c-14 (radiocarbon) is radioactive and decays into n-14 with a half-life of 5730 years. you have measured the number of atoms of c-14 in a piece of charcoal from an archeological site and find 125 atoms. assuming the charcoal started with 1000 atoms of c-14, how old is the charcoal?
Answers
c-14 (radiocarbon) is radioactive and decays into n-14 with a half-life of 5730 years. you have measured the number of atoms of c-14 in a piece of charcoal from an archeological site and find 125 atoms. assuming the charcoal started with 1000 atoms of c-14, the charcoal is 17190 years.
Radiocarbon dating is the process of determining the age of an object made of organic material by utilising the properties of radiocarbon, a radioactive isotope of carbon (also known as carbon dating or carbon-14 dating). The method was developed in the late 1940s at the University of Chicago by Willard Libby. Its foundation is the ongoing production of radiocarbon (14C) in the Earth's atmosphere, which is caused by a combination of cosmic rays and atmospheric nitrogen. The procedure results in the production of radioactive carbon dioxide, which is then mixed with atmospheric oxygen to produce 14C. The radioactive carbon dioxide that is created by plants using this 14C is ultimately consumed by mammals. When an animal or plant dies, it ceases emitting carbon dioxide into the atmosphere.
To learn more about Radiocarbon dating click on the given link: https://brainly.com/question/11913067
#SPJ4
Related Questions
WILL GIEV BRAINLIST 2 BEST ASNWER
4. What should Sue have done to avoid an accident?
class safey
2 sentances
Answers
Answer:
By not playing with sharp objects.
By not running on a slippery floor.
By not jumping on the desks and benches.
Explanation:
Hang safety posters in common areas such as the lunchroom, and office, near the restrooms and in the front hallway. Implement safety training for all students. Ask teachers to conduct classroom safety training based on the rules.
what is the expected major organic product from the treatment of 4-methyl-2-pentyne with excess hydrogen in the presence of a platinum catalyst? 4-methylpentane
Answers
4-methylpentane
The response of 4-methyl-2-pentyne with extra hydrogen in the presence of a platinum catalyst is a hydrogenation reaction, which entails the addition of hydrogen atoms across the triple bond of the alkyne. The expected foremost organic product is 4-methylpentane, which is formed through the complete discount of the triple bond to a single bond.
The hydrogenation of 4-methyl-2-pentyne proceeds through a stepwise addition of hydrogen atoms to the triple bond, forming an intermediate alkene and then a saturated alkane. However, the presence of extra hydrogen ensures that the alkene intermediate is quickly decreased to the alkane product, which is the extra thermodynamically secure form.
Therefore, the anticipated main organic product of the hydrogenation reaction of 4-methyl-2-pentyne with extra hydrogen in the presence of a platinum catalyst is 4-methylpentane
learn more about hydrogenation here:brainly.com/question/27594211
#SPJ4
The below table compares the number of electrons in two neutral atoms.
atom:
Ne 10
Na 11
Compare the electron configuration and atomic radius of these two atoms and use that to explain the difference in their reactivity.
Answers
Consider the reaction: 2HgO(s) → 2Hg() + O2(g) Which of the following statements is correct?
A. Mercury is reduced.
B. All of these statements are correct.
C. Oxygen is oxidized,
D. Mercury(II) ion is the oxidizing agent.
Answers
The Oxygen is oxidized is the correct option.
The correct option for the given statement: Consider the reaction: 2HgO(s) → 2Hg() + O2(g) is "Oxygen is oxidized.
The given chemical equation for the reaction is:
2HgO(s) → 2Hg() + O2(g)According to the given chemical equation, the reactant HgO loses oxygen and forms elemental mercury and oxygen gas. Therefore, it can be concluded that Oxygen is oxidized.
Mercury(II) ion is the reducing agent: Reducing agents are the substances that undergo oxidation during a redox reaction, and their oxidation state decreases.
The reducing agent gets oxidized and reduces the other compound.Oxygen is the oxidizing agent:
Oxidizing agents are the substances that undergo reduction during a redox reaction, and their oxidation state increases. The oxidizing agent gets reduced and oxidizes the other compound.Mercury is reduced:
In the given chemical reaction, mercury is produced in its elemental form; this implies that it has undergone reduction.
Hence mercury is reduced.
Therefore, Oxygen is oxidized is the correct option.
Learn more about oxygen with the given link,
https://brainly.com/question/26073928
#SPJ11
Write a balanced symbol equation for the formation of phosphorus pentachloride from 72g of phosphorus trichloride and excess oxygen. Use this to calculate the mass of phosphorous pentachloride produced. State and explain the atom economy for this reaction.
Answers
Chlorine gas has the the chemical formula \(Cl_{2}\) ,Solid phosphorus has the chemical formula \(P_{4}\) ,Phosphorous pentachloride has the chemical formula \(PCl_{5}\), The final balanced equation is \(P_{4}\) + 10 \(Cl_{2}\) ...........> 4 \(PCl_{5}\),
What effects does chlorine gas have on people?distorted vision blisters, redness, and burning sensation on the skin after exposure to gas. If skin is subjected to liquid chlorine, it may suffer injuries resembling frostbite. feeling of burning in the eyes, throat, and nose.
Is chlorine gas palatable?The taste and smell of chlorine gas are both disagreeable. In concentrations as low as 1 part of every million, it can be detected (ppm). As an oxidizing agent, chlorine corrodes the metals used in plumbing and kitchen appliances. Equipment gaskets might become brittle due to corrosion from chlorine.
To learn more about Chlorine visit:
https://brainly.com/question/18094198
#SPJ1
chemical compound banned in most countries because of link to stratospheric ozone depletion is called
Answers
The chemical compound banned in most countries due to its link to stratospheric ozone depletion is called chlorofluorocarbons (CFCs).
CFCs were commonly used in refrigeration and air conditioning systems, as well as aerosol sprays, but their release into the atmosphere has been found to have devastating effects on the ozone layer.
When CFCs reach the upper atmosphere, they break down and release chlorine atoms, which react with ozone molecules, leading to the destruction of the ozone layer.
This depletion allows harmful ultraviolet radiation to reach the Earth's surface, increasing the risk of skin cancer and other health problems. As a result, CFCs have been banned in most countries since the signing of the Montreal Protocol in 1987, which aimed to phase out the use of ozone-depleting substances.
For more questions like CFCs click the link below:
https://brainly.com/question/29573682
#SPJ11
Which one of the following is least chemically reactive?A. ArB. OC. SD. TiE. U
Answers
The least chemically reactive element among the given options is Argon (A).
Chemical reactivity refers to the tendency of an element to undergo chemical reactions. The reactivity of an element is influenced by various factors, including its electron configuration, atomic size, and electronegativity.
Among the options given, the element that is least chemically reactive is Argon (A). Argon belongs to the group of noble gases, which are known for their low chemical reactivity. Noble gases have a full valence shell of electrons, making them stable and unlikely to form chemical bonds.
On the other hand, elements such as oxygen (O), sulfur (S), titanium (Ti), and uranium (U) are more chemically reactive compared to noble gases. They have incomplete valence shells and tend to form chemical bonds with other elements.
Therefore, among the options given, Argon (A) is the least chemically reactive element.
Learn more:About chemically reactive here:
https://brainly.com/question/30465703
#SPJ11
The least chemically reactive of the available elements is Ar (argon). Therefore, the correct option is A.
Argon is a member of the Noble Gas family of the periodic table, distinguished by its low reactivity. Noble gases are chemically inert under normal conditions because they have a stable electron structure with a full outer shell. Specifically, argon has a filled octet of electrons, and its electron configuration is \(1s^2 2s^2 2p^6 3s^2 3p^6\).
It does not readily combine with other elements to form compounds or conduct chemical reactions. Because of this characteristic, argon is useful for a wide range of uses, including creating an inert atmosphere in scientific settings and filling gas bulbs for lighting.
Therefore, the correct option is A.
Learn more about Noble gases, here:
https://brainly.com/question/19024000
#SPJ4
The half-life of thorium-232 is 14 billion years. A rock with 50% of its thorium-232 remaining is: 3. 5 billion years old. 14 billion years old. 28 billion years old
Answers
Answer: 28 billion
Explanation: To get the age of the rock, the half-life of thorium-232, which is 14 billion years, is to be multiplied to the number of half-lives it has passed. The answer is 28 billion.
which of the following pure substances has an unusually high normal boiling point? which of the following pure substances has an unusually high normal boiling point? hcl ch3och3 ch3sh ch3cl ch3nh2
Answers
The pure substance that has an unusually high normal boiling point among the given options is ch3nh2. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid.
A pure substance is one that is made up of only one type of atom or molecule, such as ch3nh2. The boiling point of a substance is determined by the intermolecular forces that exist between its molecules.In ch3nh2, the nitrogen atom has a lone pair of electrons that causes hydrogen bonding to occur between its molecules. As a result, the intermolecular forces in ch3nh2 are stronger than those in the other pure substances listed. This means that more energy is required to separate ch3nh2 molecules from each other during boiling, resulting in a higher boiling point. In terms of intermolecular forces, hydrogen bonding is stronger than the dipole-dipole, dispersion, and van der Waals forces present in the other listed substances. Thus, ch3nh2 has an unusually high boiling point due to hydrogen bonding.The normal boiling point of ch3nh2 is about 95°C, while the boiling points of the other pure substances range from -23.8°C to -10°C (ch3cl) and -24.8°C (ch3och3). As a result, ch3nh2 has a boiling point that is much higher than that of the other pure substances.
Thus, the pure substance that has an unusually high normal boiling point among the given options is ch3nh2, and this is due to the hydrogen bonding that occurs between its molecules.
To learn more about temperature click:
brainly.com/question/7510619
#SPJ11
A sealed flask contains 3.82 x 1024 molecules of CO, How many moles of Co, are in the flask?
Answers
Answer:
if you meant 3.82* 10^24, the answer is 6.34551495 moles
if you meant 3.28*1024, the answer is 6.49780731*10^-21 moles
since you're going from molecules to moles, you need to divide by Avogadro's number which is 6.02*10^23
if a wave has a frequency of 7.20 x 10^14 Hz and travels at the speed of light (speed of light = 3.0 x 10^8 m/s), what is the wavelength?
Answers
If a wave has a frequency of 7.20 x 10^14 Hz and travels at the speed of light (speed of light = 3.0 x 10^8 m/s), the wavelength is 0.42 × 10⁻⁶ m.
What do you mean by wavelength ?The term wavelength of light is defined as the distance between the two successive crests or troughs of the light wave. Wavelength is denoted by the Greek letter lambda (λ).
Given:
frequency = 7.20 x 10^14 Hz
speed of light = 3.0 x 10^8 m/s
Wavelength = ?
Wavelength = speed of light/ frequency
λ = ν / f
= 3.0 x 10^8 / 7.20 x 10^14
= 0.42 × 10⁻⁶ m
Thus, if a wave has a frequency of 7.20 x 10^14 Hz and travels at the speed of light (speed of light = 3.0 x 10^8 m/s), the wavelength is 0.42 × 10⁻⁶ m.
To learn more about the wavelength, follow the link;
https://brainly.com/question/13533093
#SPJ1
PLEASE HELP
Which reagent is the limiting reagent in a reaction?

Answers
Answer:
I believe B
Explanation:
Hope this helps
The atomic number of Oxygen is 8. Therefore, which of the following answers would correspond to what would be in the nucleus of an oxygen atom
Answers
Answer:
Its official chemical symbol is O, and its atomic number is 8, which means that an oxygen atom has eight protons in its nucleus. ... Oxygen is normally found as a
E Unit 1 Review Part 2
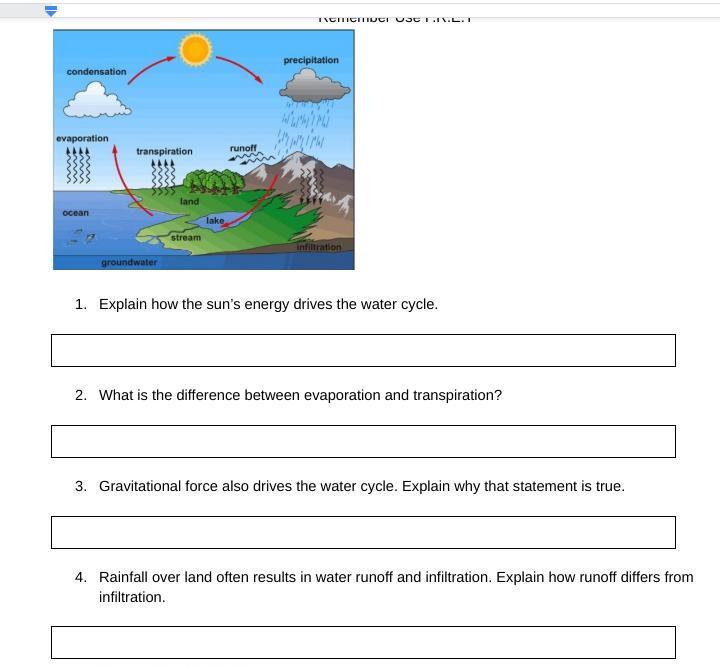
Answers
1) The energy from the sun causes the evaporation of water
2) Evaporation is the loss of surface water while transpiration is the loss of water from leaves
3) Gravitation falls is what enables the rain to fall down to the earth.
4) Run off is water draining into the water bodies while infiltration is water entering into the ground.
What is the water cycle?We know that the water cycle has to do with the movement of water in the ecosystem. The water cycle is one of the biogeochemical cycles that we find in the universe. We know that water moves out by the processes of transpiration and evaporation and enters by the means of precipitation and rainfall.
The energy from the sun causes the water that is on the surface of the earth to evaporate and then condense and fall back to the earth as rainfall. Gravitation is what makes the rain drops to fall to the ground.
Learn more about water cycle:https://brainly.com/question/1151425
#SPJ1
When water particles in their gaseous state (X) lose enough energy, then the gaseous state of water converts to liquid state as the kinetic energy of particles ...
Answers
When water particles in their gaseous state lose enough energy, the gaseous state of water converts to the liquid state as the kinetic energy of particles decreases.
In the gaseous state, water molecules have higher kinetic energy compared to the liquid state. When water particles lose energy, typically through cooling or condensation, their kinetic energy decreases. As a result, the water molecules slow down and come closer together, forming intermolecular forces that enable them to condense into the liquid state.
This phase transition occurs when the average kinetic energy of the water particles decreases below a certain threshold, allowing them to transition from the highly mobile and energetic gaseous state to the more ordered and cohesive liquid state.
To learn more about kinetic energy, Visit:
https://brainly.com/question/8101588
#SPJ11
7. The equation for this reaction is shown below.
4CuO(s) + CH4(g) → 4Cu(s) + 2H2O(g) + CO2(g)
-
The water and carbon dioxide produced escapes from the test tube.
Use information from the equation to explain why.
Answers
Answer:
because the lighted splint is burnt and the water and carbon dioxide starts to explode
write an Argument for people of westfield identifying the reddish brown substance. Plz answer this is due tomorrow!!
Answers
Answer:
The reddish-brown substance is a different color than the fertilizer, which is white, and the substance that makes up the pipe, which is gray. This means that they cannot be the same substance because they do not have the same properties. The atomic models show that the reddish-brown substance is made up of different repeating groups of atoms than the fertilizer and the pipe substance. The difference in atoms caused the difference in properties.
Explanation:
lul
People of westfield identifying the reddish brown substance and that substance is rust i.e. iron oxide.
What is rust?Rust is a chemical compound which is formed by the oxidation reaction between iron and oxygen for the formation of iron oxide.
People of westfield observed the reddish brown color in the water, that is the rust compound which is obtained by the chemical reaction occur between the iron pipes and the compound present in the fertilizers that are present in water.
Hence, the reddish brown substance in rust.
To know more about rust, visit the below link:
https://brainly.com/question/16771450
The electron in a hydrogen atom can undergo a transition from n = 4 to n = 3, emitting a photon with energy 1.06 × 10 –19J. Use this transition to answer the following questions.
i. What is the wavelength of this transition?
ii. How does this transition show that the position of the electron is quantized?
Answers
Answer:
Wavelength (λ) = 1.875 × 10⁻⁶ m
Explanation:
Given:
Energy (e) = 1.06 × 10⁻¹⁹ J
Find:
Wavelength (λ) = ?
Computation:
e = hc / λ
λ = hc / e
where c = 3 × 10⁸
Planck's constant (h) = 6.625 × 10⁻³⁴
So,
Wavelength (λ) = (6.625 × 10⁻³⁴)(3 × 10⁸) / (1.06 × 10⁻¹⁹)
1. Wavelength (λ) = 1.875 × 10⁻⁶ m
2. Given n = 4 to n = 3 both are integer not fraction so, electron is quantize
Jessica is holding a ball. What happens when she lets go of the ball as a result of forces acting on it?
Answers
What do these two changes have in common?
water boiling on a stove
water vapor condensing on a bathroom mirror
Answers
In both cases of water boiling on a stove and water vapor condensing on a bathroom mirror, the water remains chemically the same; only its physical state changes.
Boiling occurs when water reaches its boiling point, which is the temperature at which its vapor pressure equals atmospheric pressure. This causes the water to rapidly convert from a liquid to a gas, releasing steam.
Water vapor condenses on a bathroom mirror when the water vapor in the air comes into contact with the cooler surface of the mirror. The cooling effect of the mirror causes the water vapor to lose energy, which causes the water molecules to come together and form droplets.
This causes the water to rapidly convert from a gas to a liquid, creating condensation. In both cases, the changes in the water's physical state are due to changes in the temperature and pressure of the environment.
For more such questions on water, click on:
https://brainly.com/question/26306578
#SPJ11
Give another example of electrical energy being transferred into light
Answers
Answer: lightbulb, lamp, nightlight
Explanation:
Which of the following is an empirical formula?
H6O2
H402
H2O
H2O2
Answers
Answer:
hola responderé
Explanation:
el h2o es agua y el agua es mojada gracias por su atención
\( {\qquad\qquad\huge\underline{{\sf Answer}}} \)
What does an empirical formula mean ?- Empirical formula represents a molecule with its elements combined in simplest ratio, it's not necessarily same as the form in which the compound really exists in the nature. Hence we can say that empirical formula shows the simple ratios in which atoms combined to form a molecule.
For example : Hydrogen peroxide
- Hydrogen peroxide exists in nature as \(\sf H_2O_2 \), which is its molecular formula.
but it's empirical formula will be H0 [ simplest ratio of 1 : 1 ]
Therefore, the correct choice is :
C. \( \sf H_2O \)Hydrogen bonds are very stable and give water specific properties. Select two properties of water that are due to hydrogen bonds.
Answers
Answer:
Due to the extensive hydrogen bonding, water has some emergent properties that impact life on Earth in many ways. These include: Cohesion, Adhesion, High surface tension, High specific heat, High Heat of vaporization, and the fact that ice floats (Ice is less dense as a solid than liquid water).
4. (a) Draw resonating structure of Phenol. 5. What happens when (write the reactions involved) (a) Cyclohexanol reacts with concentrated sulfuric acid and resulting product is ozonolyzed (b) Phenol is heated with CH3COCl (c) Propyne reacts with hydrogeniodide in presence of benzene peroxide (d) Propoxypropane is reacted with access of NH3
Answers
(a) Draw resonating structure of PhenolPhenol is a common organic molecule. It consists of a phenyl group (C6H5) attached to a hydroxyl group (OH). The hydroxyl group is connected to the benzene ring at the para position, denoted as p-phenol.
The two main resonating structures of Phenol are shown in the figure below: This reaction takes place by cleaving the double bond of Cyclohexene using ozone, followed by a reductive workup step.
(b) Phenol is heated with CH3COCl:When Phenol is heated with Acetyl Chloride, it forms Acetophenone. The reaction is as follows:Phenol reacts with Acetyl Chloride to form Acetophenone, with the elimination of HCl as a by-product.(c) Propyne reacts with hydrogeniodide in the presence of benzene peroxide:Propyne reacts with hydrogen iodide in the presence of benzene peroxide to form 2-Iodopropane.
The reaction proceeds via a radical mechanism, as shown below:The chain initiation step:This step involves the homolytic cleavage of the benzene peroxide bond to generate benzene and two free radicals. These free radicals then interact with hydrogen iodide to form iodine radicals.The chain propagation step:The chain propagation steps involve the following sequence of reactions:The chain termination step:
This reaction involves the formation of 2-Iodopropane.(d) Propoxypropane is reacted with access of NH3:Propoxypropane is reacted with excess of NH3 to form Propylamine. The reaction is as follows:Propoxypropane undergoes nucleophilic substitution with ammonia, followed by deprotonation, to form the corresponding amine. Excess ammonia is required to drive the reaction to completion.
To know more about organic molecule visit:-
https://brainly.com/question/31574152
#SPJ11
A new element has been discovered. Scientists are trying to determine where it should it be placed on the periodic table. In order to do this they must determine the following about the element:
Answers
Elements are placed in order on the periodic table based on their atomic number, how many protons they have. In a neutral atom, the number of electrons will equal the number of protons, so we can easily determine electron number from atomic number.
A researcher is using 4.21 x 1023 molecules of chlorine gas (Cl2) in an experiment. How many grams of chlorine is the researcher using? Remember to include units (abbreviated appropriately) and the substance in your answer. Round your answer to the nearest 0.01.
Answers
The amount of chlorine gas released throughout the experiment weighs 49.70 g.
How many moles are there in total?A mole (mol) is the amount of a substance that has exactly as many particles as there are atoms in 12 grams of carbon-12.
The number of moles is obtained by dividing the specified mass of a substance by its molar mass. The weight of one mole of a substance is its molar mass, which is expressed in grams per mole.
Having said that,
The number of molecules in 1 mole of chlorine gas would be 6.02 * 1023.
Chlorine gas would contain 4.21 * 1023 molecules per mole.
Learn more about moles:brainly.com/question/30885025
#SPJ1
Help please 20 points and brainliest

Answers
Answer:
For homogeneous:
Filteration, vaporization, and a reverse chemical reaction
Explanation:
1) precipitation/crystallization reactions - if one of the components can form a solid salt when the solution undergoes several factors (concentration change, temperature change, etc); separation can be done either by filtration, centrifugation, or simple decantation.
(2) chromatography - separation can be based on the mobility of the phases (liquid chromatography, gas chromatography, ion-exchange, etc.)
(3) solvent extraction - if the components are both liquid, separation based on their relative solubilities
(4) distillation - separation based on different boiling points.
(5) magnetic separation - if one of the components have magnetic properties.
(6) electrophoresis - separation of organic molecules based on the type of gel used
heterogeneous:
Main methods of separation of heterogeneous mixtures
- Magnetic separation
- Sublimation
- Decantation
Liquid-solid mixture
Liquid-liquid mixture
- Filtration
- Centrifugation
Themes of interest
when stronger bases such as sodium methoxide are used in place of sodium 2-naphthoxide, a second product of 1-butene could be formed under the conditions used. Explain briefly where that product comes from and how it would be formed
Answers
When stronger bases such as sodium methoxide are used instead of sodium 2-naphthoxide, a second product, 1-butene, can be formed due to the elimination reaction, it occurs through the E2 mechanism.
The stronger base facilitates the deprotonation of the β-carbon in the presence of a leaving group, leading to the formation of an alkene. In this case, the leaving group is the hydrogen atom on the β-carbon of the starting material. The elimination reaction involves the removal of the leaving group and the adjacent proton, resulting in the formation of a double bond.
In the specific example mentioned, sodium methoxide would deprotonate the β-carbon of the starting material, resulting in the formation of the 1-butene product. This occurs because the stronger base enhances the rate of the elimination reaction, favoring the formation of the alkene product.
To learn more about sodium, click here:
brainly.com/question/27655686
#SPJ11
Briefly explain why adding insulation to an attic helps reduce heating costs.
Answers
Answer:
Insulation reduces the exchange of heat through a surface such as a wall, attic, duct or roof. In a well-insulated home, less warm air escapes from the house during the winter, and less cool air escapes during the summer, reducing the amount of energy needed for heating and cooling
empirical formula for C3O6
Answers
Answer:
CO2
Explanation:
You have 3 C for 6 O, then the empirical will be 1 C for 2 O
=> CO2