learn more about the Conversion factors please visit-
https://brainly.com/question/28366871
#SPJ9
How do you determine a number of each particle?
Answers
Answer:
Search Results
Featured snippet from the web
To calculate the numbers of subatomic particles in an atom, use its atomic number and mass number:
number of protons = atomic number.
number of electrons = atomic number.
number of neutrons = mass number - atomic number.
Explanation:
A sample of gas occupies a volume of 350.0 mL at 840mm Hg and 33°C. Determine the volume of this sample at 600 mm By and 52°C
Answers
Answer:
V₂ = 520.42 mL
Explanation:
Given data:
Initial volume = 350.0 mL
Initial pressure = 840 mmHg
Initial temperature = 33°C (33 +273 = 306 K)
Final temperature = 52°C (52+273 = 325 K)
Final volume = ?
Final pressure = 600 mmHg
Formula:
P₁V₁/T₁ = P₂V₂/T₂
P₁ = Initial pressure
V₁ = Initial volume
T₁ = Initial temperature
P₂ = Final pressure
V₂ = Final volume
T₂ = Final temperature
Solution:
V₂ = P₁V₁ T₂/ T₁ P₂
V₂ = 840 mmHg × 350.0 mL × 325 K / 306 K × 600 mmHg
V₂ = 95550000 mmHg.mL.K /183600 K.mmHg
V₂ = 520.42 mL
What are the characteristics of a gas?
A. no definite shape but a definite volume
B. a definite shape but no definite volume
C. a definite volume and definite shape
D. no definite shape or definite volume
Answers
Answer:
D. no definite shape or. definite volume
Explanation:
Gases do not have a definite shape or volume because the molecules in gases are very loosely packed, they have large intermolecular spaces and hence they move around. The particles of solid are closely packed and occupy less space while particles of gases are loosely packed and occupy the complete space available.
Hope this helps :)
What is 497.680g to the nearest 1g
Answers
The gram and kilogram are the units which are used to measure the mass of the objects. The unit kilogram is used to measure the mass of heavier objects. The nearest gram of 497.680g is 498 g.
What is mass?The term mass is the most basic property of the matter. The mass of an object is defined as the measure of the amount of matter present in an object. The SI unit of mass is kg.
The mass can never be zero and it is a scalar quantity.
Here 497.680g = 498 g
Thus the 497.680g to the nearest 1g is 498 g.
To know more about mass, visit;
https://brainly.com/question/28704035
#SPJ9
how do you think a device could change the sound that we hear? Make sure you use vocabulary such as frequency, energy and amplitude
Answers
Answer:
good luck tho
Explanation:
Why does the solubility of alkaline earth metal hydroxides in water increase down the group?
Answers
Answer:
The solubility of alkaline earth metal hydroxides in water increases down the group because the size of the metal cation increases as you move down the group. This increase in size results in a decrease in the cation's charge density, which makes it less able to attract and hold onto hydroxide ions. As a result, the hydroxides become more soluble in water as you move down the group. Additionally, the lattice energies of the hydroxides decrease down the group, making it easier to break apart the crystal lattice structure and dissolve the hydroxides in water.
Plate tectonics is helpful in explaining many things we observe on Earth. Which of the following is the best example of something that results from tectonic plate movement?
Answers
Answer: Seafloor spreading causes changes in coastlines
Sublimation of an element or compound is the change from a solid directly to a gas with no intermediate liquid stage. Sharada was told that if pepper seeds are placed along with camphor, the sublimation of camphor would reduce. Of these boxes, (some are open and some are closed) which two should she use to check whether this is true?
Give a detailed answer

Answers
The two which she should use to check whether the sublimation of camphor would reduce if pepper seeds are placed along with it are P and S and is denoted as option C.
What is Sublimation?This is referred to as the transition of a substance directly from the solid to the gas state, without passing through the liquid state and an example is iodine crystal.
Options P and S are both open boxes and one contains only camphor while the other contains camphor and pepper seeds which is used to compare their rate of sublimation to know if the addition of the seeds caused a reduction.
Read more about Sublimation here https://brainly.com/question/8647675
#SPJ1
Identify the element in group 15, period 3 by drawing a Lewis Dot Structure
Answers
Answer:
Phosphorus
Explanation:
Lewis Dot Structure is a structural representation of a molecule that shows valence electrons of a molecule.
The element in group 15, period 3 is Phosphorus (P). Phosphorus has an atomic number 15 and valence electrons are 5. So, the lewis structure will show 5 dots around P atom.

What is the amino group in a protein?
Answers
An amino group is a functional group consisting of an amino (-NH2) and a carboxyl (-COOH) group, which is found in proteins.
It is essential for the formation of peptide bonds between amino acids, which are the building blocks of proteins. The amino group is partially positively charged and is therefore able to interact with other molecules through hydrogen bonding and electrostatic interactions.
The amino group is also able to donate a hydrogen atom, making it an important component in many biochemical reactions.
Furthermore, it plays an important role in the folding of proteins, as the electrostatic interactions between the amino and carboxyl groups help stabilize the protein's three-dimensional structure. In addition, the presence of the amino group can influence the solubility of proteins in different solutions.
To know more about proteins, click below:
https://brainly.com/question/10058019
#SPJ4
Which of the following cannot be read directly from the periodic table?
an element's atomic mass
an element's number of neutrons
an element's symbol
an element's number of protons
Answers
find me the answer to this question

Answers
Explanation:
1. ig it's opt 3
2.
3. opt 1
4. opt 4
:)
When using vacuum filtration to separate a dissolved solid from an undissolved solid, what techniques should you use to ensure a quantitative separation
Answers
Answer: See explanation
Explanation:
Vacuum filtration is referred to as a fast filtration technique that is used in the separation of solids from liquids. It is also used to collect a desired solid. It basically uses a side-arm flask and a Buchner funnel.
Based on the question, the techniques that should be used to ensure a quantitative separation goes thus:
• Wet the filter paper before the mixture is poured into the filter funnel.
• Then, carefully rinse the flask with a little amount of water into the filter funnel.
• After that, the solid on the filter paper should be washed the with a small amount of water.
• Finally, Dry the solid on the filter paper when the separation is done.