Answers
Answer:
There are many benefits to using chemical characterization, from reducing the testing burden, saving time and money for the manufacturer, and reducing the amount of animal testing required. From an ethical standpoint, the latter benefit can be especially attractive.
Explanation:
please mark my answer in brainlist
Related Questions
Electrochemical cells generate electricity from which of the following? Select all that apply.
electron transfer
flow of electrons
dissolving an ionic compound
redox reactions
Answers
By a redox reaction that involves the transfer of electrons, often through the dissolution of an ionic substance, electrochemical cells produce electricity from the flow of electrons.
What fuels the production of energy by electrochemical cells?In electrochemistry, redox or oxidation-reduction reactions, in which electrons travel from one element to another, can produce electricity. Redox processes involve the transfer of electrons from one substance to another.
In what element are electrochemical cells made?Batteries use a very significant class of oxidation and reduction reactions to produce useable electrical energy. Using solutions of respective sulphates, copper and zinc metals can be combined to create a straightforward electrochemical cell.
To know more about electrons visit:-
https://brainly.com/question/20513633
#SPJ1
A hot metal plate at 150°C has been placed in air at room temperature. Which event would most likely take place
over the next few minutes?
Molecules in both the metal and the surrounding air will start moving at lower speeds.
Molecules in both the metal and the surrounding air will start moving at higher speeds.
The air molecules that are surrounding the metal will slow down, and the molecules in the metal will speed up.
The air molecules that are surrounding the metal will speed up, and the molecules in the metal will slow down.
Answers
Answer:
molecules will speed up
Explanation:
Determine if the following two structures are
identical, isomers, or unrelated?
CH
CH3
CH3
CH,
B
С
identical
isomers
unrelated

Answers
Answer:
Identical
Explanation:
Both compounds are identical. If you rotate the compound on the left 60 degree anticlockwise you will get the compound on the right.
These are not isomers of each other because they have same structural and molecular formulas.
Also, they are related to each other because they are the same
What chemicals have a saturation concentration less than 5 but greater than 2
Answers
Saturation concentration refers to the highest concentration of a substance that can exist in a solution in equilibrium with its solid or gaseous form. A saturation concentration less than 5 but greater than 2 is a relatively broad range, and many different chemicals could fall within this range depending on the conditions of the solution.
Examples of chemicals with saturation concentration less than 5 but greater than 2 include:
Calcium carbonate, which has a saturation concentration of around 3.5 g/L at room temperature and normal pressure
Iron(III) hydroxide, which has a saturation concentration of around 3.5 g/L at room temperature and normal pressure
Potassium chloride, which has a saturation concentration of around 3.5 g/L at room temperature and normal pressure
Ammonium chloride, which has a saturation concentration of around 2.5 g/L at room temperature and normal pressure
Sodium sulfate, which has a saturation concentration of around 3.5 g/L at room temperature and normal pressure
It's important to note that these are just examples, the saturation concentration of a chemical can vary depending on temperature, pressure, and other factors.
The boiling point at 1.00 atm of an aqueous solution of CaCl2 is 105.3 °C. What is the concentration of CaCl2
in the solution? Kb (H2O) = 0.512 °C/m. Assume an ideal van't Hoff factor for CaCl2.
Answers
d.2.93m.CaCl2 is present in the solution with a 2.93m concentration.
The boiling point of a solution is directly related to its concentration. The boiling point elevation of a solution, ΔTb, is equal to the product of the van't Hoff factor (i) and the molality of the solution (m).The quantity of moles of solute per kilogramme of solvent is known as molality.
Therefore, we can solve for the molality of the solution using the following equation:
ΔTb
\(= i *m\\105.3\°C= i * m\\\)
\(m =\frac{ 105.3 \°C }{i}\)
Assuming an ideal van't Hoff factor for CaCl2 (i = 2), the molality of the solution is:
\(m =\frac{ 105.3 \°C }{ 2}\\m = 52.65 m = 52.65 mol/kg\)
The concentration of CaCl2 in the solution is then:
\(C = m * Kb\\C = 52.65 mol/kg * 0.512 \°C/m\\C = 2.93 mol/kg\)
Therefore,The concentration of CaCl2 in the solution is 2.93m.
learn more about boiling point refer:brainly.com/question/24168079
#SPJ1
complete question:The boiling point at 1.00 atm of an aqueous solution of CaCl2 is 105.3 °C. What is the concentration of CaCl2 in the solution? Kb (H2O) = 0.512 °C/m. Assume an ideal van't Hoff factor for CaCl2.
a.3.45m
b.4.40m
c.8.79m
d.2.93m
What's the partial pressure of carbon dioxide in a container that holds .35 atm of nitrogen, and .12 atm of hydrogen and has a total pressure of 1.05 atm
Answers
Answer:
0.58 atm
Explanation:
Step 1: Given data
Total pressure of the gaseous mixture (P): 1.05 atmPartial pressure of N₂ (pN₂): 0.35 atmPartial pressure of H₂ (pH₂): 0.12 atmPartial pressure of CO₂ (pCO₂): ?Step 2: Calculate the partial pressure of CO₂
The total pressure of the gaseous mixture is equal to the sum of the partial pressures of the individual gases.
P = pN₂ + pO₂ + pCO₂
pCO₂ = P - pN₂ - pO₂
pCO₂ = 1.05 atm - 0.35 atm - 0.12 atm = 0.58 atm
what properties of a natural resource make it useful for humans as a materials or energy source?
Answers
The properties of a natural resource that make it useful for humans as a material or energy source is the ability to convert mass into energy and vice versa.
What are natural resources?The expression natural resources make reference to all types of matter and energy extracted from nature that can be used to produce goods and services.
Some examples of natural resources include for example irreversible resources such as fossil fuels (i.e., oil, or coal, gas, minerals such as metals, rocks, etc) as well as those based on the use of reversible energy such as eolic air energy, solar radiation or sunlight, soil and hydric resources or water.
Therefore, with this data, we can see that natural resources can be defined as any material and or energy obtained from nature that may be irreversible or reversibly used to produce goods and services.
Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ1
CHEMISTRY
please help me!!!

Answers
The complete product and balance equation is-
a. Fe + 2HCl → FeCl2 + H2 b. Ni + 2HOH → Ni(OH)2 + H2
c. 2Ag + 2HCl → 2AgCl + H2 d. K+ + HOH → KOH + H2
What is the product in a balanced equation?Products are substances that remain after a reaction. A chemical equation that is balanced has the same number of each type of atoms on both sides of the equation. Subscripts are a component of the chemical formulae for the reactants and products that show how many atoms of the previous element there are in each.
Which four steps are involved in balancing equations?Here are the procedures: Count the atoms on each side first. Next, alter one of the compounds' coefficients. Third, count the atoms once more, and then repeat steps two and three until the equation is balanced.
to know more about balanced equations here:
brainly.com/question/7181548
#SPJ1
The enzyme provided is a stock solution which should be diluted 50-fold with 0.001M HCl immediately before use.
Enzyme :1mg/ml chymotrypsin A in 0.001M HCl
How do I work out this calculation, if I want the 5ml of the diluted enzyme?
Answers
In order to prepare a 50-fold diluted enzyme, 0.1 mL of the stock solution of the enzyme is taken and made up to 5.0 mL with 0.001M HCl.
What is dilution?Dilution is a process whereby a solution of lower concentration is prepared from one of a higher concentration.
Dilution is done by using the dilution formula below:
C1V1 = C2V2C1; Initial concentration of enzyme = 1 mg/mL
C2; Final concentration of enzyme = 1/50 mg/ml = 0.02 mg/ml
V1; Initial volume of enzyme = y
V2; Final volume = 5.0 ml
V1 = C2V2/C1
V1 = 0.02 * 5/1 = 0.1 mL
Therefore, in order to prepare a 50-fold diluted enzyme, 0.1 mL of the stock solution of the enzyme is taken and made up to 5.0 mL with 0.001M HCl.
Learn more about dilution at: https://brainly.com/question/24881505
#SPJ1
Suppose you heat a metal object with a mass of 34.5 g to 95.5 °C and transfer it to a calorimeter containing 100.0 g of water at 17.5 °C. The water and metal reach a final temperature of 24.9 °C.
Metal in a covered cup with a thermometer and heat indicated as leaving the metal.
What is the specific heat of the metal in J/g⋅∘C
Answers
q = m × c × ΔT
where q is the heat transferred, m is the mass of the metal, c is the specific heat of the metal, and ΔT is the change in temperature.
First, we need to calculate the heat transferred from the metal to the water:
q = m × c × ΔT
q = (34.5 g) × c × (95.5 °C - 24.9 °C)
q = 224,085 J
Next, we can calculate the heat absorbed by the water:
q = m × c × ΔT
q = (100.0 g) × (4.184 J/g⋅∘C) × (24.9 °C - 17.5 °C)
q = 3,073 J
Since the heat transferred from the metal to the water is equal to the heat absorbed by the water, we can set the two equations equal to each other and solve for c:
m × c × ΔT = m × c × ΔT
(34.5 g) × c × (95.5 °C - 24.9 °C) = (100.0 g) × (4.184 J/g⋅∘C) × (24.9 °C - 17.5 °C)
c = 0.385 J/g⋅∘C
Therefore, the specific heat of the metal is 0.385 J/g⋅∘C.
When the nuclide polonium-218 undergoes alpha decay:
a. The name of the product nuclide is _____.
b. The symbol for the product nuclide is _____.
Write a balanced nuclear equation for the following: The nuclide polonium-218 undergoes alpha emission.
Answers
Answer:
a): The name of the product nuclide is lead-214
b): The symbol of the product nuclide is Pb-218
Explanation:
There are three types of decay processes:
Alpha decayBeta decayGamma decayAlpha decay is the decay process that happens when a heavy nucleus decays into a light nucleus with the release of an alpha particle. This alpha particle carries a charge of +2 units and has a mass of 4 units. It is also known as the helium nucleus. The general equation for this decay process is:
\(_Z^A\textrm{X} → _{Z-2}^{A-4}\textrm{Y}+_2^4\alpha\)
The nuclear equation for the alpha decay of Po-218 follows:
\(_{84}^{218}\textrm{Po}\rightarrow _{82}^{214}\textrm{Pb}+_2^4\alpha\)
Hence, the name of the product nuclide is lead-214 and the symbol is Pb-218.
Rank the following compounds in order of decreasing vapor pressure.
CH3CH2CH2CH2CH3
CH4
CH3CH-CH3CH2CH3
CH3CH2CH2OH
Answers
Decreasing vapour pressure for the following compounds is as follow:
CH4
CH3CH-CH3CH2CH3
CH3CH2CH2CH2CH3
CH3CH2CH2OH
Vapour Pressure of the compound:
The pressure characteristic of a pure compound's vapour at any given temperature when it is in equilibrium with its liquid or solid state is known as the vapour pressure. Compound molecules that bond well with one another will have a low vapor pressure (less inclination to escape to the vapor phase), whereas compounds that connect poorly with one another would have a high vapor pressure. Vapor pressure is a measure of a compound's capacity to bond with itself.
To learn more about the Vapor Pressure:
https://brainly.com/question/4463307
#SPJ1
In the SOLID state of matter ,particles have enough energy to move freely but not enough energy to overcome their attraction for each other
Answers
In the solid state of matter, particles, such as atoms, ions, or molecules, are closely packed and held together by strong intermolecular forces, such as ionic bonds, metallic bonds, or covalent bonds.
In a solid, particles have enough energy to vibrate around fixed positions but do not have enough energy to overcome the attractive forces between them. These attractive forces, also known as cohesive forces, arise from the electrostatic interactions between particles or the sharing of electrons in covalent bonds.
The energy of the particles in a solid is typically much lower than in the liquid or gaseous states, resulting in a fixed arrangement of particles.
The movement of particles in a solid is characterized by vibrations or oscillations around their equilibrium positions.
These vibrations occur due to the thermal energy present in the solid, but the particles remain relatively fixed in their positions due to the strong attractive forces. The amplitude of the vibrations increases with increasing temperature, as the particles gain more thermal energy.
However, the particles in a solid do not have enough energy to break the intermolecular bonds and move freely throughout the entire solid. Instead, they can only move within their local vicinity or lattice positions.
This restricted movement is what distinguishes the solid state from the liquid or gaseous states, where particles have enough energy to overcome intermolecular forces and move more freely.
For more such questions on intermolecular forces visit:
https://brainly.com/question/12243368
#SPJ8
Help!!
Which set of Earth components is arranged in order from solid to liquid to gas?
hydrosphere, atmosphere, lithosphere
hydrosphere, lithosphere, atmosphere
lithosphere, atmosphere, hydrosphere
lithosphere, hydrosphere, atmosphere
Answers
Answer:
Lithosphere, Hydrosphere, atmosphere
The set of Earth components that is arranged in order from solid to liquid to gas is D. lithosphere, hydrosphere, atmosphere
Lithosphere refers to the solid, outer part of the Earth. It is also known as the Rocky part of the Earth. It consists of the top part of the upper mantle and the brittle crust.Hydrosphere simply refers to the water on the Earth, underground and those that are in the air. This can be seen in ocean, seas, rivers, etc.Atmosphere refers to the layers of gases that can be found on Earth.ut contains gases like nitrogen, oxygen, etc.Read related link on:
https://brainly.com/question/19541638
Iodine-129 is a product of nuclear fission, whether from an atomic bomb or a nuclear power plant. It is a B- emitter with a half-life of 1.7 × 10^7 years. How many disintegrations per second would occur in a sample containing 1.00 mg I (mass 129 amu)?
Answers
The sample containing 1.00 mg of iodine-129 would have an activity of 1900 disintegrations per second.
The first step in solving this problem is to determine the number of iodine-129 atoms present in 1.00 mg of the sample. We can use the Avogadro's number (6.022 × 10^23 atoms per mole) and the molar mass of iodine-129 (129 g/mol) to calculate this:
Number of iodine-129 atoms = (1.00 mg / 129 g/mol) x (6.022 × 10^23 atoms/mol)
= 4.66 × 10^16 atoms
Next, we can use the half-life of iodine-129 (1.7 × 10^7 years) to calculate the decay constant (λ) using the following equation:
λ = ln(2) / half-life
λ = ln(2) / 1.7 × 10^7 years
= 4.09 × 10^-8 per year
Now, we can use the decay constant and the number of iodine-129 atoms to calculate the activity (A) in disintegrations per second (Bq):
A = λ x N
A = (4.09 × 10^-8 per year) x (4.66 × 10^16 atoms)
= 1900 Bq
For more question on iodine-129 click on
https://brainly.com/question/28947143
#SPJ11
Seeds Roots Cones Stem Spores Leaves 1. Mongo 2. Moss 3. Ferns 4. Pine Tree 5. Guava 6. Mango 7. Coconut 8. Katakataka 9. Potato 10. Okra 14

Answers
The method of propagation of the given organisms is shown below:
1. Mongo ---> seeds
2. Moss ---> spores
3. Ferns ---> stem
4. Pine Tree ---> cones
5. Guava ---> seeds
6. Mango ---> seeds
7. Coconut ---> seeds
8. Katakataka ---> leaves
9. Potato ---> stem
10. Okra ---> seeds
Definition of reproductionRepr0duction is the process by which living organisms produce young ones of their kind.
Repr0duction can either involve two parents through the joining of male and female gametes to produce a zygote i.e. seeds in plants and embryo in animals or can involve only one parent and occurs through vegetative parts such as roots, stem, or leaves.
List of organisms and their propagation partsThe table below gives organisms and their propagation parts:
1. Mongo ---> seed
2. Moss ---> spores
3. Ferns ---> stem
4. Pine Tree ---> cones
5. Guava ---> seeds
6. Mango ---> seeds
7. Coconut ---> seeds
8. Katakataka ---> leaves
9. Potato ---> stem
10. Okra ---> seeds
Learn more about propagation and repr0duction at: https://brainly.com/question/13876478
1)Grignard reagent when reacted with methanol will yield A) ethanol (B) secondary alcohols (C) tertiary alcohols (D ropanol (E) primary alcohol
Answers
When the reaction of Grignard reagent reacted with methanol will yield a tertiary alcohol. Therefore, Option C tertiary alcohol is correct.
Contains a carbon-metal link, Grignard reagents are chemicals used in catalysis. They generally result from the anhydrous reaction of magnesium metal with an alkyl or aryl halide. Because of their high reactivity, Grignard reagents frequently act as nucleophiles in organic reactions.
An alkyl group from a Grignard reagent binds to the oxygen atom of methanol (CH3OH) when it interacts with the methanol, breaking the carbon-metal connection. A precursor alkoxide is created as a result. The equivalent alcohol is then produced by protonating the intermediate alkoxide.
The reaction of a Grignard reagent with methanol leads to the formation of a tertiary alcohol.
Learn more about reagents here:
https://brainly.com/question/29729676
A solution that is neutral has a pH of:
0
14
10
1
7
Answers
A solution is made by dissolving 38.81 grams of nickel (II) sulfate, NiSO4, in enough water to make 0.467
liters of solution. Calculate the molarity of this solution.
Answers
The molarity of the NiSO₄ solution made by dissolving 38.81 grams of nickel (ii) sulfate, NiSO₄, in enough water to make 0.467 liters of solution is 0.535 M
How do i determine the molarity of the solution?First, we shall obtain the mole of 38.81 grams of nickel (ii) sulfate, NiSO₄. Details below:
Mass of NiSO₄ = 38.81 grams Molar mass of NiSO₄ = 154.75 g/molMole of NiSO₄ = ?Mole of NiSO₄ = mass / molar mass
= 38.81 / 154.75
= 0.25 mole
Now, we shall determine the molarity of the solution. Details below:
Mole of NiSO₄ = 0.25 moleVolume of solution = 10.467 LMolarity of solution = ?Molarity of solution = mole / volume
= 0.25 / 0.467
= 0.535 M
Thus, the molarity of the solution is 0.535 M
Learn more about molarity:
https://brainly.com/question/16073358
#SPJ1
Brainiest and 10 Points
Which has a HIGHER frequency?
A. Visible
B. Ultraviolet
Answers
Answer:
B. Ultraviolet
Explanation:
UV has a higher frequency and shorter wavelength than visible light
a gas mixture at 20.0 C and 2.0 atm contains 0.40 mol of H2, 0.15 mol of O2, and 0.50 mol of N2. Assuming ideal behavior, what is the partial pressure of hydrogen gar [H2] in the mixture?
Answers
The partial pressure of hydrogen gas in the mixture is:0.53334 atm.
What is partial pressure?
partial pressure. noun. the pressure that a gas would have if it took up the entire volume that the mixture of gases currently occupies.
P total,and the total number of moles in the mixture would be,
n total=nH2+nO2+nN2
This means that you could write
P total.V=ntotal.RT
p total=n total.RT/V
This is equivalent to
p total=[nH2+nO2+nN2].RT/V
therefore,
P total =PH2+PO2+pN2
now, to get the partial pressure of,let's Hydrogen gas
RT/V=Ptotal/nH2+nO2+nN2
therefore
PN2=XN2.p total
similarly,
PO2=XO2.p total
PN2=XN.p total
The total number of moles will be
\(ntotal\) = 0.40+0.15+0.50=1.50 moles.
\(PH2\)=0.40/1.50*2.0 atm =0.5334 atm.
\(PO2\)=0.15/1.50*2.0 atm =0.2 atm.
\(PN2\)=0.50/1.50*2.0 atm =0.66 atm.
Hence, the partial pressure of hydrogen gas in the mixture is:0.53334 atm.
To learn more about the partial pressure of H2 from the given link:
https://brainly.com/question/19813237
#SPJ9
To assist with the self-administration of eye drops, you should refer to the manufacturer’s instructions because some eye drops need to be:
Answers
Before administering eye drops, make sure to wash your hands thoroughly with soap and water to minimize the risk of introducing any contaminants.
Tilting your head back or lying down can help create a better angle for administering the eye drops and prevent them from running out of your eye.
Use your index finger to gently pull down the lower eyelid, creating a small pocket.
Carefully hold the dropper tip close to your eye, without touching your eye or eyelashes.
Follow the specific instructions provided with your eye drops to determine the appropriate number of drops to use. Squeeze the dropper to release the drops into the pocket created by pulling down your lower eyelid.
If any excess solution has spilled onto your skin, gently wipe it away with a clean tissue or cloth to avoid irritation.
To learn more about the eye drop, follow the link:
https://brainly.com/question/29061402
#SPJ1
Best answer gets brainliest

Answers
Answer:
C) Mercury based thermometer
Explanation:
Answer:
Mercury based thermometer
Explanation:
When you touch a lightbulb it is warm. Hairdryer produces heat to dry your hair. Stove heats up to warm food and liquids up.
Roasting battle, Bored xD
Answers
Answer: ok lets battle XD
Explanation:
Answer:
hmmmmm
Explanation:
seems kinda sus
those questions in pictures

Answers
For this section:
The acids that would be completely dissociated when dissolved in water are A, (H O)₂SO and B, (H O)IO₃.(i) pH of 7(ii) pH of 4.763I₂ + 2PBr₃ → 6IBr + P₂4LiH + GaCl₃ → 4LiCl + GaH₃NH₃ + BCl₃ → NH₃BCl₃How to determine pH and products?(d) To determine which acid would be completely dissociated when dissolved in water, consider the strength of the acid and its ability to ionize completely. Strong acids are those that ionize completely in water, while weak acids only partially dissociate.
Among the given options:
(H O)₂SO is sulfuric acid (H₂SO₄), which is a strong acid and completely dissociates in water.
(H O)IO₃ is iodic acid (HIO₃), which is also a strong acid and completely dissociates in water.
(H O)₂SO₂ is not a known acid and cannot be evaluated for dissociation.
HCO₂H is formic acid (HCOOH), which is a weak acid and only partially dissociates in water.
Therefore, the acids that would be completely dissociated when dissolved in water are (H O)₂SO and (H O)IO₃.
(e) To estimate the pH of the given solutions formed by titration, compare the moles of the acid and base used in the reaction.
(i) For the titration of 25.0 mL of 0.10 M aqueous NaOH with 25.0 mL of 0.10 M aqueous HCl, the reaction between a strong acid (HCl) and a strong base (NaOH) forms a neutral salt (NaCl) and water. The resulting solution would have a pH of 7, indicating neutrality.
(ii) For the titration of 25.0 mL of 0.10 M aqueous NaOH with 25.0 mL of 0.10 M aqueous acetic acid, consider the ionization of acetic acid and the formation of its conjugate base. The pKa of acetic acid is given as 4.76.
Since the volumes and concentrations of the acid and base are equal, we have a 1:1 mole ratio between them. This means that half of the acetic acid will be neutralized, and the remaining half will be in the form of the conjugate base (acetate ion, CH₃COO-). The resulting solution will be a buffer solution with a pH close to the pKa of acetic acid, which is 4.76.
(f) To predict the products formed from mixing the given reagents, we need to consider the reactions and the possible chemical reactions that occur.
(i) 3I₂ + PBr₃: This reaction involves the combination of iodine (I₂) with phosphorus tribromide (PBr₃). The balanced equation is:
3I₂ + 2PBr₃ → 6IBr + P₂
(ii) 4LiH + GaCl₃: This reaction involves the combination of lithium hydride (LiH) with gallium trichloride (GaCl₃). The balanced equation is:
4LiH + GaCl₃ → 4LiCl + GaH₃
(iii) NH₃ + BCl₃: This reaction involves the combination of ammonia (NH₃) with boron trichloride (BCl₃). The balanced equation is:
NH₃ + BCl₃ → NH₃BCl₃
Find out more on pH here: https://brainly.com/question/26424076
#SPJ1
i need help on this one

Answers
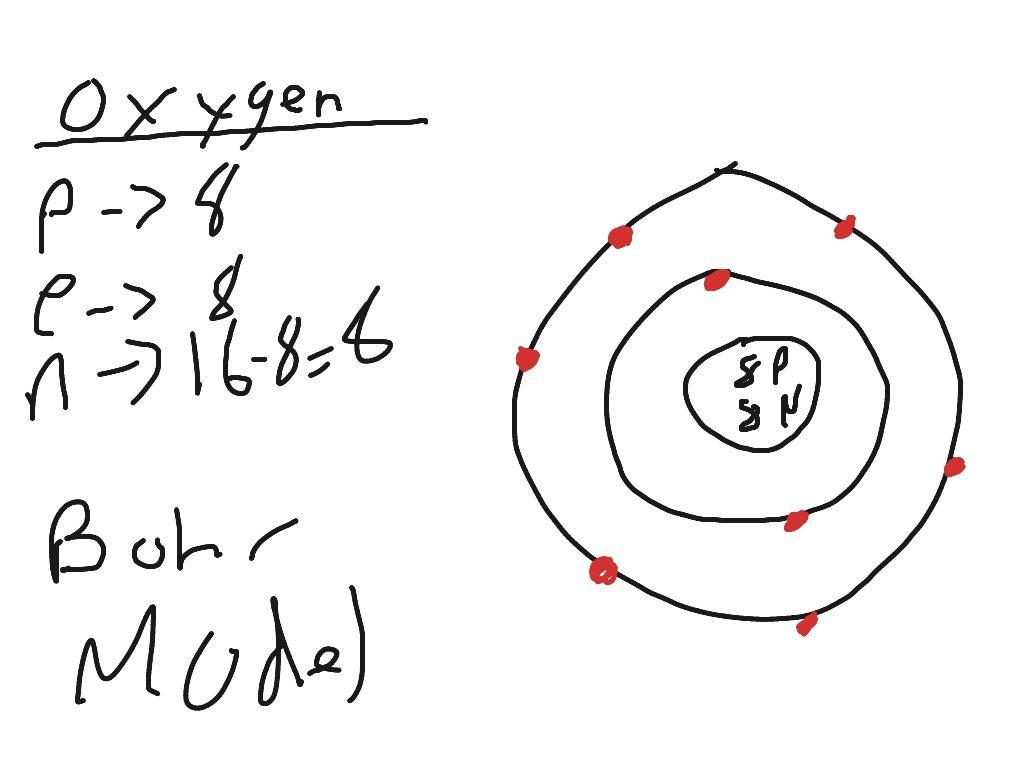
Answer:
Ok so first it wants the atomic number is the atomic number would be 8.
Atomic mass is next which is 15.9
number of protons : 8 and the number of electrons is also 8
Now to find the number of neutrons ( have no charge) you have to subtract your atomic mass number and the total of protons and electrons so 15.999-16=0.001
Explanation:
I hope this helps this is 100% right I went over it many times.
Answer:
here
Explanation:
A solution contains 3.5 mol NaCl and 4.2 mol MgCl₂. How many equivalents of chloride ion are present?
Answers
There are 15.4 equivalents of chloride ion present in the solution
To calculate the number of equivalents per mole of chloride ionWe need to multiply the total number of moles of chloride ion in the solution by the number of equivalents.
The molar mass of NaCl is 58.44 g/mol, so 3.5 mol of NaCl contains :
3.5 mol NaCl x 2 mol Cl⁻/1 mol NaCl = 7 mol Cl⁻
Similarly, the molar mass of MgCl₂ is 95.21 g/mol, so 4.2 mol of MgCl₂ contains:
4.2 mol MgCl₂ x 2 mol Cl⁻/1 mol MgCl₂ = 8.4 mol Cl⁻
Therefore, the total number of moles of chloride ion in the solution is:
7 mol Cl⁻ + 8.4 mol Cl⁻ = 15.4 mol Cl⁻
By dividing the total number of moles by the number of equivalents per mole, we can finally determine how many equivalents of the chloride ion there are. There is one equivalent of the chloride ion per mole since it has a valency of -1.
15.4 mol Cl⁻ x 1 eq/mol = 15.4 eq
So there are 15.4 equivalents of chloride ion present in the solution.
Learn more about mole here : brainly.com/question/30337257
#SPJ1
A net force of 66 newtons accelerates a child at 2.0 m/s? down a smooth playground slide. What is the mass of the
child?
A 130 kg
B68 kg
© 64 kg
D 33 kg
Answers
Answer:The answer is D 33
Explanation:
The mass of the child accelerating at 2 m/s² by a net force of 66 N is 33 Kg (Option D)
Relationship between force and accelerationForce is related to acceleration according to the following equation
Force (F) = mass (m) × acceleration (a)
F = ma
With the above formula, we can obtain the mass of the child
How to determine the mass of the child Acceleration (a) = 2 m/s²Force (F) = 66 NMass (m) =?F = ma
66 = m × 2
Divide both side by 2
m = 66 / 2
m = 33 Kg
Thus, the mass of the child is 33 Kg
Learn more about acceleration:
https://brainly.com/question/491732
Select all of the following that are combustion reactions.

Answers
Answer:
Explanation:
1,2,4,
The equations that show combustion are equations A, B and D.
What is combustion?When we talk about combustion, the idea is that the substance would be burnt in oxygen. In other words, the combustion can be taken to be an oxidation reaction. It is an oxidation reaction in the sense that the oxidation number of the substance that is reacting with the oxygen would become increased.
When we look at the equations that we have, it is quite easy to pick out among the balanced reaction equations that are shown here the ones that has to do with the burning of the substance in oxygen and a consequent rise in the oxidation number.
Learn more about combustion:https://brainly.com/question/15117038
#SPJ1
A chemist wants to make a 17.85 %(m/m) solution of NaCl using a only 50.0 g of the salt. How much water is needed to make the solution? Numerical answer only. No units.
Answers
The mass percentage is an important method which is used to calculate the concentration of a solution. The amount of water needed to add in order to make 17.85 % NaCl solution is 230.1 g.
What is mass percentage?The mass percentage of a component in a solution is defined as the mass in grams of that component present per 100 g of the solution. The term mass percentage is denoted as w/w. It is used to calculate the concentration of a binary solution.
Mass percentage = Mass of the component in the solution / Total mass of solution × 100
17.85 = 50.0 / 50.0 + x × 100
0.1785 (50.0 + x) = 50.0
8.925 + 0.1785 x = 50.0
0.1785 x = 41.075
x = 230.1 g
Thus the amount of water added to make the solution is 230.1 g.
To know more about mass percentage, visit;
https://brainly.com/question/27429978
#SPJ2