Answers
Answer:
\(10\, {\rm Fe}\rm SO_4 + 2\, \rm K {Mn} O_4 + 8\, H_2SO_4\\ \to 5\, {Fe} (SO_4)_3 + K_2SO_4 + 2\, {Mn}SO_4 + 8\, H_2O\).
Explanation:
Identify the elements with oxidation state changes:
Oxidation states of iron, \(\rm Fe\):
\(+2\) in \(\rm FeSO_4\) among the reactants.\(+3\) in \(\rm Fe_2(SO_4)_3\) among the products.Change to the oxidation state: \(+1\) (oxidation) for each \(\rm Fe\) atom.Oxidation state of manganese, \(\rm Mn\):
\(+7\) in \(\rm KMnO_4\) among the reactants.\(+2\) in \(\rm MnSO_4\) among the products.Change to the oxidation state: \((-5)\) (reduction) for each \(\rm Mn\) atom.The change in the oxidation state of \(\rm Mn\) is five times the opposite of the change to the oxidation state of \(\rm Fe\). If there are one mole of \(\rm Mn\!\) atoms in each mole of this reaction, there would be five times as many \(\rm Fe\!\) atoms per mole reaction. In other words:
\(\displaystyle 5\, \overset{+2}{\rm Fe}\rm SO_4 + 1\, \rm K \overset{+7}{Mn} O_4 + ?\, H_2SO_4\\ \to \frac{5}{2}\, \overset{+3}{Fe} (SO_4)_3 + ?\, K_2SO_4 + 1\, \overset{+2}{Mn}SO_4 + ?\, H_2O\).
(Notice that each mole of this reaction would include five times as many \(\rm Fe\) atoms as \(\rm Mn\) atoms.)
Multiply the coefficients by \(2\) to eliminate the fraction:
\(\displaystyle 10\, {\rm Fe}\rm SO_4 + 2\, \rm K {Mn} O_4 + ?\, H_2SO_4\\ \to 5\, {Fe} (SO_4)_3 + ?\, K_2SO_4 + 2\, {Mn}SO_4 + ?\, H_2O\).
Find the unknown coefficients using the conservation of atoms.
Reactants:
\(2\) potassium \(\rm K\) atoms in two \(\rm K_2SO_4\) formula units.Therefore, among the products:
\(2\) potassium \(\rm K\) atoms in one \(\rm K_2SO_4\) formula unit.\(\displaystyle 10\, {\rm Fe}\rm SO_4 + 2\, \rm K {Mn} O_4 + ?\, H_2SO_4\\ \to 5\, {Fe} (SO_4)_3 + {1}\, K_2SO_4 + 2\, {Mn}SO_4 + ?\, H_2O\).
Products:
\(5 \times 3 + 2 + 1 = 18\) sulfur \(\rm S\) atoms in five \(\rm Fe_2(SO_4)_3\) formula units, two \(\rm K_2 SO_4\) formula units, and one \(\rm MnSO_4\) formula unit.Reactants:
There are already ten \(\rm S\) atoms in that ten \(\rm Fe(SO_4)_2\) formula units. The other \(18 - 10 = 8\) formula units would correspond to eight \(\rm H_2SO_4\) molecules among the reactants of this reaction.\(\displaystyle 10\, {\rm Fe}\rm SO_4 + 2\, \rm K {Mn} O_4 + 8\, H_2SO_4\\ \to 5\, {Fe} (SO_4)_3 + {1}\, K_2SO_4 + 2\, {Mn}SO_4 + ?\, H_2O\).
Products:
There are \(8 \times 2 = 16\) hydrogen \(\rm H\) atoms in that eight \(\rm H_2SO_4\) molecules.Therefore, among the products:
There would be \(16 / 2 = 8\) molecules of \(\rm H_2O\), with two \(\rm H\) atoms in each \(\rm H_2O\!\) molecule.\(\displaystyle 10\, {\rm Fe}\rm SO_4 + 2\, \rm K {Mn} O_4 + 8\, H_2SO_4\\ \to 5\, {Fe} (SO_4)_3 + {1}\, K_2SO_4 + 2\, {Mn}SO_4 + 8\, H_2O\).
Related Questions
A 2.498M solution contains 245 g of H2SO4. What is the volume of the solution?
Answers
The volume of a 2.498M solution that contains 245 g of sulfuric acid is 1L.
How to calculate volume?The volume of a solution can be calculated from the molarity using the following expression:
Molarity = no of moles ÷ volume
Molarity refers to the concentration of a substance in solution, expressed as the number moles of solute per litre of solution.
no of moles of sulfuric acid = 245g ÷ 98g/mol
no of moles of sulfuric acid = 2.5moles
volume = 2.5moles ÷ 2.498M
volume = 1L
Therefore, 1L is the volume of the sulfuric acid solution.
Learn more about volume at: https://brainly.com/question/28698457
#SPJ1
12. What structures does the fish have that are NOT present in the fly?
Answers
What is the average mass (in grams) of a single chlorine dioxide molecule?
Answers
Answer:
35.5 is the answer
Explanation:
pls mark me brainlest
? Which types of batteries are commonly used in laboratory equipments
Answers
Answer:
your question is not very clear but I think the answer is either
Primary (non-rechargeable)
Secondary (rechargeable) or
Lithium-ion battery.
Rechargeable li-ion button cell.
C c Why did they work an average brightness for each length of graphite tested?
Answers
An average brightness was calculated for each length of graphite tested to get a better understanding of the relationship between the length of the graphite and the brightness of the line it produced.
What is the use of graphite?Graphite has many uses in various industries. Some of its uses include: Pencils, Lubricants, Refractories, Batteries, Electrodes, Nuclear reactors, Aerospace industry.
By calculating the average, it is possible to see if there is a trend in the data and if longer or shorter lengths of graphite produce brighter or duller lines. This information can be useful in determining the best length of graphite to use for a particular task or project.
Find out more on graphite here: https://brainly.com/question/27860158
#SPJ1
1. As elements go across from left to right in a period,
they hold their electrons more tightly, because they
have more protons in the nucleus attracting the
orbitting electrons in the electron cloud. So across a
period, does atomic radius increase or decrease?
Answers
Answer:
decrease.
Explanation:
WHY? - As you go across a period, electrons are added to the same energy level. ... The concentration of more protons in the nucleus creates a "higher effective nuclear charge." In other words, there is a stronger force of attraction pulling the electrons closer to the nucleus resulting in a smaller atomic radius.
1. Write the formula for the following compounds a. Bismuth(III) Nitrate:b. Ammonium Chlorate:c. Dichlorine Heptoxide:d. Aluminum Phosphide:e. Hypochlorous Acid:f. Diphosphorus Pentoxide:
Answers
A chemical formula is a way of identifying a compound by placing all the elements present in the compound together using their acronyms
1.
A) Bi(NO3)3
B) NH4ClO3
C) Cl2O7
D) AlP
E) HClO
F) P2O5
If 1.500 g of sodium phosphate (Na3PO4) is dissolved in water, and the total volume of the solution is 1.00 L, what is the molar concentration of sodium ion [Na+] in this solution? (MW(Na3PO4) = 163.94 g/mole)
Answers
In this solution, sodium ion [Na+] has a molar concentration of 0.02745 M. (or 27.45 mM).
What is sodium phosphate?A salt combining sodium and phosphate, sodium phosphate has the chemical formula Na3PO4. It is an inorganic chemical that comes in a variety of shapes, including hydrated and anhydrous versions.
How do you determine it?The amount of sodium phosphate in the solution must first be determined in moles:
Number of moles of Na3PO4 = Mass of Na3PO4 / Molecular weight of Na3PO4
Number of moles of Na3PO4 = 1.500 g / 163.94 g/mol
Number of moles of Na3PO4 = 0.00915 mol
The total number of moles of sodium ions in the solution is because one molecule of sodium phosphate (Na3PO4) contains three sodium ions (Na+).
Number of moles of Na+= 3 x Number of moles of Na3PO4
Number of moles of Na+ = 3 x 0.00915 mol
Number of moles of Na+ = 0.02745 mol
The sodium ion [Na+] molar concentration in the solution can then be calculated as follows:
Moles of Na+ / volume of solution = number of moles of Na+
Na+ has a molar concentration = 0.02745 mol / 1.00 L.
Na+ has a molecular weight = 0.02745 M.
As a result, this solution has a molar sodium ion concentration of 0.02745 M. (or 27.45 mM).
To know more about sodium phosphate, visit:
https://brainly.com/question/10559079
#SPJ1
How do the physical and chemical properties the halogens compare with those of the noble gases?
Answers
Explanation:
To form bonds with noble gases, a lot of energy is required to form those bonds. Halogens, on the other hand, are extremely reactive. ... The halogens tend to be very reactive, while the noble gases are in no way reactive and don't bond easily, if at all.
Vinegar is sold at the grocery store with a concentration of 5.0 % acetic acid. How many grams of acetic acid are in 28 g of Vinegar?
Answers
White vinegar typically consists of 93%–96% water and 4–7% acetic acid. It can be used to cooking, bake, cleaning, and get rid of weeds. It can also help you lose weight and lower your blood sugar and cholesterol. Consumption is safe in moderation, but excessive consumption or when combined with certain medications could be harmful.
Apple cider vinegar is widely used in cooking and as a salad dressing because it contains acetic acid and nutrients like vitamins C and B vitamins. But at the same time, it's been utilized customarily as medication. It helps in losing weight.
Learn more about vinegar, here:
https://brainly.com/question/23700611
#SPJ1
Think about how you could design a robot to propel itself across an ice rink by applying the same principles that cause rockets to move. Describe what materials you would use and how the robot would work. What are some material limitations that you would need to consider for a robot moving on ice?
please help
Answers
Answer:
To design a robot that propels itself across an ice rink using the same principles as rockets, I would start by considering the materials that would be suitable for use on ice. Some materials that might work well for this purpose include plastic, rubber, and certain types of metal, such as aluminum or stainless steel.
Explanation:
The robot would work by using a propulsion system to generate a force that propels it forward. This could be achieved using a variety of methods, such as by using a jet engine or a rocket engine to produce a stream of hot gases that exits through a nozzle, creating a thrust force in the opposite direction.One material limitation to consider when designing a robot that moves on ice is the coefficient of friction between the robot's surface and the ice. A material with a low coefficient of friction, such as rubber or plastic, would be better suited for movement on ice, as it would provide less resistance and allow the robot to move more easily. In contrast, a material with a high coefficient of friction, such as steel, would be more difficult to move on ice, as it would generate more resistance and require more force to overcome.Other material limitations to consider when designing a robot for movement on ice might include the robot's weight and shape, as well as the overall stability and balance of the robot. It would also be important to consider the durability and wear resistance of the materials used, as the robot may need to withstand repeated movement on the ice over time.Answer:
Explanation:
Students learn about humankind’s search for life in outer space and how it connects to robotics and engineering. NASA is interested in sending exploratory missions to one of Jupiter’s moons, Europa, which requires a lot of preparatory research and development on Earth before it can happen. One robot currently being engineered as a proof of concept for a possible trip to explore Europa is the Icefin, which is an innovative robot that can explore under ice and in water, which are the believed conditions on Europa. This lesson provides students with intriguing information about far off (distance and time!) space missions and field robotics, and also sets up two associated robotics and arts integration activities to follow. The lesson can be used individually to provide new information to students, or as a precursor to the associated activities. A PowerPoint® presentation and worksheet are provided
Accuracy of which of the following equipment will be analyzed in this experiment: (If you are unsure; consult the lab experiment write-up:) Select one or more: A. Thermometer 50 mL B. Graduated Cylinder 10 mL C. Pipet Analytical Balance Buret 10 mL D. Graduated Cylinder
Answers
C. Pipet Analytical balance,It is expected that the accuracy of the following equipment will be examined depending on the equipment listed: Thermometer Graduated Cylinder Thermometer Buret C.
Pipet Analytical balance, part E These tools are frequently used in scientific experiments to measure volume, temperature, and weight. It's also crucial to understand the difference between accuracy and precision. While precision refers to the reproducibility of a series of measurements, accuracy relates to how near a measurement is to the real value of the quantity being measured.While 10 ml graduated cylinders feature marks every tenth of a milliliter, most 50 ml graduated cylinders have markings spaced every milliliter. Our measurement should be more precise if we use a 10 ml graduated container than if we use a 50 ml graduated container. We refer to an instrument as being ACCURATE if it produces values that are extremely near to the genuine value. Example: The same sample was measured three times using a graduated cylinder, yielding readings of 566 mL, 584 mL, and 541 mL. These three figures add out to 563.7 mL on average.
learn more about cylinder here:
https://brainly.com/question/16134180
#SPJ4
2. On a separate piece of paper balance each of the following equations by adding appropriatecoefficient. Show all your work for each.a.NaOH +(NH4)3PO4 →Na3PO4 +H2O +NH3b.C2H50 +02 →CO2 +H2OC.Mg3N2 +H2O →Mg(OH)2 +NH3d.C8H18 +O2 →CO2 +H2Oe.C12H22011 +O2 →CO2 +H2Of.H2C2O4 +CaCl2 →CaC2O4 +HCI

Answers
A. 3 NaOH + (NH4)3PO4 -> Na3PO4 + 3 H2O + 3 NH3
B. C2H6O + 3 O2 -> 2 CO2 + 3 H2O
C. Mg3N2 + 6 H2O -> 3 Mg(OH)2 + 2 NH3
D. 2 C8H18 + 25 O2 -> 16 CO2 + 18 H2O
E. C12H22O11 + 12 O2 -> 12 CO2 + 11 H2O
F. H2C2O4 + CaCl2 -> CaC2O4 + 2 HCl
you are exploring a system of caves. You have ignored safety regulations and have not brought a partner. While spelunking you drop your flashlight and the on/off button breaks. in order to repair it, you must find something that can conduct electricity and complete the circuit in the flashlight. You feel around you in the darkness and find a few different items. Which could work ?
A. you find a small mineral formation behind you that is yellow and smells, are are sure it is sulfur(S).
B. you have a flare for emergencies, which has a strip of magnesium (Mg) in it that you could remove.
C. You find a match. The head of a match is mostly covered in phosphorus (P).
D. you have a bottle of iodine (I) tablets as a vitamin supplement
Answers
Answer:
i think js b...........
While spelunking you drop your flashlight and the on/off button breaks. in order to repair it, "you have a flair for emergencies, which has a strip of magnesium (Mg) in it that you could remove."
What is spelunking?The main distinction between the two must be why each person is just exploring the cave. Spelunking would be the term used when someone enters a cave purely for fun and an adrenaline rush. On the other side, caving would be the practice of exploring caves for biological or conservation purposes.
What is magnesium?Magnesium is a kind of element which has the atomic number 12.
you are exploring a system of caves. You have ignored safety regulations and have not brought a partner. While spelunking you drop your flashlight and the on/off button breaks. in order to repair it, you must find something that can conduct electricity and complete the circuit in the flashlight. You feel around you in the darkness and find a few different items. You have a flair for emergencies, which has a strip of magnesium (Mg) in it that you could remove.
To know more about Spelunking and magnesium
https://brainly.com/question/6317313
#SPJ2
What characteristic of a light wave in a medium determines the index of refraction of that
medium?
Answers
Answer:
The refractive index can be seen as the factor by which the speed and the wavelength of the radiation are reduced with respect to their vacuum values: the speed of light in a medium is v = c/n, and similarly the wavelength in that medium is λ = λ0/n, where λ0 is the wavelength of that light in vacuum.
Explanation:
Explanation: i need points
The most abundant element in the earth's crust is ____________ .
Answers
Answer:
Hey mate.....
Explanation:
This is ur answer....
OxygenHope it helps!
Brainliest pls!
Follow me! :D
Express your answer as a chemical equation. Enter and no reaction if no participate is formed

Answers
From the balanced chemical equation above, we can see that:
• A precipitate may form wh ( BaSO4(s) ) forms.
,• Therefore, ,there is a reaction, that took place here.
What is the molarity of a 4.447 L solution
that is made from 13.53 g of NaCl?
Answer in units of M.
Answers
Answer:52M
Explanation:
use S= 1000w/ MV
Here, s= molarity
w= the amount of NaCl
M= the atomic mass of NaCl
V= the volume of solution
What is the blood alcohol level in mass percent if 8.33 mL of 0.04988 M K2Cr2O7 is required for titration of a 9.9950 g sample of blood?
Answers
The blood alcohol level in mass percent is 0.0956% if 8.33 mL of 0.04988 M \(K2Cr2O7\) is required for titration of a 9.9950 g sample of blood.
To calculate the blood alcohol level in mass percent, we need to first determine the amount of ethanol present in the blood sample. This can be done by using a redox titration with potassium dichromate \((K2Cr2O7)\), which oxidizes the ethanol to acetic acid.
The balanced chemical equation for the reaction is:
\(C2H5OH + 2Cr2O7^2- + 16H+ → 2CO2 + 4Cr^3+ + 11H2O\)
From the balanced equation, we can see that the stoichiometric ratio between \(K2Cr2O7\) and ethanol is 2:1. Therefore, the moles of ethanol in the blood sample can be calculated as:
moles of ethanol = 0.5 × moles of \(K2Cr2O7\)
The moles of \(K2Cr2O7\)can be determined from its concentration and volume used in the titration:
\(moles of K2Cr2O7 = 0.04988 mol/L × 8.33 × 10^-3 L = 4.15 × 10^-4 mol\)
Substituting this value into the equation above, we get:
moles of ethanol = 0.5 × 4.15 × 10^-4 mol = 2.075 × 10^-4 mol
The mass of ethanol in the blood sample can be calculated from its molar mass:
\(mass of ethanol = 2.075 × 10^-4 mol × 46.07 g/mol = 9.551 × 10^-3 g\)
Finally, the blood alcohol level in mass percent can be titration calculated as:
mass percent = mass of ethanol / mass of blood sample × 100%
mass percent = \(9.551 × 10^-3 g / 9.9950 g × 100%\)
mass percent = 0.0956%
Therefore, the blood alcohol level in mass percent is 0.0956%.
Learn more about titration here
https://brainly.com/question/31229711
#SPJ1
Fhd the missing
lumb
x + 6 = -3(x - 2)
Submit
Answers
Answer:
wala kang jowa sabi ko haha
State the law of conservation of mass in your own words
Answers
Answer:
The law of conservation of mass states that mass is neither created nor destroyed by chemical reactions or physical transformations. So basically, the mass of the products in a chemical reaction must equal the mass of the reactants
Answer: law of conservation of mass says mass can’t be created or destroyed
Explanation:
the true definition is “the law of conservation of mass states that mass can neither be created nor destroyed in a chemical reaction. Thus, the amount of matter can not change
just change the more mature words into child proof words
Given that oxygen -16 and oxygen -18 both havean atomic number of 8, how many electrons, protons and neutrons do these oxygen atoms contain
Answers
Oxygen-16: 8 protons, 8 neutrons, 8 electrons
Oxygen-18: 8 protons, 10 neutrons, 8 electrons
Explanation:Using atomic and mass numbers, we can find information about the makeup of atoms.
Protons
Every element has a unique number of protons, and every atom of the same element will have the same number of protons. This means that both oxygen atoms must have the same number of protons. The number of protons in an element is equal to its atomic number. Since oxygen has an atomic number of 8, all oxygen atoms will have 8 protons. This means that oxygen-16 and oxygen-18 have 8 protons.
Neutrons
The number of neutrons an atom of a certain element can have varies. Atoms of the same element that have different numbers of neutrons are known as isotopes. The number of neutrons can be found through the mass number. The mass number is the number that follows the dash in the name of the isotope. This number is equal to the protons plus the neutrons. So, to find the number of neutrons in an oxygen atom, subtract 8 protons from the mass number. This means oxygen-16 has 8 neutrons and oxygen-18 has 10 neutrons.
Electrons
Remember that electrons are negatively charged, protons are positively charged, and neutrons have no charge. The number of electrons in an atom determines the charge of the atom. If the number of electrons equals the number of protons, then the charge of the atom will equal 0. If there are more electrons, then the atom will be negatively charged and vice versa. Since neither of the atoms has any indication of a nonzero charge, the number of electrons must equal the number of protons. So, both oxygen atoms have 8 electrons.
All of the following increase variation in a population except
A: habitat destruction
B: sexual reproduction
C:mutation
D:environmental
pressures
Answers
Answer:
B
Explanation:
Complete the following equation of nuclear transmutation.
23892U + 126C → 24498Cf + 6 ______
Complete the following equation of nuclear transmutation.
U + C → Cf + 6 ______
A) 1n
B) 0 e
C) 0 e
D) 1H
E) 0g 0 -1 +1 1 0
Answers
Answer:
Option A. 1 0n
Explanation:
Details on how to balanced the equation for the reaction given in the question above can be found in the attached photo.
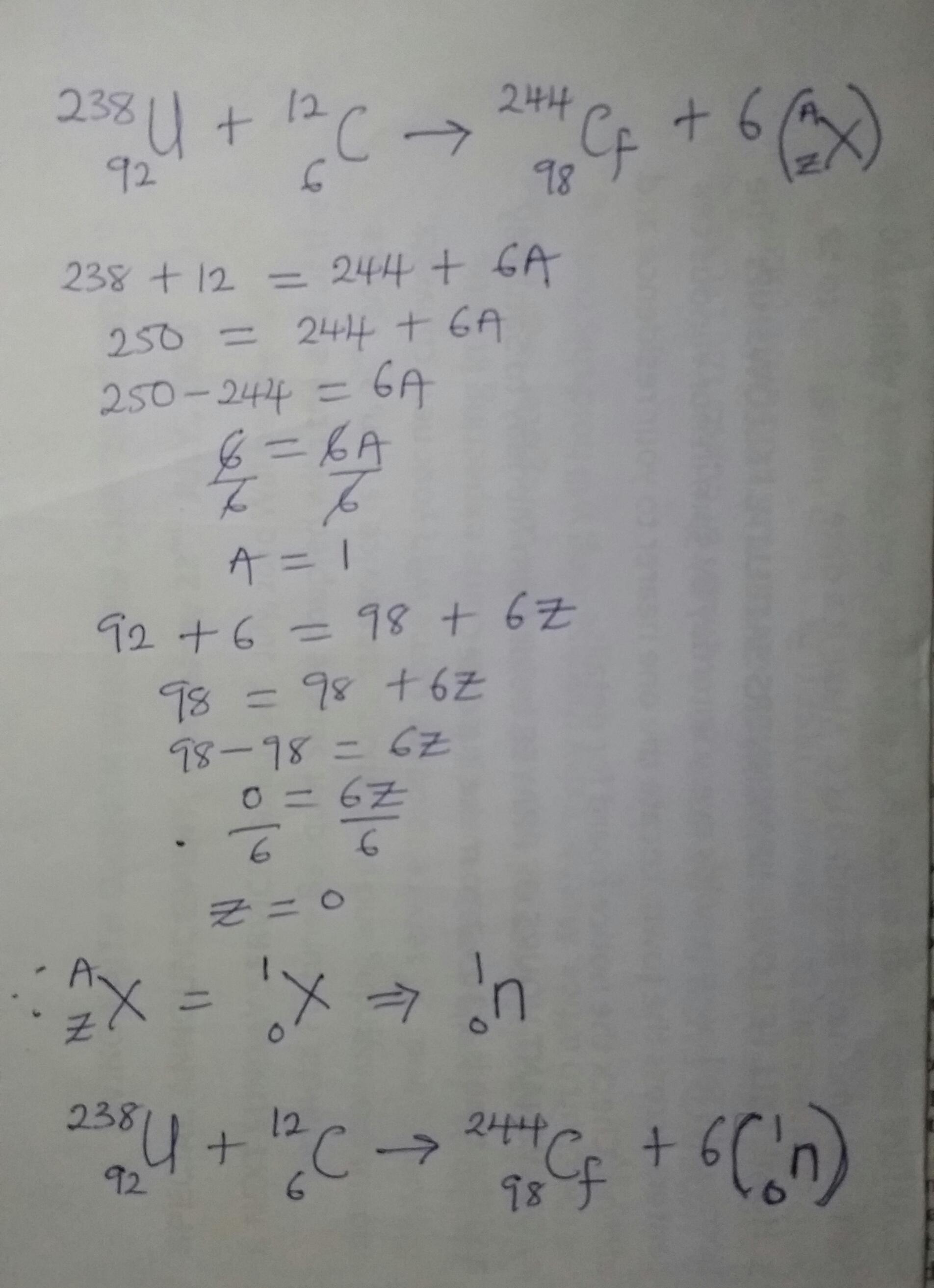
The missing part of the transmutation equation as it has been shown is 1/o n. Option A
What is nuclear transmutation?Nuclear transmutation is the process of shifting the number of protons in an atom's nucleus to change one element into another. Nuclear processes that change one atomic nucleus into another with a different atomic number are involved.
The production of nuclear energy, radioactive decay, and the creation of new isotopes for use in science and industry all depend on nuclear transmutation, a fundamental idea in nuclear physics.
We have the equation as;
238/92 U + 12/6 C ----> 244/98 Cf + 6 1/0 n
Learn more about nuclear transmutation:https://brainly.com/question/30078683
#SPJ6
How many protons, neutrons, and electrons in Cl-?
O 17 protons; 17 electrons; 17 neutrons
O 17 protons; 16 electrons; 18 neutrons
O 16 protons; 17 electrons; 18 neutrons
O 17 protons; 18 electrons; 18 neutrons
Answers
Answer:
hello . proton:17 electron:18 neutron :18
Explanation:
it's obvious and you must memorise that Cl has 17 protons .
because it has one negative so electrons which are negative should be one more than protons I mean 18
neutron doesn't need to look at it here
electron shows the answer.
hope u understand
if u have any questions don't hesitate to ask.
3Ba(ClO3)2 number of atoms for each element
Answers
Answer:
3 atoms of Ba
2 x 1 = 2 atoms of Cl
2 x 3 = 6 atoms of O
So, the total number of atoms for each element in 3Ba(ClO3)2 are:
Barium (Ba): 3 atoms
Chlorine (Cl): 2 atoms
Oxygen (O): 6 atoms
Explanation:
Molecules have ?
: A) both potential and kinetic energy. B) only kinetic energy. C) only potential energy. D) neither kinetic nor potential energy.
Answers
Answer:
C) only potential energy.
Tin is widely used for plating steel cans used as food containers, in metals used for bearings, and in solder. What will be the final temperature of a 163.79 g piece of tin that is at an initial temperature of 27.4oC and requires 2432.0 J of heat? The specific heat of tin is 0.226 g/J oC.
Answers
We can use the formula for heat transfer to find the final temperature of the tin:
Q = mcΔT
where Q is the heat transferred, m is the mass of the tin, c is the specific heat of tin, and ΔT is the change in temperature.
We want to solve for ΔT, which we can rearrange as:
ΔT = Q / (mc)
Plugging in the values we know:
m = 163.79 g
c = 0.226 g/J oC
Q = 2432.0 J
ΔT = 2432.0 J / (163.79 g * 0.226 g/J oC)
ΔT = 6.44 oC
Therefore, the final temperature of the tin will be:
Final temperature = Initial temperature + ΔT
Final temperature = 27.4 oC + 6.44 oC
Final temperature = 33.84 oC
So the final temperature of the tin will be 33.84 oC.
PLS SOMONE HELP I KNOW THE PHOTO ISNT THAT GOOD BUT I REALLY NEED HELP I WILL MARK AS BRAINLIEST AND GIVE 5 STARS ASWELL AS A HEART PLSS IM BEGGING YOU

Answers
The statement that best explains why the chemical equation provided for combustion of methane supports law of conservation of mass is as follows: There is same number of each kind of atom before and after the chemical reaction and the total mass remains the same (option D).
What is law of conservation of mass?The law of conservation of mass is a conservation law that states that the total mass of a closed system remains constant regardless of the chemical or physical changes that take place within it.
The law of conservation of mass further explains that in a chemical reaction, the amount of each atom on both sides of the equation is the same.
According to this question, methane gets combusted in air. To fulfill the law of conservation of mass, the same number of each kind of atom before and after the chemical reaction and the total mass remains the same.
Learn more about law of conservation of mass at: https://brainly.com/question/28711001
#SPJ1
Does silver form a gas? Yes or no.
Answers
Answer:
Yes it does
Explanation:
Silver is a chemical element with the symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal.[citation needed] The metal is found in the Earth's crust in the pure, free elemental form ("native silver"), as an alloy with gold and other metals, and in minerals such as argentite and chlorargyrite. Most silver is produced as a byproduct of copper, gold, lead, and zinc refining.