Kinetic Energy in Solids, Liquids, and Gases
Particles of solids, liquids, and gases have kinetic energy because the particles
are in constant motion.
The particles of solids have extremely _ kinetic energy
The particles of liquids
have extremely_ kinetic energy
The particles of gases
have_kinetic energy
Answers
Answer:
The amount of motion of these particles depends on the kinetic energy they possess. Particles with more kinetic energy move faster and farther apart. Particles with less energy move more slowly and stay closer together. The total kinetic energy of all the particles in a sample of matter is called thermal energy. Solids have the lowest kinetic energy as they are tightly packed and vibrate in place. Liquids have comparatively higher kinetic energy so the particles slide past each other. Gases have the most kinetic energy as a result they float around in the air.
Liquids have more kinetic energy than solids. When a substance increases in temperature, heat is being added, and its particles are gaining kinetic energy. Because of their close proximity to one another, liquid and solid particles experience inter-molecular forces.

Related Questions
What is the solvent in blood?
Answers
Answer:
Water serves to suspend the red blood cells to carry oxygen to the cells. It is the solvent for the electrolytes and nutrients needed by the cells, and also the solvent to carry waste material away from the cells. With water as the solvent, osmotic pressure acts to transport the needed water into cells.
Explanation:
you add 5.6g of iron to 23.60ml if water and observe that the volume of iron and water together is 24.31 ml. calculate the density
Answers
Answer:
8g/mL
Explanation:
Density = Mass/Volume
There is 5.6g of Iron added to the water of 23.60mL and it rose by 0.7mL.
Density = 5.6g/0.7mL of Iron.
Suppose that a beaker of water is 15°C and you raise the
temperature by 5°C. Use the graph above to calculate the percent decrease in the amount of dissolved O2 gas.

Answers
The percentage decrease in the amount of dissolved oxygen is 10%
Percent yield is the percent ratio of actual yield to the theoretical yield. It is calculated to be the experimental yield divided by theoretical yield multiplied by 100%. If the actual and theoretical yield are the same, the percent yield is 100%
In chemistry, yield is a measure of the quantity of moles of a product formed in relation to the reactant consumed, obtained in a chemical reaction, usually expressed as a percentage.
From the graph,
The amount of dissolved oxygen at 15°C is 10 mg/L
The amount of dissolved oxygen at 20°C is 9 mg/L
The decrease in the amount of dissolved oxygen is 1mg/L
The percentage decrease = (1/10) × 100 = 10%
Learn more about Percentage yield, here:
https://brainly.com/question/30794636
#SPJ1
In a laboratory demonstration, a balloon filled with methane and oxygen was exposed to a flame. The result was a brief, large flame. The students were asked to formulate an equation for the reaction. One answer is below.
CH4 + O2 CO2
Is this equation correct? Explain your reasoning.
Answers
Answer:
The equation is not correct:
[CH4 + O2 → CO2 + 2H2O]
Explanation:
The chemical formulas for methane, oxygen, and carbon dioxide are correct, but the equation is not balanced, so we really don't know idf the overall reaction is written correctly.
Let's balance the reaction:
CH4 + O2 → CO2
We need to have the same numbers of atoms on both sides.
Count the numbers of each atom:
Element Reactants Products Difference
C 1 1 0 Balanced
H 4 0 -4 XXX!!!!
O 2 2 0 Balanced
The hydrogens aren't showing up in the products. In fact, there is a product missing entirely from the reaction, H2O, water. The basic reaction is:
CH4 + O2 → CO2 + H2O
Now balance this reaction:
Element Reactants Products Difference
C 1 1 0 Balanced
H 4 2 -2 Hmmmm . . .
O 2 3 1 Uh = oh . . .
This reaction is not possible - there are either too many or too few element atoms (the "Difference").
We can adjust the coefficients for each of the molecules to balanced the equation. This is a bit tricky, but it consists of trying some coefficients that would make things balance. Only whole number coefficients are allowed in the final answer, although fractions might be used in the process to help find the whole numbers.
We need 2 H2O molecules to account for the 4 hydrogens on a single methane molecule
Step 1: CH4 + O2 → CO2 + 2H2O
That leads to:
Element Reactants Products Difference
C 1 1 0 Balanced
H 4 4 0 Balanced
O 2 4 2 Darn
We need more oxygen atoms, so:
Step 2: CH4 + 2O2 → CO2 + 2H2O
This leads to:
Element Reactants Products Difference
C 1 1 0 Balanced
H 4 4 0 Balanced
O 4 4 0 Balanced
This is a balanced reaction, and the correct chemical equation.
For the following question, consider the following equation: 2Mg+O2→2MgO
The number of moles of oxygen gas needed to react with 4.0 moles of Mg is
A) 1.0 mole
B) 2.0 moles
C) 3.0 moles
D) 4.0 moles
E) 6.0 moles
Answers
The number of moles of oxygen gas needed to react with 4.0 moles of Mg is 2.0 moles.
Equation: 2Mg + O2 → 2MgO
Step 1: Identify the mole ratio between Mg and O2 from the equation. For every 2 moles of Mg, there is 1 mole of O2 needed (2Mg:1O2).
Step 2: You have 4.0 moles of Mg. To find the number of moles of O2 needed, we can set up a proportion based on the mole ratio:
(4.0 moles Mg) / (x moles O2) = (2 moles Mg) / (1 mole O2)
Step 3: Solve for x moles O2 by cross-multiplying:
4.0 moles Mg * 1 mole O2 = 2 moles Mg * x moles O2
4.0 moles O2 = 2x moles O2
Step 4: Divide both sides by 2 to get the number of moles of O2:
x = 4.0 moles O2 / 2
x = 2.0 moles O2
The number of moles of oxygen gas needed to react with 4.0 moles of Mg is 2.0 moles (Option B).
learn more about moles here
https://brainly.com/question/15356425
#SPJ11
at what points does the object accelerate please explain
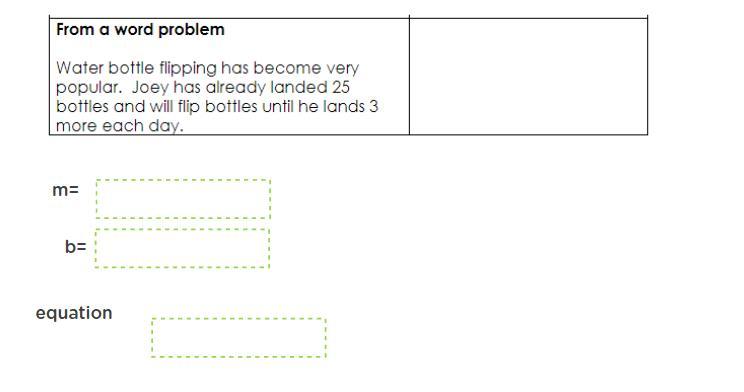
Answers
Answer:
M=3
B=25
Equation= 3x+25
Explanation:
M is your slope and if you follow the slope- intercept formula (y=m+b) you just need to plug in the numbers.
Thus your answer:
M=3
B=25
Equation= 3x+25
What happens when a base dissolves?OA. It increases the concentration of H* in solution.OB. It is no longer dangerous.OC. It increases the concentration of OH in solution.о D. It adds oxygen to the solution.
Answers
A base, according to the Arrhenius definition, is any species that increases the concentration of OH- in the solution. Some examples of bases are, NaOH, KOH, LiOH, all these substances will increase the concentration of OH when dissolved. Letter C
How are wavelength and frequency related to one another?
Answers
Explain why Mineral water is a mixture
Answers
minerals are disbursed through the water
Explanation:
say you have a bottle of water and you leave it in a car when you go in to a store you come back and you see some particles in the water well that's mixture water is made of minerals so when it says the particles so when the mix the minerals get disbursed through out the water and they become invisible. that's why minerals water is a mixture
What volume of 0.175 M Na3PO4 solution is required to completely react with 126 mL of 0.150 M0.102 M CuCl2?
2 Na3PO4 (aq) + 3 CuCl2 (aq) → Cu3(PO4)2 (s) + 6 NaCl (aq)
Answers
The volume of 0.175 M \(Na_3PO_4\) solution required to completely react with 126 mL, 0.150 M \(CuCl_2\) is 0.72 L 0r 720 mL.
Stoichiometric problemFrom the balanced equation of the reaction, the mole ratio of \(Na_3PO_4\) and \(CuCl_2\) is 2:3.
Recall that: mole = molarity x volume
126 mL = 126/1000 = 0.126 L
Mole of 126 mL, 0.150 M \(CuCl_2\) = 0.150 x 0.126
= 0.0189 moles
Equivalent mole of \(Na_3PO_4\) from the mole ratio = 2/3 x 0.0189
= 0.0126 moles
Volume = mole/molarity
Volume of 0.0126 moles, 0.175 M \(Na_3PO_4\) = 0.126/0.175
= 0.72 L
0.72 L = 0.72 x 1000
= 720 mL
In other words, 0.72 L 0r 720 mL of 0.175 M \(Na_3PO_4\) is required to completely react with 126 mL of 0.150 M \(CuCl_2\).
More on stoichiometric problems can be found here: https://brainly.com/question/15047541
#SPJ1
in the titration of 50.0 ml of 0.400 m hcooh with 0.150 m lioh, how many ml of lioh are required to reach the equivalence point?'
Answers
In the titration of 50.0 ml of 0.400 m hcooh with 0.150 m lioh, the equivalence point is the point at which the moles of acid and base are equal. 133 mL of 0.150 M lioh are required to reach the equivalence point in the titration of 50.0 mL of 0.400 M hcooh.
To find the volume of lioh required to reach the equivalence point, we can use the equation:
n(hcooh) = n(lioh)
Where n is the number of moles of each compound. The number of moles of hcooh is:
n(hcooh) = C(hcooh) x V(hcooh)
n(hcooh) = 0.400 mol/L x 0.0500 L
n(hcooh) = 0.0200 mol
At the equivalence point, the number of moles of lioh added is equal to the number of moles of hcooh. So:
n(lioh) = 0.0200 mol
We can use the equation above to find the volume of lioh required:
n(lioh) = C(lioh) x V(lioh)
V(lioh) = n(lioh) / C(lioh)
V(lioh) = 0.0200 mol / 0.150 mol/L
V(lioh) = 0.133 L or 133 mL
Learn more about equivalence point here:
brainly.com/question/31375551
#SPJ11
Horsetails are helpful for treating
bee stings.
fevers.
urinary tract infections.
bleeding injuries.
Answers
Answer:
urinary tract infections. B
Explanation:
a mixture of gases contains 12.0 grams of n2 and 15.0 grams of ar and has a total pressure of 1.32 atm. what is the partial pressure of n2?
Answers
12.0 grams od n2 & 15.0 gram of ar are present in a gas mixture with a pressure head of 1.32 atm. 0.704 atm is the pressure drop of n2.
How does pressure work?Another of the fundamental observable characteristics of the this phase of matter, pressure (P) is understood to be the force of all gas particulate interactions divided by the size of the wall.
What connections does pressure have to chemistry?Pressure has a major impact on every aspect of our life, from of the weather to flying. It is particularly crucial during chemical reactions. Chemists can compel chemical reactions to happen and hasten the transitions among solids, fluids, or gases by adjusting pressure.
Briefing:N=12.0, Ar=15.0
Total pressure=1.32
Partial pressure= n2
WN₂=12.0
WAr =15.0
nN₂=12/28 =0.42
nAr=15/40 =0.37
Xn₂=0.42/0.42+0.37 =0.53
Pn=Xn2×Ptotal
=0.53×1.32=0.69atm that ie nearly equal to 0.7atm.
To know more about pressure visit:
https://brainly.com/question/14760196
#SPJ4
a client is admitted to the emergency department with a headache, weakness, and slight confusion. the physician diagnoses carbon monoxide poisoning. what should the nurse do first?
Answers
When a patient is admitted to the emergency department with symptoms of headache, weakness, and slight confusion, the physician diagnoses carbon monoxide poisoning. In this case, the nurse should first administer oxygen therapy to the client.
Carbon monoxide poisoning is a medical emergency that occurs when carbon monoxide (CO) gas is breathed in.
It may cause serious harm or death, making it important to identify and manage the condition as soon as possible.
CO is a colorless, odorless, tasteless gas that is produced by burning fuels like coal, wood, charcoal, oil, kerosene, natural gas, and propane.
Carbon monoxide poisoning is a medical emergency that can lead to death if not treated quickly.
Symptoms of carbon monoxide poisoning include:
Headache
Dizziness
Nausea and vomiting
Weakness
Fatigue
Chest pain
Shortness of breath
Confusion
Loss of consciousness
The treatment for carbon monoxide poisoning involves removing the person from the source of CO gas and providing oxygen therapy.
Administering 100% oxygen through a mask is the preferred method of treatment, as it helps to reduce the amount of carbon monoxide in the blood and tissues.
This helps to restore oxygen to the body's tissues and organs, and it also helps to prevent the formation of carbon monoxide in the bloodstream.
The nurse should first administer oxygen therapy to the client because it is the first and most important step in treating carbon monoxide poisoning.
To know more about carbon monoxide visit:
https://brainly.com/question/10193078
#SPJ11
The number of protons in an atom is known as its .......... ...........?
Answers
Answer:
Atomic number
Explanation:
Atomic number is the number of protons, and therefore also the total positive charge, in the atomic nucleus. The Rutherford–Bohr model of the hydrogen atom (Z = 1) or a hydrogen-like ion (Z > 1).
Which element is most similar to fluorine in the way it reacts with other elements?
Answers
Answer:
The answer is chlorine
Explanation:
Por favor ayudenme con este ejercicio Una disolución de ácido sulfúrico de concentración 330 g/l tiene una densidad de 1,4 g/ml. ¿Cuánto vale su concentración en % en masa
Answers
Answer:
23.5 %
Explanation:
La fórmula del ácido sulfúrico es H₂SO₄, mientras que su masa molar es de 98 g/mol.
Siendo la densidad 1.4 g/mL, quiere decir que en 1 mL de solución tenemos 1.4 g de solución. Como la concentración es de 330 g/L entendemos que la solución contiene 330 gramos de soluto en 1L de solución.
Entonces en 1000 mL de solución (lo que equivale a 1 litro), tendremos:
1.4 g/mL = masa de solución / 1000 mL
masa de solución = 1.4 g/mL . 1000 mL = 1400 g
Como % en masa es la concentración que define la masa de soluto en 100 gramos de solución, para esta solución de H₂SO₄ es:
(330 g / 1400 g) . 100 = 23.5 %
__________ is always involved in hydrolysis reactions.
O None of the listed responses is correct.
O Water
O ATP
O H+ and OH-
O Synthesis
Answers
Water is always involved in hydrolysis reactions in the given options.
A hydrolysis reaction: what is it?The term "hydrolysis" refers to the breakage of chemical bonds by the addition of water and represents the reaction of an organic chemical with water to produce two or more new chemicals. Some occurrences of hydrolysis include the formation of hydronium and bisulfate compounds when sulphuric dissolves in water or a salt of a weak acid or base is mixed with water.
What is the term for sugar hydrolysis?Inversion is the mechanism of hydrolyzing sucrose to produce glucose and fructose. Invert sugar is the consequence of the hydrolysis of sucrose, which causes the sign of rotation to alter from dextro (+) to laevo (-).
Learn more about hydrolysis reactions here:
brainly.com/question/16715756
#SPJ4
What does the speaker in the poem mean by the phrase "my heart leaps up"?
A.
He is having a heart attack.
B.
He is exercising.
C.
He feels excited and happy.
D.
His heart continues to beat.

Answers
Answer:
C. He feels excited and happy
Explanation:
In a 0.57 M solution of propanoic acid, HOC6H5, 0.0684% of the acid has dissociated. a. Find the concentrations of all aqueous species in the solution at equilibrium. b. Find the pH of the solution. c. What concentration of HBr would produce a solution with the same pH as a 0.57 M solution of propanoic acid, HOC6H5? Justify your answer.
Answers
a) [HOC₆H₅] ≈ 0.57 M ; b) pH of the solution is approximately 3.41.c) concentration of [HBr] = (3.9012 × 10⁻⁴)²/Ka ≈ 2
What is propanoic acid?Propanoic acid is a carboxylic acid with chemical formula as C₃H₆O₂ and is also known as propionic acid.
a.) HOC₆H₅(aq) + H₂O(l) ⇌ H₃O⁺(aq) + OC₆H₅⁻(aq)
[H₃O⁺] = [OC₆H₅⁻] = 0.0684/100 × 0.57 M = 3.9012 × 10⁻⁴ M
Initial concentration of HOC₆H₅ is 0.57 M, and since only small fraction of it has dissociated, we can assume that its concentration at equilibrium is approximately equal to initial concentration. Therefore:
[HOC₆H₅] ≈ 0.57 M
b.) pH = - ㏒ [H₃O⁺]
pH = - ㏒ (3.9012 × 10⁻⁴) ≈ 3.41
Therefore, pH of the solution is approximately 3.41.
c.) pH = pKa + ㏒([OC₆H₅⁻]/[HOC₆H₅])
The pKa of propanoic acid is 4.87, so:
3.41 = 4.87 + ㏒([OC₆H₅⁻]/[HOC₆H₅])
㏒([OC₆H₅⁻]/[HOC₆H₅]) = -1.46
[OC₆H₅⁻]/[HOC₆H₅] = 3.47 × 10⁻²
[OC₆H₅⁻] = (3.47 × 10⁻²) × 0.57 M ≈ 1.97 × 10⁻² M
HBr(aq) + H₂O(l) ⇌ H₃O⁺(aq) + Br⁻(aq)
Ka = [H₃O⁺][Br⁻]/[HBr]
Ka = (3.9012 × 10⁻⁴)²/1.97 × 10⁻² ≈ 7.69 × 10⁻⁹
Ka = [H₃O⁺][Br⁻]/[HBr] = [H₃O⁺]²/[HBr]
[H₃O⁺] = √(Ka[HBr])
3.9012 × 10⁻⁴ = √(Ka[HBr])
[HBr] = (3.9012 × 10⁻⁴)²/Ka ≈ 2
To know more about propanoic acid, refer
https://brainly.com/question/15241093
#SPJ1
WHAT DO THESE DIAGRAMS MEAN PLS HELP

Answers
Answer:
(I). Evaporation
(ii). Sublimation
Please help! Please!!!
Look at the picture down below to answer the question!
>>Select the 2 that apply.
Q: An unsaturated hydrocarbon is represented by which structural formula(s)?
>>Choose two from the picture below that matches with the question above!

Answers
Answer:
A and D
Explanation:
unsaturated hydrocarbons have double or triple bonds
21.One calcium atom (Ca+2) will combine with which of the following atoms in a one-to-one ratio?Select one:a. Be+2b. O-2c. Cl-d. Na+
Answers
O2- Option B is correct
Explanations:
Calcium atom is a group 2 elements that gives out two electrons in order to form a stable configuration and a Ca2+ ion. These two electrons are transferred to an atom that required two electrons to complete its outer shell.
Since oxygen required 2 electron to attain its octet configuration, hence they can easily accepts the two electrons from calcium atoms to form CaO which is composed of Ca2+ and O2- ions in the ratio of one Ca2+ ion to one O2− ions.
A ground test utilizing an auxiliary current electrode and an auxiliary potential electrode is known as the______.
Answers
Answer:
The three-point test
Explanation:
The three-point test refers to a ground test utilizing an auxiliary current electrode and an auxiliary potential electrode.
how can we predict if a single replacement reaction will occur
Answers
A single replacement reaction is a type of chemical reaction that takes place when one element in a compound is replaced by another element. In other words, in this reaction, one element is replaced by another element. There are a few ways to predict whether or not a single replacement reaction will occur.
These ways are explained below: Paying Attention to ReactantsThe first way to predict if a single replacement reaction will occur is by paying attention to the reactants. In a single replacement reaction, a more reactive element will replace a less reactive element. For example, if a metal element is mixed with an aqueous solution that contains ions of another metal, a single replacement reaction will occur if the metal in the solid state is more reactive than the metal in the solution state. For instance, if you put zinc in copper sulfate, a reaction will occur because zinc is more reactive than copper. Therefore, it will replace copper, producing zinc sulfate and copper.Using the Activity SeriesAnother way to predict if a single replacement reaction will occur is by using the activity series. The activity series is a list of metals and their ability to replace other metals from their compounds. This list is arranged in order of decreasing activity. Therefore, if a metal is more active than another metal on the activity series, it will replace that metal from its compound. For example, if you put magnesium in silver nitrate, a reaction will occur because magnesium is more reactive than silver. Therefore, magnesium will replace the silver, producing magnesium nitrate and silver. If the metal is less active than another metal on the activity series, no reaction will occur.Using Electrochemical SeriesThe electrochemical series is another way to predict if a single replacement reaction will occur. The electrochemical series lists the elements in order of their standard reduction potentials. A metal with a higher reduction potential will replace a metal with a lower reduction potential from its compound. For instance, if you put copper in magnesium sulfate, no reaction will occur because copper has a lower reduction potential than magnesium. Therefore, magnesium will not replace copper from its compound.
To know more about single replacement reaction visit :
brainly.com/question/29224660
#SPJ11
What happens to pyruvate in the absence of oxygen?
Answers
Pyruvate undergoes fermentation, which transforms it into lactic acid inside the absence of oxygen.
The breakdown of the drug occurs throughout this process. similar to how pyruvate is converted into lactic acid.
An organic acid contains lactic acid. Its chemical formula is CH 3CH(OH)COOH.
It serves as a synthesis precursor inside a number of biochemical as well as molecular synthesis industries.
The final result of the glycolysis pathway, pyruvate, is transformed into lactic acid inside the lack of oxygen.
Glycolysis
The process through which glucose gets broken down to provide energy is known as glycolysis.
Learn more about Fermentation here :-
https://brainly.com/question/13050729r
#SPJ4
The reaction between sodium and chlorine that forms table salt is shown
_Na(s) + Cl2 (g) → _NaCl (3)
What coefficient should be added to the blanks to balance the equation?
А.
2
В.
1
2.
2
Answers
Answer: 2 Na (s) + Cl(g) -> 2 NaCl (s)
Explanation:
43. (a) Define the terms mixture and compound. Give three differences between them. Classify the following substances as an element, a mixture or a compound. (i) Limestone (ix)Clay (ii) Diamond (x) Urea (iii) Sand (xi) Antimony (iv) Soil (xii) Soap (v) Urine (xiii) Milk (vi) Bronze (xiv) Air (vii) Sugar (xv) Neon (viii) Gold (xvi) Iron
Answers
Mixture: A combination of two or more substances that are physically intermingled. Can be separated by physical methods.
Compound: A substance composed of two or more elements chemically bonded together. Can only be separated by chemical means.
Classification:
(i) Limestone - Mixture
(ix) Clay - Mixture
(ii) Diamond - Element
(x) Urea - Compound
(iii) Sand - Mixture
(xi) Antimony - Element
(iv) Soil - Mixture
(xii) Soap - Compound
(v) Urine - Mixture
(xiii) Milk - Mixture
(vi) Bronze - Mixture
(xiv) Air - Mixture
(vii) Sugar - Compound
(xv) Neon - Element
(viii) Gold - Element
(xvi) Iron - Element
Differences between mixtures and compounds:
Composition: Mixtures have variable compositions, meaning the ratio of substances can vary. Compounds have a fixed composition with a specific ratio of elements.
Separation: Mixtures can be separated into their components using physical methods. Compounds can only be separated into elements through chemical reactions.
Properties: Mixtures exhibit the properties of their individual components and can have varying physical properties throughout. Compounds have unique properties different from the elements they are composed of.
Classification of substances:
(i) Limestone: Mixture (contains minerals and impurities)
(ii) Diamond: Element (composed of carbon atoms)
(iii) Sand: Mixture (combination of mineral particles)
(iv) Soil: Mixture (combination of organic matter, minerals, water, and air)
(v) Urine: Mixture (contains various dissolved substances)
(vi) Bronze: Mixture (alloy of copper and tin)
(vii) Sugar: Compound (composed of carbon, hydrogen, and oxygen)
(viii) Gold: Element
(ix) Clay: Mixture (combination of minerals and organic matter)
(x) Urea: Compound (composed of carbon, hydrogen, nitrogen, and oxygen)
(xi) Antimony: Element
(xii) Soap: Compound (formed by the reaction of fatty acids with an alkali)
(xiii) Milk: Mixture (contains water, proteins, fats, sugars, and minerals)
(xiv) Air: Mixture (combination of gases like nitrogen, oxygen, carbon dioxide, etc.)
(xv) Neon: Element
(xvi) Iron: Element
It's important to note that some substances listed may have impurities or variations in composition, which can affect their classification.
Know more about Compound here:
https://brainly.com/question/14782984
#SPJ8
Diiodine pentaoxide is used in respirators to remove carbon monoxide from air:
I2O5(s) + 5CO(g) -----> I2(s) + 5CO2(g)
(a) What mass of carbon monoxide could be removed from air
by a respirator that contains 50.0 g of diiodine pentaoxide?
(b) What mass of I2 would remain in the respirator?
Answers
A respirator containing 50.0 g of diiodine pentaoxide can remove approximately 20.95 g of carbon monoxide from the air, leaving approximately 190.08 g of diiodine (\(I_2\)) remaining.
We use stoichiometry and the given balanced equation:
\(I_2O_5(s) + 5CO(g)\)→\(I_2(s) + 5CO_2(g)\)
(a) The mass of carbon monoxide (CO) that can be removed, we need to determine the stoichiometric ratio between diiodine pentaoxide\((I_2O_5)\) and CO. From the balanced equation, we can see that 1 mole of \((I_2O_5)\) reacts with 5 moles of CO.
We calculate the number of moles of \((I_2O_5)\):
Number of moles = mass / molar mass
Molar mass of \((I_2O_5)\) = 2(I) + 5(O) = 2(126.9 g/mol) + 5(16.0 g/mol) = 333.8 g/mol
Number of moles of \((I_2O_5)\) = 50.0 g / 333.8 g/mol ≈ 0.15 mol
Since the stoichiometric ratio is 1:5 between \((I_2O_5)\) and CO, the number of moles of CO that can be removed is:
Number of moles of CO = 0.15 mol × 5 = 0.75 mol
The mass of CO, we multiply the number of moles by its molar mass:
Mass of CO = number of moles × molar mass
Molar mass of CO = 12.0 g/mol + 16.0 g/mol = 28.0 g/mol
Mass of CO = 0.75 mol × 28.0 g/mol ≈ 21.0 g
Therefore, approximately 21.0 grams of carbon monoxide could be removed from the air by a respirator containing 50.0 grams of diiodine pentaoxide.
(b) To find the mass of \(I_2\) remaining in the respirator, we use the stoichiometric ratio of 1 mole of \((I_2O_5)\) to 1 mole of\(I_2\)
Number of moles of \(I_2\) remaining = 0.15 mol
To calculate the mass of \(I_2\), we multiply the number of moles by its molar mass:
Mass of \(I_2\) = number of moles × molar mass
Molar mass of \(I_2\) = 2(I) = 2(126.9 g/mol) = 253.8 g/mol
Mass of \(I_2\) = 0.15 mol × 253.8 g/mol ≈ 38.1 g
Therefore, approximately 38.1 grams of \(I_2\) would remain in the respirator.
To know more about carbon monoxide refer here
https://brainly.com/question/14698881#
#SPJ11
what quantity in moles of chlorine gas at 120.0 °c and 33.3 atm would occupy a vessel of 12.0 l?
Answers
The quantity in moles of chlorine gas at 120.0 °C and 33.3 atm that would occupy a vessel of 12.0 L is 0.754 moles.
We can use the Ideal Gas Law to solve for the quantity of moles of chlorine gas. The formula for the Ideal Gas Law is PV = nRT, where P is pressure, V is volume, n is the quantity in moles, R is the gas constant, and T is temperature in Kelvin.
First, we need to convert the temperature from Celsius to Kelvin: 120.0°C + 273.15 = 393.15 K.
Next, we can rearrange the Ideal Gas Law to solve for n:
n = PV/RT
We plug in the values given in the problem:
P = 33.3 atm
V = 12.0 L
R = 0.0821 L•atm/mol•K (gas constant)
T = 393.15 K
n = (33.3 atm x 12.0 L) / (0.0821 L•atm/mol•K x 393.15 K)
n = 0.754 moles
Therefore, the quantity in moles of chlorine gas at 120.0°C and 33.3 atm that would occupy a vessel of 12.0 L is 0.754 moles.
To Know more about moles, visit
https://brainly.com/question/29367909
#SPJ11