both low explosives and high explosives will typically create a crater in the spot where the bomb detonates. true or false
Answers
False. While both low explosives and high explosives release energy upon detonation, high explosives generally create a crater due to their faster and more violent energy release. Low explosives, on the other hand, burn at a slower rate and typically do not produce a crater.
An explosive event that occurs at, immediately above, or below the surface can cause material to be ejected from the ground's surface, creating an explosion crater.
An explosive event causes material from the ground to be displaced and ejected, creating a crater. Usually, it has a bowl-like shape. Three processes—high-pressure gas, shock waves—are in charge of making the crater:
-Ground deformation caused by plasticity.
-Ejecta, or material that is thrown up by an explosion, from the ground.
-The ground's surface spalling.
Visit here to learn more about energy : brainly.com/question/11399976
#SPJ11
Yes, both low explosives and high explosives have the ability to create a crater in the spot where the bomb detonates. This is because when explosives are detonated, they rapidly release large amounts of energy, which generates a high-pressure shock wave.
This shock wave then travels outward from the point of detonation, causing the surrounding ground to be displaced and ejected, resulting in a crater. However, the size and depth of the crater will depend on the type and amount of explosives used, as well as the nature of the surrounding soil and terrain. High explosives, which typically contain a higher percentage of energetic materials, are generally more powerful and capable of creating larger and deeper craters compared to low explosives.
To know more about low explosives and high explosives:
https://brainly.com/question/31544631
#SPJ11
Related Questions
Blank moles of carbon dioxide are required to make 7.2 moles of glucose. A plant using 618 grams of carbon dioxide and plenty of water can make
Answers
Answer:
43.2 moles of carbon dioxide are required and 421g of glucose could be produced
Explanation:
Based on the reaction:
6CO2 + 6H2O → C6H12O6 + 6O2
1 mole of glucose, C6H12O6, requires 6 moles of carbon dioxide. 7.2moles of glucose requires:
7.2mol C6H12O6 * (6mol CO2 / 1mol C6H12O6) =
43.2 moles of carbon dioxide are required618g of CO2 -Molar mass: 44.01g/mol- are:
618g * (1mol / 44.01g) = 14.04moles CO2
Moles C6H12O6:
14.04moles CO2 * (1mol C6H12O6 / 6mol CO2) = 2.34moles C6H12O6
Mass glucose -Molar mass: 180.156g/mol-
2.34moles C6H12O6 * (180.156g / mol) =
421g of glucose could be producedThe gas formed when coal is heated in the absence of air_____________
Answers
Which compound(s) exhibit hydrogen bonding forces?
(Select all that apply.)
a. HF
b. XeF4
c. SO2
d. CH4
e. BrF3
f. NH3
Answers
The compounds that exhibit hydrogen bonding forces are a. HF and f. NH₃.
Hydrogen bonding is a type of chemical bonding that occurs between a hydrogen atom covalently bonded to an electronegative atom (such as nitrogen, oxygen, or fluorine) and another electronegative atom in a different molecule or region of the same molecule.
In HF, the hydrogen is bonded to fluorine, and in NH₃, the hydrogen is bonded to nitrogen. Both of these compounds exhibit hydrogen bonding forces.
The other compounds listed, XeF₄, SO₂, CH₄, and BrF₃, do not have hydrogen atoms bonded to highly electronegative atoms, so they do not exhibit hydrogen bonding forces.
Learn more about Hydrogen bonding here: https://brainly.com/question/1420470.
#SPJ11
Arsenic, hydraulic fracturing, lead, and PFAS present chemical threats to global drinking water supplies in different ways. For each problem, describe: (a) the origin of exposure, (b) human health consequences, (c) drivers of continued exposure, and (d) examples of modern solutions.
Answers
Arsenic, hydraulic fracturing, lead, and PFAS present chemical threats to global drinking water supplies in different ways.
Let's discuss each of them in detail:
(a) Arsenic - The origin of arsenic exposure is natural deposits or contamination from agricultural or industrial practices. Human health consequences include skin, lung, liver, and bladder cancers. It can also lead to cardiovascular diseases, skin lesions, and neurodevelopmental effects. Drivers of continued exposure include poor regulation and monitoring. Modern solutions include rainwater harvesting and treatment.
(b) Hydraulic fracturing - Hydraulic fracturing involves using a mixture of chemicals, water, and sand to extract natural gas and oil from shale rock formations. The origin of exposure is contaminated surface and groundwater due to the release of chemicals from fracking fluids and other sources. Human health consequences include skin, eye, and respiratory irritation, headaches, dizziness, and reproductive and developmental problems. Drivers of continued exposure include lack of regulation and poor oversight. Modern solutions include alternative energy sources and regulation of the industry.
(c) Lead - Lead contamination in drinking water can occur due to corrosion of plumbing materials. Human health consequences include neurological damage, developmental delays, anemia, and hypertension. Drivers of continued exposure include aging infrastructure and poor maintenance. Modern solutions include replacing lead service lines, testing for lead levels, and implementing corrosion control.
(d) PFAS - PFAS (per- and polyfluoroalkyl substances) are human-made chemicals used in a variety of consumer and industrial products. They can enter the water supply through wastewater discharges, firefighting foams, and other sources. Human health consequences include developmental effects, immune system damage, cancer, and thyroid hormone disruption. Drivers of continued exposure include the continued use of PFAS in consumer and industrial products. Modern solutions include reducing the use of PFAS in products and treatment methods such as granular activated carbon.
To know more about hydraulic fracturing visit:
https://brainly.com/question/31032804
#SPJ11
if 50g copper oxide is added to 0.5mol sulphuric acid, calculate the unreacted copper oxide
Answers
Answer: 10.3 grams of Copper oxide is left unreacted.
Explanation:
To calculate the moles :
\(\text{Moles of solute}=\frac{\text{given mass}}{\text{Molar Mass}}\)
\(\text{Moles of} CuO=\frac{50g}{79.5g}=0.63moles\)
The balanced chemical reaction is:
\(CuO+H_2SO_4\rightarrow CuSO_4+H_2O\)
According to stoichiometry :
1 moles of \(H_2SO_4\) require = 1 mole of \(CuO\)
Thus 0.5 moles of \(H_2SO_4\) will require=\(\frac{1}{1}\times 0.5=0.5moles\) of \(CuO\)
Thus \(H_2SO_4\) is the limiting reagent as it limits the formation of product and \(CuO\) is the excess reagent.
moles of CuO left = (0.63-0.5) mol= 0.13 mol
Mass of \(CuO\) left =\(moles\times {\text {Molar mass}}=0.13moles\times 79.5g/mol=10.3g\)
10.3 grams of Copper oxide is left unreacted.
Ocean where prevailing winds pass throug
Answers
The ocean where prevailing winds pass through is the Pacific Ocean.
\(\huge{\mathfrak{\colorbox{black}{\textcolor{lime}{I\:hope\:this\:helps\:!\:\:}}}}\)
♥️ \(\large{\textcolor{red}{\underline{\mathcal{SUMIT\:\:ROY\:\:(:\:\:}}}}\)
Which of the following is an example of a force that acts at a distance?
Answers
Answer:
Explanation:
Gravitational force
Answer:
1 gravity
2 magnetism
3 static electricity
Explanation:
Who was the first person to come up with the idea of the "atom?"
Answers
Answer:
Democritus
Explanation:
Democritus first introduced the idea of the atom almost 2500 years ago. Democritus was an important philosopher however if your talking about how the atom is today look at first rudimentary pudding model by JJ Thomson first design then its Neils Bohr whos model we look at today
Which property is shared by both acids and bases?
1. changes red litmus paper to blue
2. feels slippery
3. conducts electricity in aqueous solution
4. tastes sour
O 1
2
4
3
Answers
\(\huge{\textbf{\textsf{{\color{navy}{An}}{\purple{sw}}{\pink{er}} {\color{pink}{:}}}}}\)
3. Conducts electricity in aqueous solution.
ThanksHope it helps.what is the immediate source of the colored light produced by neon signs
Answers
The immediate source of the colored light produced by neon signs is the excitation of the neon atoms.
According to the Bohr theory, when energy is supplied to an atom, the electrons in the atom become excited and move from a lower to a higher energy level. The source of energy that causes excitation may be heat, electric discharge, etc.
As the excited atoms return to ground state, they emit energy in the form of visible light corresponding to a particular wavelength.
The source of the colored light produced by neon signs is the transition of neon atoms from higher to a lower energy level.
Learn more: https://brainly.com/question/3964366
A sample of water is heated and increases in temperature from 45°C to 125°C. What mass
of water can by the 4.5x10^8 J of energy needed to raise the temperature?
Answers
Answer:
2.691*10^6 g
Explanation:

Explain why malting point and poiling of alkane increae with increaing number of carbon atom
Answers
A lot of energy is required to overcome these forces and melt or vaporize the alkane
The melting point and boiling point of alkanes (a type of hydrocarbon with only single bonds between the carbon atoms) generally increase as the number of carbon atoms in the molecule increases. This is because the larger the molecule, the more Van der Waals forces (also known as London dispersion forces) that are present. These forces are a type of weak attractive force that can exist between any two molecules and are caused by the fluctuating dipoles (temporary separations of positive and negative charge) that occur within a molecule. The more carbon atoms a molecule has, the more fluctuating dipoles it has, and the stronger the Van der Waals forces are. As a result, more energy is required to overcome these forces and melt or vaporize the substance.
In addition to the size of the molecule, the type of bond present in the molecule can also affect the melting point and boiling point. Single bonds, like those found in alkanes, are weaker than double bonds or triple bonds. As a result, molecules with double or triple bonds have higher melting points and boiling points than molecules with the same number of carbon atoms but only single bonds.
Learn more about Alkanes here: brainly.com/question/3648919
#SPJ4
What is a solvent? please answer
A. The material that is dissolved
B. The material that increases the speed of the dissolution
C. The material that reduces how much can be dissolved
D. The material that is dissolving another material
WILL MARK BRAINLIEST
Answers
Answer:
D
Explanation:
Solvent is the material that is dissolving another material. Thus, option D is correct.
A solvent is a substance that dissolves another substance to form a solution. The substance that is being dissolved is called the solute, and the substance that is doing the dissolving is called the solvent. The solute is usually present in a smaller amount than the solvent, and it is the solvent that determines the physical properties of the solution, such as its density, viscosity, and boiling point.
For example, when salt is dissolved in water, the salt is the solute and the water is the solvent. The water molecules surround the salt molecules and break them apart, so that the salt ions are free to move around in the solution. The solution is then a homogeneous mixture of salt ions and water molecules.
Thus, option D is correct.
Learn more about solvent on:
https://brainly.com/question/11985826
#SPJ3
how are the electrons that are lost by the chlorophyll molecules replaced
Answers
By dividing water, the electron that was lost from the chlorophyll special pair is replaced. Protons are pumped from the stroma to the thylakoid lumen as a result of the electron passing through the first link in the electron transport chain.
The electrons from photosystem II that have traveled via the electron transport chain replace the electrons lost by these chlorophyll molecules. The two chlorophyll "a" molecules of photosystem II are compelled by light energy to go up an energy level.
The energy storage molecule ATP and the reduced electron carrier NADPH are produced by the light-dependent processes using light energy, which is required for the subsequent stage of photosynthesis. Light reactions in plants occur in the thylakoid membranes of organelles referred to as chloroplasts in chlorophyll.
The light reactions are largely controlled by photosystems, sizable complexes of proteins, and pigments (light-absorbing molecules) that are designed to capture light. Photosystem I (PSI) and Photosystem II are the two different types of photosystems (PSII). Via a process known as photolysis, the chlorophyll molecule regains the lost electron from a water molecule, releasing dioxygen (O2) in the process.
To know more about chlorophyll:
brainly.com/question/15867555
#SPJ4
Which of these common substances is a homogeneous mixture?
O
A. table salt
B. pure water
C. whole milk
D. maple syrup
Answers
Answer:
whole milk ,table salt , maple syrup
Answer:
d
Explanation:
(a) Barium ions are poisonous. Patients with digestive tract problems are sometimes given
an X-ray after they have swallowed a ‘barium meal’, consisting of a suspension of
BaSO4 in water. The [Ba2+(aq)] in a saturated solution of BaSO4 is too low to cause
problems of toxicity.
(i) Write an expression for the solubility product, Ksp, for BaSO4, including its units.
...................................................................................................................................
(ii) The numerical value of Ksp is 1.30 × 10–10. Calculate [Ba2+(aq)] in a saturated
solution of BaSO4.
...................................................................................................................................
...................................................................................................................................
(iii) The numerical value of Ksp for BaCO3 (5 × 10–10) is not significantly higher than
that for BaSO4, but barium carbonate is very poisonous if ingested. Suggest a
reason why this might be so.
...................................................................................................................................
............................................................................
QUESTION NUMBER (b)(iii) and (ii) PLEASE....
Answers
The numerical value of the Ksp of \(BaSO_{4}\) is 1.69 * 10^-20.
What is the Ksp?The Ksp is an equilibrium constant that shows the extent to which a substance is soluble in water. Now consider the fact that \(BaSO_{4}\) is almost insoluble in water.
i) The Ksp of the \(BaSO_{4}\) solution can be obtained from the relation;
Ksp = [\(Ba^{2+}\)] [\(SO_{4}^{2-}\)]
ii) The numerical value of the Ksp is obtained from; [1.30 × 10–10]^2 = 1.69 * 10^-20
iii) The reason for the toxicity of \(BaCO_{3}\) even though it is not more soluble that barium sulfate is that \(BaCO_{3}\) can dissolve in the gastrointestinal tract which is acidic leading to barium poisoning.
Learn more about barium poisoning:https://brainly.com/question/14593131
#SPJ1
Why do you add hydroxide to your hexanediamine solution? what would occur if you did not add it?.
Answers
In the polymerization reaction, the lone pair electrons on the NH₂ groups of hexanediamine attack the C=O groups of the dicarboxylic acid in a nucleophilic substitution reaction as shown in the image.
Hydroxide is added to remove any H⁺ ions present and keep the hexanediamine in the deprotonated form, so that the NH₂ lone pair electrons are available for reaction.
What if you don't add it ?If hydroxide is not added, the NH₂ groups will get protonated by H⁺ ions present to give NH₃⁺ groups, which cannot react.
You can also check out the questions based on Hydroxies through the link:
https://brainly.com/question/15683618
#SPJ4

Which of the following has the greatest influence on the size and force of waves?
A.
seasons
B.
Moon’s gravity
C.
wind speed
D.
Earth’s gravity
Answers
when do electrons release photons
Answers
Answer:
Later when they are older
Explanation:
brainliest/
What is the term for the chromosomes that are identical after DNA replication?
Answers
Sister chromatids is the term for the chromosomes that are identical after DNA replication.
Sister chromatids are identical chromosomes that are created during the process of DNA replication. DNA replication is the process where a DNA strand is copied, creating a new strand. During this process, each chromosome is split in half, forming two identical halves.
These halves are known as sister chromatids and are held together by a centromere. Sister chromatids are attached to one another during the interphase stage of the cell cycle and are separated during the metaphase stage of the cell cycle.
For more questions like Chromatids click the link below:
https://brainly.com/question/29213129
#SPJ4
When metals bond with other elements to create new compounds, which type of element do they mainly react with?.
Answers
Answer:
Nonmetal elements
Explanation:
Elements combine to form chemical compounds that are often divided into two categories. Metals often react with nonmetals to form ionic compounds.
which of the following processes will likely result in a precipitation reaction? (a) mixing a nano3 solution with a cuso4 solution. (b) mixing a bacl2 solution with a k2so4 solution. write a net ionic equation for the precipitation reaction
Answers
The process that will likely result in a precipitation reaction is (b) mixing a BaCl2 solution with a K2SO4 solution.
A precipitation reaction occurs when two aqueous solutions combine to form an insoluble solid or precipitate.
The balanced ionic equation for this precipitation reaction is:
Ba2+ (aq) + SO42- (aq) → BaSO4 (s)K+ (aq) + Cl- (aq) → KCl (aq)
The net ionic equation for this precipitation reaction is:
Ba2+ (aq) + SO42- (aq) → BaSO4 (s)
The net ionic equation only includes the ions that take part in the reaction. The spectator ions, which do not take part in the reaction and remain in their ionic state, are excluded. In this case, K+ and Cl- are spectator ions.
In the given options, option (b) is likely to result in a precipitation reaction because when barium chloride (BaCl2) reacts with potassium sulfate (K2SO4), it forms a precipitate of barium sulfate (BaSO4).
Learn more about precipitation on:
https://brainly.com/question/14330965
#SPJ11
Which material is an insulator against electric current?
copper wire
a rubber tire
a silver necklace
a light bulb filament
Answers
Answer:
b) a rubber tire
Explanation:
Answer: B - a rubber tire
Explanation: Rubber is an insulator
> A Moving to another question will save this response. Question 1 What is the mass number of an atom of potassium that has 20 neutrons?
a. 35
b. 59
c. 39
d. 15
e. 19
Answers
The mass number of an atom of potassium that has 20 neutrons is option C. 39
Mass number-
Mass number is the total number of protons and neutrons in the nucleus of an atom is called the mass number. It is represented by the symbol A. In other words, mass number refers to the sum of the number of protons and neutrons in the nucleus of an atom.
Atom-
Atoms are tiny particles that make up everything in the world. Everything in the world is made up of tiny particles known as atoms. An atom is the basic unit of matter. The term "atom" comes from the Greek word atomos, which means indivisible.
The basic building blocks of all matter are atoms, which are made up of three types of particles: protons, neutrons, and electrons.
In a neutral atom, the number of electrons is equal to the number of protons, thus the overall charge on the atom is zero. However, the mass number of the atom is equal to the sum of the number of protons and neutrons.
Learn more about the mass number of an atom from the given link-
https://brainly.com/question/26160840
#SPJ11
A d orbital can
hold ____ electrons.
A. 2
B. 6
C. 10
D. 14
Answers
Answer:
10 electrons
Explanation:
2. Explain brightness of light using the wave model of light.
Answers
Answer:
the wave model of light is useful for explaining brightness, color, and the frequency-dependent bending of light at a surface between media. For example, students could observe some of the wave behaviors or light by observing that when light passes through a small opening the waves spread out. They could observe that if the wavelength is short, the waves spread out very little, whereas longer wavelengths spread out more
Explanation:
write difference between metallic and non metallic minerals
Answers
Metallic Minerals will be minerals in which metal components are available in their crude structure. Non-metallic minerals don't contain any metal substances in them. At the point when metallic minerals are dissolved another item is shaped. Non-metallic minerals are frequently discovered installed in youthful overlay mountains and sedimentary rocks.
Draw the alkyl bromide(s) you would use in a malonic ester synthesis of ethyl 2-methyl-4-pentenoate
Answers
The structure of the alkyl bromides used in a malonic ester synthesis of ethyl 2-methyl-4-pentenoate are as drawn in the attached image.
Ethyl 2-methyl-4-pentenoate by Malonic ester synthesis.The malonic ester synthesis is a chemical reaction characterized by the alkylation of diethyl malonate or a similar ester of malonic acid at the carbon alpha (directly adjacent) to both carbonyl groups, and subsequently converted to a substituted acetic acid.
Hence, it follows from the structure of Ethyl 2-methyl-4-pentenoate that the alkyl bromides used are Ethyl bromide and methyl bromide.
Read more on Malonic ester synthesis;
https://brainly.com/question/17237043
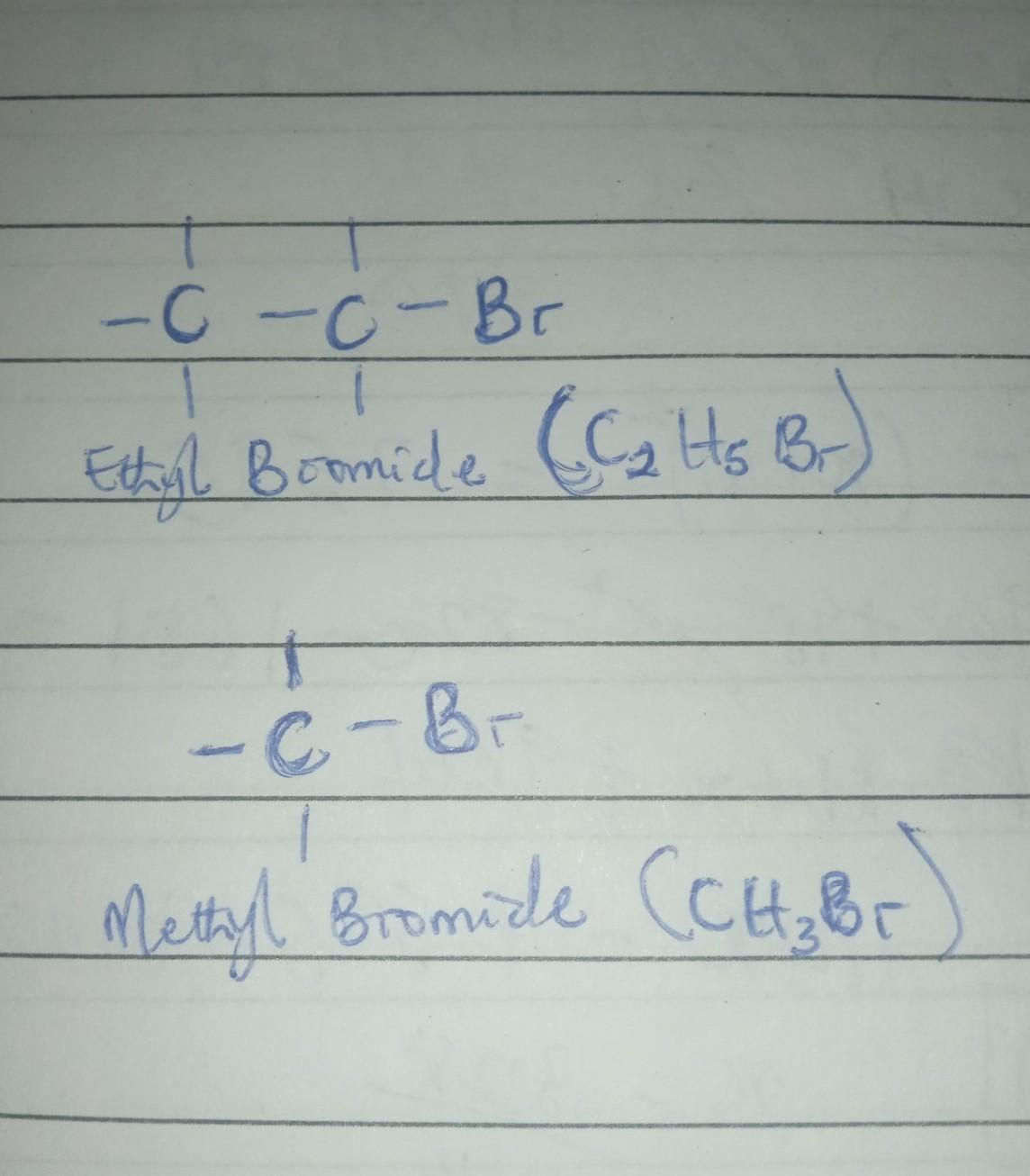
The structure of alkyl bromide that would be used in a malonic ester synthesis of ethyl 2-methyl-4-pentenoate are ethyl bromide and methyl bromide.
What is alkyl bromide?
When halogens such as Br, Cl, I, attched to the sp3 carbon atom of alkyl group, they called alkyl halides.
Here, bromide is attached to an alkyl group.
Ethyl bromide and methyl bromide are the alkyl halides used in synthesis of ethyl 2-methyl-4-pentenoate
Thus, ethyl bromide and methyl bromide are the alkyl bromides.
Learn more about alkyl bromide
https://brainly.com/question/14126879
#SPJ4


How many grams are equal to
413 kg?
Answers
Answer:
413000
Explanation:
you multiply it by a thousand
413 x 1000
cholrine is an active non-metal.why?