Answers
Answer:
If it loses 2 electrons, the net charge on the atom will be 2+
If the atom instead gains 4 electrons, the net charge will be 4-
Explanation:
It is based on adding and subtracting charges. Protons are +1 and electrons are -1
If the atom has 9 protons and 9 electrons, the net charge is +9 + (-9) = 0. The +9 is the 9 protons and the -9 is from the 9 electrons.
If two electrons are taken away, there would be 9-2 or 7 electrons with 9 protons. The net charge would then be +9 + (-7) = +2. +9 comes from the 9 electrons and -7 is from the 7 electrons.
So, if two electrons are taken away, the net charge is +2.
Similarly, if the atom gains 4 electrons, there will be 9+4 or 12 electrons and 9 protons. The net charge would then be +9 + (-12) = -4. +9 comes from the 9 protons and -12 comes from the 12 electrons.
So, if 4 electrons are added, the net charge is -4.
Related Questions
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. According to kinetic-molecular theory, which of the following statements is true? Check all that apply. If the temperatures of both containers are equal, container A has greater pressure than container B. If the volume of container A decreased, its pressure would decrease. If the pressure in both containers is equal, container A has a lower temperature than container B. Two containers are shown. Container A is square, and Container B is the same height, but is about twice as wide. Each container holds 6 gas particles distributed randomly.
Answers
The kinetic-molecular theory's true assertions are as follows:
If both containers' temperatures are the same, container A will have a higher pressure than container B.
Container A has a lower temperature than container B if the pressure in both containers is equal.
What is the kinetic theory of molecules?The molecules that make up a gas are always moving randomly, colliding with one another and the container walls, according to the kinetic molecular hypothesis. Remember that high temperature and low pressure are the only conditions in which perfect gases can exist.
The kinetic-molecular theory's true assertions are as follows:
If both containers' temperatures are the same, container A will have a higher pressure than container B.
If both are under pressure
Learn more about kinetic molecular theory:brainly.com/question/12025712
#SPJ1
How does the plasma membrane contribute to the structure and function of the cell?
It forms a boundary that controls what enters and leaves the cell.
It forms a rigid structure that only allows materials to exit the cell
It forms a boundary that only allows materials to enter a cell.
It forms a rigid structure around the cell that provides support to the cell
Answers
Answer:
A.It forms a boundary that controls what enters and leaves the cell.
Explanation:
i took the test on k12
if the reaction used up 2.35 moles of H2 , how many moles of NH3 were produced? use this eqationN2 + 3 H2 → 2 NH3
Answers
Answer:
mark me brilliant
Explanation:
According to the balanced chemical equation, for every 3 moles of H2 consumed, 2 moles of NH3 are produced.
Therefore, to find the number of moles of NH3 produced, we need to determine the ratio of H2 to NH3 based on the balanced equation:
3 moles H2 : 2 moles NH3
If 3 moles of H2 produces 2 moles of NH3, then 2.35 moles of H2 would produce:
(2 moles NH3 / 3 moles H2) x 2.35 moles H2 = 1.57 moles NH3
So, 1.57 moles of NH3 would be produced if 2.35 moles of H2 were consumed in this reaction.
Every compound is a molecule, but every molecule is not a compound. Why is this? Give an example with your answer.
Answers
Answer:
Explanation:
A molecule is a basic unit of a chemical substance made up of two or more atoms that are bonded together. A compound, on the other hand, is a substance made up of two or more different elements that are chemically bonded together.
Every compound is a molecule because it is made up of at least two chemically bonded atoms, but not every molecule is a compound because a molecule can be made up of the same type of atoms and not be a compound.
For example, molecular oxygen (O2) is made up of two oxygen atoms, but it is not a compound because both atoms are the same element. On the other hand, water (H2O) is a compound made up of hydrogen and oxygen atoms.
A Brainly star would be appreciated if it helped you!
How many electrons does the ion 3517C1- have?

Answers
Answer:
18
Explanation:
Which set of molecule representations correctly match.

Answers
Answer:
D
Explanation:
THERE ARE 3 HYDROGEN ATOMS AND 1 NITROGEN SUPPORTING THE FORMULA OF AMMONIA NH3 AND TOTAL ATOMS IS ALSO 4.
what are two ways to increase thermal energy are
Answers
understanding coefficients

Answers
Answer:
Mg-9
N-6
C-2
O-4
Ca-9
P-6
O-24
Explanation:
A 105.5mg sample of a white substance, suspecte of being cocaine (C17 H21 NO4), from 279.3mg of H2O on combustion chemical analysis shows that the compound contain 4.68%N by mass. would you conclude that the white solid is cocain?
Answers
Yes, we can conclude that a white solid that weighs 105.5 mg and is presumed to be cocaine (C₁₇H₂₁NO₄) produces 279.3 mg of CO2 and 66.46 mg of water upon combustion.
Briefing:Cocaine is a tropane alkaloid that has both local and central nervous system (CNS) stimulating effects. Cocaine inhibits the re-uptake of dopamine, serotonin, and norepinephrine into pre-synaptic neurons by binding to the transport proteins for these neurotransmitters. The salt form of cocaine is called cocaine hydrochloride. The taste is bitter and numbing, and it is a fine white powder.
Types Of Cocaine:Pure CocaineCrack CocaineSynthetic CocainePink CocaineBlack CocaineFish Scale CocaineCocaine HydrochlorideYellow CocaineBrown CocaineTo know more about Cocaine visit:
https://brainly.com/question/15865826
#SPJ9
The complete question is -
A 105.5mg sample of a white substance, suspected of being cocaine (C17H21NO4), forms 279.3mg of CO2 and 66.46mg of water on combustion. Chemical analysis shows that the compound contains 4.68% N by mass. Would you conclude that the white solid is cocaine?
if B is completely insoluble in water. Your description should include the volume of solvent required.6b) Assuming that 2 mg of the impurity B are present along with 100 mg of A, describe how you can purify A if B has the same solubility behavior as A. Will one crystallization produce pure A
Answers
Answer:
1st step : mix the mixture with water that way A will dissolve while B will remain insoluble.
2nd step :To get B from the solution, filter the mixture and get B
3rd step : To get A from the solution evaporate the new solution
Explanation:
Assuming 2mg of impurity B to be present
100 mg of A is present as well
Method of purifying A given that B is of same solubility
Dissolve the 100 mg of A with 30 mL
1st step : mix the mixture with water that way A will dissolve while B will remain insoluble.
2nd step :To get B from the solution, filter the mixture and get B
3rd step : To get A from the solution evaporate the new solution
Determine whether the compounds below could be used to prepare an buffer solution. Items (6 items) (Drag and drop into the appropriate area below) acetic acid Ka-1.8x10-5 ammonia Kb= 1.8x10 carbonic acid Ka-4.3x107 chlorous acid: calcium Ka-1.1x10-2 sulfuric acid hydroxide Categories CANNOT be used Drag and drop here Drag and drop here
Answers
Answer:
Acetic acid, carbonic acid and chlorous acid with calcium hydroxide
Ammonia with sulfuric acid
Explanation:
A buffer is an aqueous mixture of a weak acid and its conjugate base or vice versa.
Weak acids reacts with strong bases to produce the conjugate base. In the right amount, you can produce a buffer. In the same way, you can produce a buffer from the mixture of weak bases with strong acids.
In the problem, you have weak acids (acetic acid, carbonic acid, chlorous acid), one weak base (ammonia), one strong base (calcium hydroxide) and one strong acid (Sulfuric acid).
Thus, the mixtures that can produce a buffer are:
Acetic acid, carbonic acid and chlorous acid with calcium hydroxide
And:
Ammonia with sulfuric acid
List three ways you can contribute toLab safetyin our classroom.
Answers
EXPLANATION:
There are several ways to contribute to the safety of a laboratory
1. Ensure you read, understand, and obey the rules and regulations guiding the laboratory
2. Ensure you are always on your personal protective equipment (PPE) when working in the laboratory.
3. Always direct contact with chemicals.
Question 2
A proton and an electron move further apart from each other. Does the electrostatic
potential energy associated with their interaction increase or decrease?
O Increase
O Decrease
Answers
Answer:
decrease
Explanation:
because they're not the same
A gas has a volume of 50.0 mL at a temperature of 10.0 K and a pressure of 760. kPa. What will be the new volume when the temperature is changed to 20.0 K and the pressure is changed to 380. kPa?
Answers
To solve this problem using the gas laws, we need to use the Ideal Gas Law. This law states that the product of the pressure and the volume of a gas is proportional to the absolute temperature.
The equation of the Ideal Gas Law is the following:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{\dfrac{P_1V_1}{T_1}=\frac{P_2V_2}{T_2} } \end{gathered}$} }\)
Where:
P₁ = initial pressure = 760 kPaV₁ = initial volume = 50.0 mL = 0.050 LT₁ = initial temperature = 10.0 KP₂ = Final pressure = 380 kPaT₂ = final temperature = 20.0 KV₂ = Final volume = ?We clear for V₂:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{P_1V_1T_2}{P_2T_1 } } \end{gathered}$} }\)
Where:
P₁ = initial pressure V₁ = initial volumeT₁ = initial temperatureP₂ = Final pressureT₂ = final temperatureV₂ = Final volumeSubstituting the known values:
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{760\not{kPa}\times0.050 \ L\times20.0\not{k} }{ 380\not{kPa}\times10.0\not{k} } } \end{gathered}$} }\)
\(\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2=\frac{760 \ L}{3800 } } \end{gathered}$} }\)
\(\boxed{\boxed{\large\displaystyle\text{$\begin{gathered}\sf \bf{V_2\approx0.2 \ Liters} \end{gathered}$} }}\)
When the temperature changes to 20.0 K and the pressure changes to 380 kPa, the new volume will be approximately 0.2 L (200.0 mL).How many µg of mercury are contained in 27.2 mL of a 14.10 ppm solution?
Answers
mass (in µg) = volume (in mL) x concentration (in ppm) x atomic mass (in g/mol) / 1000
The atomic mass of mercury (Hg) is 200.59 g/mol.
Substituting the given values into the formula, we get:
mass = 27.2 mL x 14.10 ppm x 200.59 g/mol / 1000
mass = 76.9 µg
Therefore, there are 76.9 µg of mercury in 27.2 mL of a 14.10 ppm solution.
What is the mass number for an atom of xenon containing 54 protons and 75 neutrons?
Answers
Answer:
Answer should 133
Explanation:
all you have to do is add the protons and neutrons
The mass number for an atom of xenon containing 54 protons and 75 neutrons is 129 amu.
What is an Atomic mass of an atom?The Atomic mass of any atom may be defined as the mass of an atom that is togetherly constituted by protons and neurons in the nucleus of an atom. It is approximately equivalent to the number of protons and neutrons in the atom (the mass number).
The mass number of Xenon is calculated by the following formula:
The Number of neutrons = Mass number - Atomic number.
where, number of neutrons = 75, and number of protons = 54.
75 = Mass number - 54 (number of protons = atomic number).
Mass number = 75 + 54 = 129 amu.
Therefore, the mass number for an atom of xenon containing 54 protons and 75 neutrons is 129 amu.
To learn more about the Mass number, refer to the link:
https://brainly.com/question/3187640
#SPJ2
if the negative and the positive attract why are the proton and the electron not attracted to each other?
Answers
Answer:
They do attract
Explanation:
Protons and neutrons aren't electrically attracted to each other but when they get close enough they can exchange particles and become bound together by a strong force.
Earth is the only planet in the Solar System with known, sustained life. Which of these things most likely enabled life on Earth to develop? (Choose 3) Select 3 correct answer(s) Question 4 options: Presence of liquid water Size of the Milky Way Galaxy Proximity of the Sun Composition of the atmosphere Oxygen-rich atmosphere
Answers
The three things that most likely enabled life on Earth to develop are the presence of liquid water, the composition of the atmosphere, and an oxygen-rich atmosphere, which are the first, fourth, and fifth options.
The development of life on Earth is believed to have occurred due to a combination of factors, including the presence of liquid water, the composition of the atmosphere, and the evolution of an oxygen-rich atmosphere. Water is essential for life as we know it. It provides a medium for biochemical reactions and is necessary for the functioning of many cellular processes. Earth's surface temperature and atmospheric pressure are such that water exists in its liquid state, which is ideal for life to thrive.
Learn more about the earth here.
https://brainly.com/question/31436357
#SPJ1
According to the Arrhenius equation, changing which factors will affect the
rate constant?
OA. The constant A and the temperature
B. Temperature and activation energy
C. Temperature and the ideal gas constant
D. The activation energy and the constant A
SUBMIT
Answers
The correct answer is B: Temperature and activation energy are the factors that affect the rate constant according to the Arrhenius equation.
According to the Arrhenius equation, which describes the relationship between the rate constant (k) of a chemical reaction and temperature, changing the factors of temperature and activation energy will affect the rate constant.
The Arrhenius equation is given by:
k = A * e^(-Ea/RT),
where k is the rate constant, A is the pre-exponential factor or the frequency factor, Ea is the activation energy, R is the ideal gas constant, T is the temperature in Kelvin, and e is the base of the natural logarithm.
From the equation, it is evident that the rate constant is directly influenced by temperature and activation energy. Increasing the temperature results in a higher rate constant, as the exponential term in the equation becomes larger. This is because higher temperatures provide more kinetic energy to the reacting molecules, leading to more frequent successful collisions and increased reaction rates.
Similarly, the activation energy affects the rate constant. A higher activation energy results in a lower rate constant, as the exponential term becomes smaller. Activation energy represents the energy barrier that reactant molecules must overcome to form products. A higher activation energy implies a slower reaction rate. option(B)
For such more questions on Temperature
https://brainly.com/question/30668924
#SPJ8
Suppose you wish to use this reaction to determine the weight percentage of TiO2 in a sample of ore. To do this you collect the O2 gas from the reaction. If you find that 1.586 grams of the TiO2 containing ore evolved 32.1 mg of oxygen gas, what is the weight percent of TiO2 and Ti in the ore
Answers
Answer:
Mass percentage of TiO2 in the ore = 5.04%
Mass percentage of Ti = 3.01%
Explanation:
Equation of reaction is given below:
3 TiO2(s) + 4 BrF3(l) ---> 3 TiF4(s) + 2 Br2(l) + 3 O2(g)
From the equation of reaction above, 3 moles of O2(g) is obtained from 3 moles of TiO2(s)
Molar mass of O2 = 32 g/mol; molar mass of TiO2 = 79.87 g/mol, molar mass of Ti = 47.87 g/mol
Number of moles of O2 in 32.1 mg or 0.0321 g of O2 = 0.0321 g/32 g/mol = 0.001 moles
Therefore, 0.001 moles of O2 will be obtained from 0.001 moles of TiO2
Hence, mass l of 0.001 moles of TiO2 = 0.001 moles x 79.87g/mol = 0.07987
Mass percentage of TiO2 in the ore = 0.07987 x 100/1.586 = 5.04%
Mass of Ti in sample = (0.07987 - 0.03210) g = 0.04777 g
Mass percentage of Ti = 0.04777 × 100/1.586 = 3.01%
Question 2 of 30
A pamphlet about a fuel that contains up to 15% ethanol claims that the fuel
produces less of some types of pollution. The amount of ethanol in the fuel
and the pollutants are regulated by the government. What can you not infer
from this information?

Answers
Answer:
the awnser is d the other fuel produce more pollutants
Explanation:
because right out the bat we know that other fuels produce more than a 15 percent fuel so the right awnser is d
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
Electroplating is a way to coat a complex metal object with a very thin (and hence inexpensive) layer of a precious metal, such as silver or gold. In essence the metal object is made the cathode of an electrolytic cell in which the precious metal cations are dissolved in aqueous solution. Suppose a current of 0.270 A is passed through an electroplating cell with an aqueous solution of Ag_2 SO_4 in the cathode compartment for 72.0 seconds. Calculate the mass of pure silver deposited on a metal object made into the cathode of the cell. Round your answer to 3 significant digits. Also, be sure your answer contains a unit symbol.
Answers
Mass of the pure silver deposited on a metal object made into the cathode of the cell is calculated to be 0.0217 gm.
What is electroplating?The process of using electrodeposition to coat an object in a layer of metal is called electroplating .
As we know that, Q = I * t
=0.270 * 72
= 19.44 C
Here Q is quantity of electricity , I is current in amperes = 0.270 A (given)
t is time in seconds (72.0 sec)
As 96500 Coulomb of electricity electrolyzes 1 mole of Ag
then,19.44 C of electricity deposits,
=1/96500 * 19.44
= 0.000201 moles of Ag
Mass of Ag is = number of moles * molar mass
= 0.000201 * 108
= 0.0217 gm
Thus, mass of pure silver deposited on a metal object made into the cathode of the cell is 0.0217 gm.
To know more about electroplating, refer
https://brainly.com/question/16266707
#SPJ4
What is chemical equilibrium?
Question 1 options:
The rearrangement of the constituent atoms to create different substances as products.
A reversible reaction.
The process by which the reactants form products and products form reactants at equal rates.
A process that involves rearrangement of the molecular or ionic structure of a substance, as opposed to a change in physical form or a nuclear reaction.
Answers
.
Can you explain why you feel warm when you are standing near a campfire?
Answers
Answer:
You feel warm when you stand near a campfire because the flames of the fire warm your body. The heated waves are hit towards you and your body feels warm.
Hope this helped :)
Explanation:
is nothing something and if so or if not how, is water wet, if 2 people dropped a piece of bread on the opposite sides of the earth at the same time does the world temporarily become a sandwich...?
Answers
Answer:
technically no
Explanation:
there are 2 definitions of sandwich. one is the food one: 'an item of food consisting of two pieces of bread with meat, cheese, or other filling between them, eaten as a light meal.' the earth isn't food so it can't be a sandwich. the 2nd definition is the verb : 'insert or squeeze (someone or something) between two other people or things, typically in a restricted space or so as to be uncomfortable.' by dropping a piece of bread on the opposite sides of the earth at the same time won't make the earth squished or uncomfortable, so no it can't be a sandwich ( or be sandwiched)
Conversion Problem (show all work):
1. A patient required 3.0 pints of blood during surgery. How many liters does this correspond
to? Show all work. Use conversion factors available in the text or the exam packet. (4)
Answers
1.42liters, which is equivalent to 3pints, of blood is required for the surgery
Pints is a unit of measurement for volume in the United States. However, it can be converted to litres using the following equation:1 US pint = 0.473 liters
Hence, according to this question which states that a patient required 3.0 pints of blood during surgery. This means that the patient required:3 × 0.473
= 1.419 liters of blood for the surgery
1.42liters, which is equivalent to 3pints, of blood is required for the surgeryLearn more at: https://brainly.com/question/24168664
1. What is the Kinetic Energy of a 150 kg object that is moving with a speed of 15 m/s?
Answers
Answer:
16875 J
Explanation:
KINETIC ENERGY EQUATION = 1/2 m v^2
= 1/2 times mass times velocity of metres per second^2 (speed)
= 1/2 times 150 times by 15^2
= 16875 J (joules)
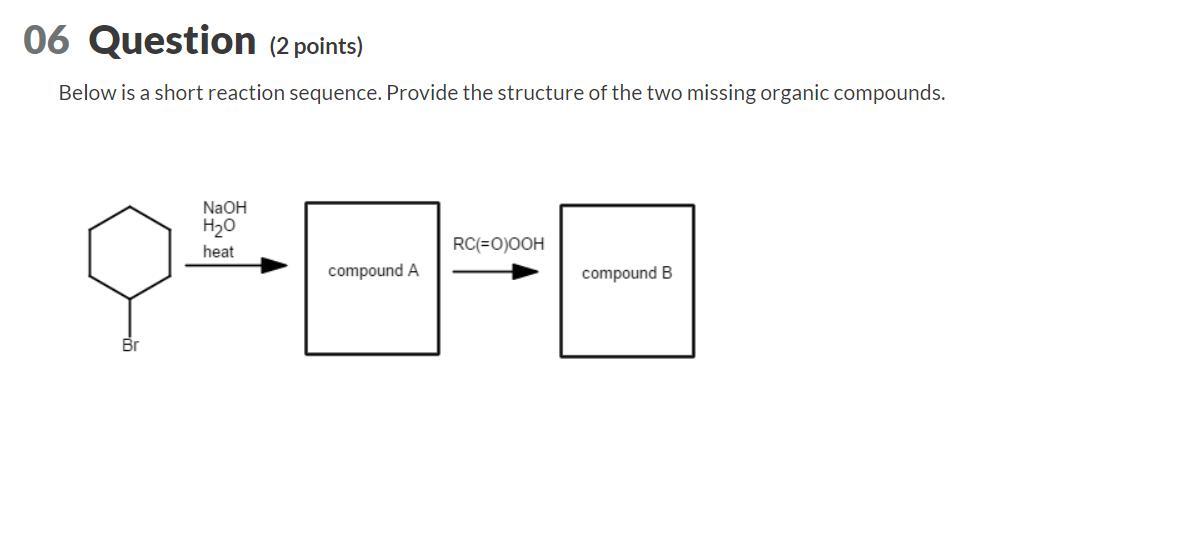
Below is a short reaction sequence. Provide the structure of the two missing organic compounds.
Answers
This reaction involves the the alkyl halide that interacts with the base.
What are the missing compounds?This reaction involves the the alkyl halide that interacts with the base. Now let us recall that the interaction of the substrate and the base leads to the formation of an alkene.
Let us recall that some reactions in chemistry just s this does not take place in a single reactive encounter. There exists a sequence of steps in which the reaction occurs and this is better understood as the mechanism of the reaction that is in question.
This alkene substrate now interacts with the peroxide as shown in the image attached to this answer. Now the interaction of the substrate and the alkene yields the epoxide product as shown.
The epoxide is the compound that oxygen bound in a three atom ring. The other two atoms that we can see in the ring are the atoms of carbon as shown
Learn more about organic reactions:https://brainly.com/question/9585105
#SPJ1
A 25-gram block of Aluminum has an initial temperature of 35 degrees Celcius. What will be the final temperature of the aluminum block with the addition of 503.38 Joules of heat?
Answers
The final temperature of aluminum is 57.37°C
Q=mcΔT
This means that the amount of energy produced is equal to the mass of the system multiplied by its change of temperature and multiplied by its specific heat
ΔT=(x−27.5) °C
503.38 = 25 × 0.9 × (x - 35)
(x - 35) = 503.3 / 25 × 0.9
x = 57.37°C
What is specific heat constant?
The specific heat capacity c [J/(kg K)] of tissue describes how much energy is required to change the temperature of 1 kg of tissue by 1 K (=1°C). For example, the lower specific heat capacity of fat compared to other soft tissue indicates, that fat requires less energy to obtain a certain temperature increase. If we multiply specific heat capacity by mass density (ρ·c [J/(m3 K)]), we obtain the energy required to raise the temperature of 1 m3 of tissue by 1 K (=1°C)—that is, a quantity equivalent to a volume-specific heat capacity
To know more about specif heat capacity, visit
brainly.com/question/27991746
#SPJ1