Calculate the amount of energy (in kJ) necessary to convert 450g of liquid water from 38°C to water vapor at 125 C. The molar heat of vaporization (Hvap) of water is 40.79 kJ/mol. The specific heat for water is 4.184 J/g C, and for steam is 1.99 J/g C. (Assume that the specific heat values do not change over the range of temperatures in the problem.)
Answers
total amount of energy required is= 132.6 + 717.9 + 45.42 = 896 kJ to convert 450g of liquid water from 38°C to water vapor at 125 C. The molar heat of vaporization (Hvap) of water is 40.79 kJ/mol. The specific heat for water is 4.184 J/g C, and for steam is 1.99 J/g C.
cslculation-heat energy required to raise the temp of liquid water to 100 C = 317 * 4.184 * 100 = 132632 J =132.6 kJ,mole of water = 317 / 18 = 17.6 mol,heat required for vaporization = 17.6 * 40.79 = 717.9 kJ,Heat required to raise the temp of water vapor to 172 C = 317 * 1.99 * 72 = 45420 J = 45.42 kJ, total heat required = 132.6 + 717.9 + 45.42 = 896 kJ. The quantity of heat necessary to increase the temperature of one unit mass of a substance by one degree Celsius. The specific heat capacity of a substance is specified as the amount of heat (J) soaked up per unit mass (kg) when its temperature rises 1 K (or 1 °C). The specific heat capacity of a substance is typically calculated by monitoring the heat capacity of a specimen of the material, generally with a calorimeter, and trying to divide by the sample's mass. The average kinetic energy of each molecule increases as the substance heats up.
Learn more about specific heat here:
https://brainly.com/question/11297584
#SPJ4
Related Questions
H2S(g) 2H2O(l)3H2(g) SO2(g) Using standard absolute entropies at 298K, calculate the entropy change for the system when 1.60 moles of H2S(g) react at standard conditions.
Answers
Answer: \(\Delta S\) = 473.92J/K.mol
Explanation: In physics, Entropy is defined as a degree of disorder in a system. Entropy change is given by the sum of all the products multiplied by their respective coeficients minus the sum of all the reagents multiplied by their respective coeficients:
\(\Delta S = m\Sigma product - n\Sigma reagent\)
The balanced reaction:
\(H_{2}S_{(g)}+2H_{2}O_{(l)}=>3H_{2}_{(g)}+SO_{2}_{(g)}\)
gives the proportion reagents react to form products, so, if 1.6 moles of \(H_{2}S_{(g)}\):
3.2 moles of water is used;
4.8 moles of hydrogen gas is formed;
1.6 moles of sulfur dioxide is also formed;
Calculating entropy change:
\(\Delta S = (4.8*131+1.6*248.8)-(1.6*205.6+3.2*70)\)
\(\Delta S=628.8+398.08-328.96-224\)
\(\Delta S\) = 473.92J/K.mol
Entropy change for the given chemical reaction is \(\Delta S\) = 473.92J/K.mol
How does a phase change affect a thermochemical equation?
O It alters the products.
O It alters the moles of reactants.
O It affects the balance of the equation.
O It can affect the AH value.
Answers
The correct answer is option D, It can affect the AH value.
What is a phase change?A phase change is a physical change in a substance in which the substance's state of matter is changed, such as from a gas to a liquid or from a liquid to a solid. It is also known as a phase transition.
Phase changes also involve changes in energy, temperature, and pressure. For example, when a solid melts to become a liquid, it absorbs energy and the temperature rises. When a liquid boils to become a gas, energy is released and the temperature decreases. Similarly, when a gas condenses to become a liquid, energy is released and the pressure increases.
Learn more about phase change here:
https://brainly.com/question/25664350
#SPJ1
A certain chemical reaction 444 kj releases of heat energy per mole of reactant consumed. Suppose some moles of the reactant are put into a calorimeter (a device for measuring heat flow). It takes of heat energy to raise the temperature of this calorimeter by . Now the reaction is run until all the reactant is gone, and the temperature of the calorimeter is found to rise by . How would you calculate the number of moles of reactant that were consumed
Answers
Complete question is;
A certain chemical reaction 444 kj releases of heat energy per mole of reactant consumed. Suppose some moles of the reactant are put into a calorimeter (a device for measuring heat flow). It takes 4.68 J of heat energy to raise the temperature of this calorimeter by 6.9 °C. Now the reaction is run until all the reactant is gone, and the temperature of the calorimeter is found to rise by . How would you calculate the number of moles of reactant that were consumed
Answer:
7.27 × 10^(-5) moles
Explanation:
We are given;
Heat energy = 444 kJ/mol
Final Temperature = 6.9 °C
Heat capacity = 4.68 J/°C
Let's calculate the heat generated from the formula;
Heat generated = heat capacity × final temperature
Heat generated = 4.68 × 6.9 = 32.292 J = 32.292 × 10^(-3) KJ
Now, the number of moles of reactant that were consumed is gotten from;
No_ of moles of rctnt consumed = heat energy generated/heat energy used = (32.292 × 10^(-3))/444 = 7.27 × 10^(-5) moles
Table salt, NaClThe name of the metal ion is _____.The name of the nonmetal ion is ______.Add them together and drop "ion"s to find the name of the compound is:______
Answers
The name of the metal ion is sodium (Na+).
The name of the nonmetal ion is chlorine (Cl-).
Add them together and drop "ion"s to find the name of the compound is: sodium chloride.
Helppppp pleaseeee xxxxxx
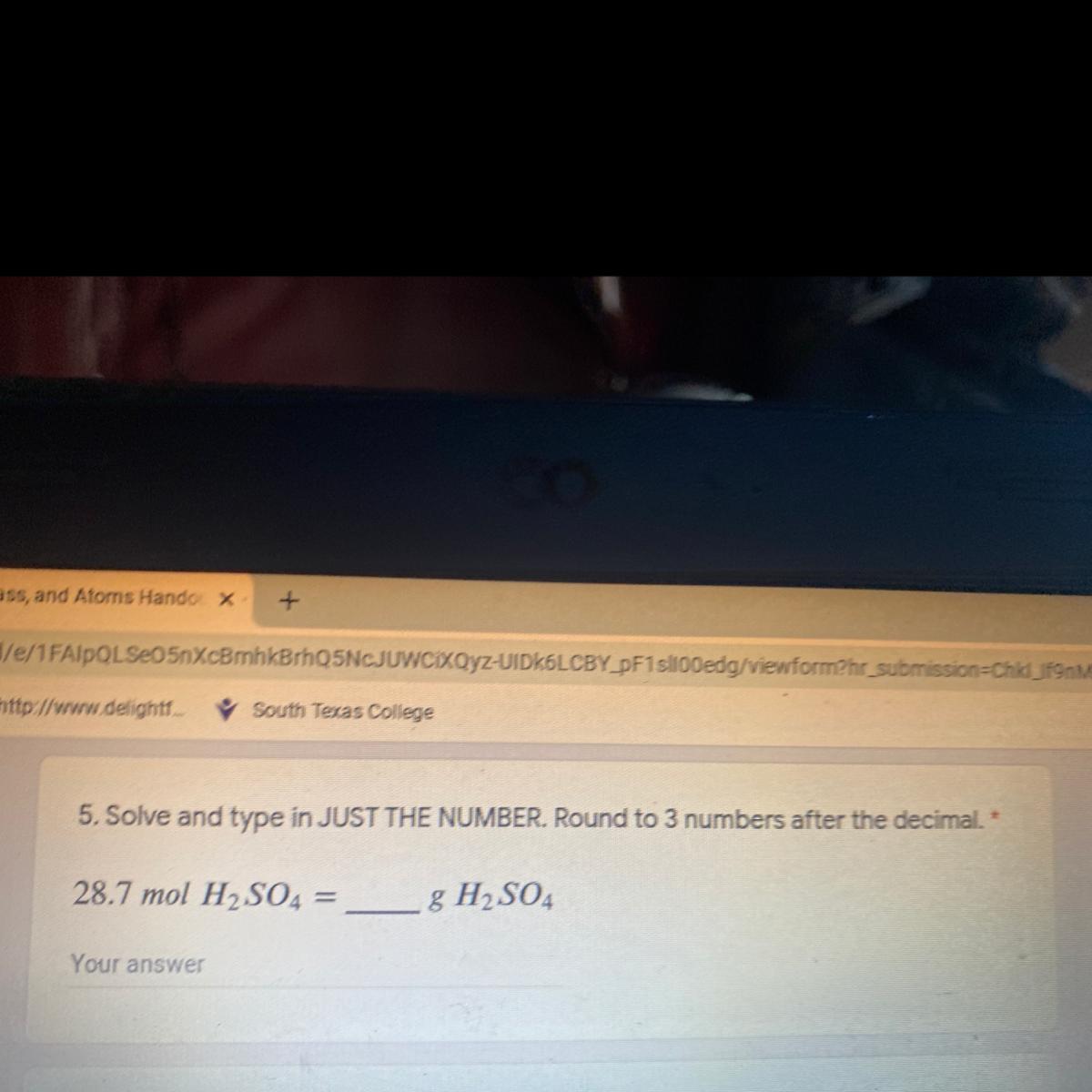
Answers
Answer:
2812.6 g of H₂SO₄
Explanation:
From the question given above, the following data were obtained:
Mole of H₂SO₄ = 28.7 moles
Mass of H₂SO₄ =?
Next, we shall determine the molar mass of H₂SO₄. This can be obtained as follow:
Molar mass of H₂SO₄ = (1×2) + 32 + (16×4)
= 2 + 32 + 64
= 98 g/mol
Finally, we shall determine the mass of H₂SO₄. This can be obtained as follow:
Mole of H₂SO₄ = 28.7 moles
Molar mass of H₂SO₄ =
Mass of H₂SO₄ =?
Mole = mass / Molar mass
28.7 = Mass of H₂SO₄ / 98
Cross multiply
Mass of H₂SO₄ = 28.7 × 98
Mass of H₂SO₄ = 2812.6 g
Thus, 28.7 mole of H₂SO₄ is equivalent to 2812.6 g of H₂SO₄
How is the pressure of a gas related to its
temperature?
Answers
arrange the following group of atoms in order of decreasing atomic size:B,Al,Ga
Answers
Answer:
B<Ga<Al
Explanation:
Hope this helps
Help me out??
H2SO4(aq) + 2 KOH(aq) --> 2 H2O(l) + K2SO4 (aq)
If you have 2 moles of H2SO4, how many moles of KOH do you need?
Answers
Answer:
4 mol KOH
Explanation:
Step 1: Write the balanced neutralization reaction.
H₂SO₄(aq) + 2 KOH(aq) ⇒ 2 H₂O(l) + K₂SO₄(aq)
Step 2: Establish the appropriate molar ratio
According to the balanced equation, the molar ratio of H₂SO₄to KOH is 1:2.
Step 3: Calculate the number of moles of KOH needed to react with 2 moles of H₂SO₄
We will use the previously established molar ratio.
2 mol H₂SO₄ × 2 mol KOH/1 mol H₂SO₄ = 4 mol KOH
You have an aqueous solution and add more and more base to it and plot the pH. You do the same experiment again, but the second time with a buffer in the solution. Compared to the solution without the buffer, for the solution with the buffer, the curve of the pH versus the amount of base added will
Answers
If a strong base is added to a buffer the pH will change only slightly. In the non-buffered solution changing the pH significantly.
If a strong base is added to a buffer, the weak acid will give up its H+ in order to transform the base (OH-) into water (H2O) and the conjugate base: HA + OH- → A- + H2O. Since the added OH- is consumed by this reaction, the pH will change only slightly.
In the non-buffered solution, the added hydronium or hydroxide ions have nothing to react with so the concentrations increase rapidly, changing the pH significantly. If a base is added to an acidic solution, the solution becomes less acidic and moves toward the middle of the pH scale.
Learn more about pH values here:
https://brainly.com/question/15289714
#SPJ4
0.73 grams of toluene was reacted with 2.0 grams of potassium permanganate in presence of 7.0 mL of 6 Molar potassium hydroxide and 30 mL of water. After refluxing for 1 hour the reaction mixture was treated with 6 Molar sulfuric acid to pH~ 2.0 followed by oxalic acid. On cooling this solution in an ice bath 0.633 grams of pure benzoic acid was obtained. Calculate the % yield of benzoic acid in this reaction.
Answers
Answer:
The value is \(k =66\%\)
Explanation:
From the question we are told that
The mass of toluene \( m_t =0.73 \ g \)
The mass of potassium permanganate is \( m =2.0 \ g \)
The volume of potassium hydroxide V = 7.0 mL
The concentration of potassium hydroxide C = 6 M
The mass of benzoic acid is \(m_b = 0.633 \ g\)
Generally the % yield of benzoic acid is mathematically represented as
\(k = \frac{m_b}{Z} * 100\)
Here Z is the theoretical yield which is mathematically represented as
\(Z = \frac{E}{W} * m_t \)
Here W is the molecular weight of product (benzoic acid) with value
W = 92.14 \ g
E is the molecular weight of reactant (toluene)with a constant value of
E = 122.12 g
So
\(Z = \frac{122.12 }{92.14} * 0.73 \)
=> \(Z = 0.968 \ g \)
So
\(k = \frac{0.633}{0.968} * 100\)
=> \(k =66\%\)
Aluminum undergoes a single-displacement reaction with copper (II) sulfate to form aluminum sulfate and _______________.
Answers
Ans cooper
Think of the different types of reactions you have learned about. How can you tell if they absorb energy or release energy, and at the molecular level, how are they different?
Answers
Answer:
? more info
Explanation:
Give users an example of the reactions you've learned about so we can have more of an idea. I'd be glad to help, if you edited your answer, meanwhile I too have homework in need of finishing.
the main function of the potato plant’s stem is to?
Answers
Answer:
support the plant
hold leaves and buds
store food
What Two Beta-Keto Esters Are Formed In The Dieckmann Reaction Of The Following Diester?
Answers
In the Dieckmann reaction, the diester undergoes a cyclization reaction to form a beta-keto ester. Two beta-keto esters are formed, one from the carboxyl group at the end of the chain, and one from the carboxyl group at the middle of the chain.
How does cyclization begin?Cyclization is started by the production of a putative cation, typically from a sp3-hybridized carbon, either by electrophilic addition to a double bond or by ionization.
The most frequent cyclization processes take place when an electrophile and a nucleophile interact. Consequently, the following are the most common reaction types: the nucleophilic change in a carbon atom that is saturated. addition by nucleophiles to an unsaturated carbon. Atomic addition-removal nucleophilic.
To know more about Cyclization visit: https://brainly.com/question/29422955
#SPJ4
, 2.2 g of Al reacts with 1.8 g of Oz.
Answers
The result of the reaction between 2.2 g of Al and 1.8 g of O2 is the formation of 3.89 g of \(Al_{2} O_{3}\).
The given masses of Al and \(O_{2}\) are in a ratio of approximately 1:1. Therefore, we can assume that all the Al reacts with all the O2, and use stoichiometry to determine the products and reactants involved.
The balanced chemical equation for the reaction between Al and O2 is:
4 Al + 3 \(O_{2}\) → 2 \(Al_{2} O_{3}\)
Using the molar masses of Al (26.98 g/mol) and \(O_{2}\) (32.00 g/mol), we can convert the given masses into moles:-
2.2 g Al × (1 mol Al/26.98 g Al) = 0.0815 mol Al
1.8 g \(O_{2}\) × (1 mol \(O_{2}\)/32.00 g \(O_{2}\)) = 0.0563 mol \(O_{2}\)
From the balanced chemical equation, we can see that 4 moles of Al react with 3 moles of \(O_{2}\) to produce 2 moles of Al2O3. Therefore, the limiting reactant is \(O_{2}\), since there are fewer moles of \(O_{2}\) than required by the stoichiometry of the reaction.
Using the mole ratio from the balanced chemical equation, we can determine the amount of \(Al_{2} O_{3}\) produced:
0.0563 mol \(O_{2}\) × (2 mol \(Al_{2} O_{3}\)/3 mol \(O_{2}\)) × (101.96 g Al2O3/mol) = 3.89 g \(Al_{2} O_{3}\).
To know more about chemical equation:
brainly.com/question/11231920
#SPJ1
Consider the reaction, CH4 (g) +202 (g) → CO₂ (g) + 2H₂O (1), AH= −890 kJ.
What will be the change in enthalpy when 3 moles of methane react in excess oxygen?
O-890 kJ
○ -2.67 × 10³ kJ
O +890 kJ
O +2.67 x 10³ kJ
Answers
The change in enthalpy when 3 moles of methane react in excess oxygen is -2.67 × 10³ kJ (Option B).
What is enthalpy?Enthalpy (H) is a thermodynamic property that describes the total heat content of a system at a constant pressure. It is a measure of the energy that is transferred as heat during a chemical reaction or physical change at constant pressure.
Enthalpy is defined mathematically as:
H = U + PV
where U is the internal energy of the system, P is the pressure, and V is the volume. Enthalpy is often measured in units of Joules (J) or kilojoules (kJ).
The given reaction is:
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l) ΔH = -890 kJ
This equation shows that when one mole of methane reacts with two moles of oxygen, it produces one mole of carbon dioxide and two moles of water while releasing 890 kJ of energy.
To determine the change in enthalpy when 3 moles of methane react in excess oxygen, we need to first calculate the amount of heat released when one mole of methane reacts with excess oxygen.
From the balanced chemical equation, we can see that 1 mole of CH4 releases 890 kJ of energy, so the energy released by 3 moles of CH4 would be 3 times that value:
Energy released by 3 moles of CH4 = 3 × (-890 kJ/mol) = -2670 kJ
To know more about internal energy, visit:
https://brainly.com/question/14668303
#SPJ1
The change in enthalpy when 3 moles of methane react in excess oxygen is -2670 kJ.
What is Enthalpy?
Enthalpy is a thermodynamic property of a system that describes the heat content of the system at constant pressure. It is denoted by the symbol "H" and is expressed in units of joules (J) or kilojoules (kJ). Enthalpy is a state function, which means that it depends only on the initial and final states of the system, not on the path taken to reach those states.
The given reaction is: CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (l), ΔH = −890 kJ
This reaction is for one mole of methane. To find the change in enthalpy when 3 moles of methane react, we need to multiply the enthalpy change by 3:
ΔH = 3 × (-890 kJ/mol) = -2670 kJ
Therefore, the change in enthalpy when 3 moles of methane react in excess oxygen is -2670 kJ.
Learn more about Enthalpy from given link
https://brainly.com/question/14047927
#SPJ1
How many moles are in 12.5 g ammonium chloride
Answers
Answer:
0.233682161601125
Explanation:
10 examples of elimination reaction
Answers
Answer:
1. Dehydration of alcohols
2. Dehydrohalogenation of alkyl halides
3. Decarboxylation of carboxylic acids
4. Pyrolysis of esters
5. Deamination of amino acids
6. Dealkylation of ethers
7. Dehalogenation of aryl halides
8. Dehydration of amides
9. Dehydrogenation of alkanes
10. Dehydrogenation of alkenes.
Explanation:
Question 6 of 10Which statements are true about balancing chemical equations?Check all that apply.I A. Single atoms should be done last.shouldB. Atoms that are in only one of the reactants and only one of theproducts should be done last.I C. Balancing chemical equations involves trial and error.D. Balancing chemical equations does not involve trial and error.

Answers
Single atoms should be done last.
Balancing chemical equations involves trial and error.
Explanations:Chemical equations are known to be balanced if the total number of elements on the reactant side is equal to the total elements on the product side.
Balancing of chemical equations may not be determined at once, hence the use of trial and method is crucial.
Also, atoms that are in only one of the reactants and only one of the products should be done first while single atoms should be done last.
I
Based on the information below, which of the following are atoms of the same element? (mark all that apply)
Atom A
Atom B
Atom C
8 protons
8 neutrons
8 electrons
10 protons
10 neutrons
10 electrons
8 protons
10 neutrons
8 electrons
end

Answers
Based on the information below . The atoms of the same element are Atom A and Atom C .
Atoms of same element have the same atomic number. so the number of protons and electron are same but they have different atomic masses called as Isotopes. so, the atoms having different number of neutron will have different masses.
In the given case ,
Atom A Atom B Atom C
8 protons 10 protons 8 protons
8 neutrons 10 neutrons 10 neutrons
8 electrons 10 electrons 8 electrons
so, In Atom A and Atom C , the number of protons and electrons are same but the have different atomic masses means having different no. of neutrons. so Atom A and Atom C are the atoms of same elements.
Hence,Based on the information below . The atoms of the same element are Atom A and Atom C.
To learn more about Atoms and Elements here
https://brainly.com/question/15173445
#SPJ1
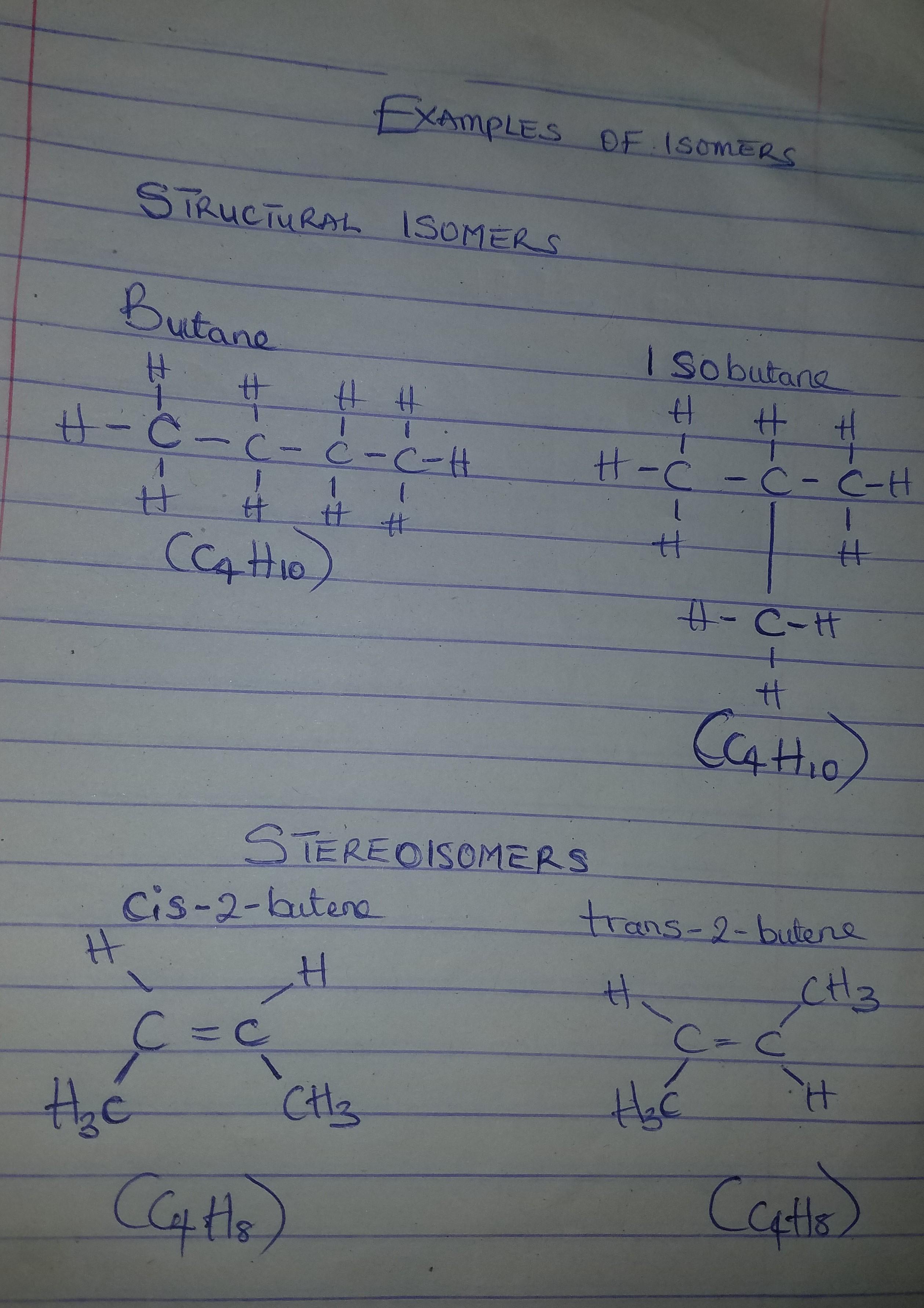
Isomers of hydrocarbons have the same _______formula but different ____formula.
Answers
Answer:
Isomers of hydrocarbons have the molecular formula but structural formula.
Explanation:
Molecules with the same structural formula, but different molecular geometries (spatial arrangement) are called isomers. These differences in the arrangement of the various atoms confer certain differences in chemical properties to the resulting hydrocarbons, even though their chemical composition is the same. There are two types of isomers:
Structural isomers: Here, each atom are connected or bonded in different ways, hence structural isomers may contain different functional groups or pattern of bonding. structural isomers are further divided into: chain, position, and functional group isomers.
Stereoisomers: Here, the connections of the atoms are the same, but the difference is in their orientation in space
If a system receives 57 J of energy as heat and does 12 J of work on the surroundings, what is the change in the internal energy of the system?
Keep in mind the sign (+,-)
Answers
The change in the internal energy of the system is +69 J, indicating that the system has gained energy.
The change in internal energy of the system can be calculated using the First Law of Thermodynamics, which states that the change in internal energy of a system is equal to the heat added to the system minus the work done by the system on its surroundings.
In this case, the system receives 57 J of energy as heat, which means that the heat is added to the system, and the value is positive (+57 J). On the other hand, the system does 12 J of work on the surroundings, which means that the work is done by the system, and the value is negative (-12 J).
Using the First Law of Thermodynamics, we can calculate the change in internal energy of the system as:
ΔU = Q - W
ΔU = (+57 J) - (-12 J)
ΔU = 69 J
Therefore, the change in the internal energy of the system is +69 J, indicating that the system has gained energy.
To know more about First Law of Thermodynamics, visit:
https://brainly.com/question/3808473
#SPJ1
What Types of food molecules make up lettuce
Answers
Consider the following reaction: 2N2O5(g) → 4NO2(g) + O2(g) Calculate the volume N2O5 that must decompose completely to produce 9.64 L nitrogen dioxide.
Answers
The volume of \(N_2O_5\) needed to produce 9.64 L of \(NO_2\) is 4.97 L, calculated using stoichiometry and the ideal gas equation.
The given chemical equation is \(2N_2O_5(g) \rightarrow 4NO_2(g) + O_2(g)\) .The volume of \(N_2O_5\) that decomposes completely to form 9.64 L of \(NO_2\) is to be calculated. For this, we can use the concept of stoichiometry. Stoichiometry is a branch of chemistry that deals with the quantitative relationships between reactants and products in a balanced chemical equation.To calculate the volume of \(N_2O_5\) that is needed to produce 9.64 L of \(NO_2\), we will first determine the number of moles of NO2 produced in the reaction. For this, we can use the ideal gas equation, PV = nRT. Here, we have the volume of NO2 and we can assume the pressure and temperature to be constant. Thus, we have PV = nRT, where P = pressure, V = volume, n = number of moles, R = ideal gas constant, and T = temperature. Substituting the given values in the ideal gas equation, we get,n = PV/RT = (1 atm × 9.64 L)/(0.0821 L atm K-1 mol-1 × 300 K) = 0.404 molFrom the chemical equation, we see that 2 moles of \(N_2O_5\) give 4 moles of \(NO_2\). Thus, 0.404 mol of \(NO_2\) must have been produced from (0.404/2) = 0.202 mol of \(N_2O_5\). Using the ideal gas equation, we can also find the volume of 0.202 mol of \(N_2O_5\) at the given conditions. Thus, V = nRT/P = (0.202 mol × 0.0821 L atm K-1 mol-1 × 300 K)/1 atm = 4.97 L. Thus, the volume of \(N_2O_5\) that must decompose completely to produce 9.64 L nitrogen dioxide is 4.97 L.For more questions on stoichiometry
https://brainly.com/question/14935523
#SPJ8
all the questions 1. What contribution did de Broglie make to the development of the modern model of the atom? (A)Observed the effect of bombarding thin gold foil (and other metal foils) with alpha radiation from radioactive substances. 60 m B. Discovery of the nucleus C. Discovered that atoms and molecules emit energy only in certain discrete quantities, or quanta. D. Discovered negatively charged particles by cathode ray tube experiment E. Described the wave properties of particles
Answers
De Broglie contributed to the development of the modern model of atoms by describing the wave properties of particles. Option E.
De Broglie's contribution to atomic theoryLouis de Broglie was a French physicist who made significant contributions to the development of quantum mechanics.
In his doctoral thesis, he proposed that particles, such as electrons, have both particle-like and wave-like properties. This idea became known as wave-particle duality and laid the foundation for the development of the modern model of the atom.
According to de Broglie's theory, particles can exhibit wave-like behavior and have a wavelength that is inversely proportional to their momentum.
This theory was later experimentally confirmed in a series of experiments that demonstrated the diffraction of electrons and other particles.
More on de Broglie can be found here: https://brainly.com/question/17295250
#SPJ1
please help!!!! Chem question

Answers
Answer : The net ionic equation will be,
\(Ba^{2+}(aq)+SO_4^{2-}(aq)\rightarrow BaSO_4(s)\)
Explanation :
In the net ionic equations, we are not include the spectator ions in the equations.
Spectator ions : The ions present on reactant and product side which do not participate in a reactions. The same ions present on both the sides.
The given balanced ionic equation will be,
\(Ba(OH)_2(aq)+H_2SO_4(aq)\rightarrow 2H_2O(aq)+BaSO_4(s)\)
The ionic equation in separated aqueous solution will be,
\(Ba^{2+}(aq)+2OH^-(aq)+2H^{+}(aq)+SO_4^{2-}(aq)\rightarrow BaSO_4(s)+2H^+(aq)+2OH^{-}(aq)\)
In this equation, \(H^+\text{ and }OH^-\) are the spectator ions.
By removing the spectator ions from the balanced ionic equation, we get the net ionic equation.
The net ionic equation will be,
\(Ba^{2+}(aq)+SO_4^{2-}(aq)\rightarrow BaSO_4(s)\)
Dana is reading about the best conditions for worm composting. Worm composting involves keeping worms in a bin with vegetable scraps from the kitchen along with other plant waste, and using the soil and drippings they produce to improve gardens. Dana decides to conduct an experiment to find the best conditions for worm composting. Which of the scenarios is the best way for Dana to conduct the scientific investigation?
Answers
For a reaction where ∆H° = +573 kJ/mol and ∆S° = +1.64 kJ/mol・K, at what temperature, in K, does X(l) → X(g) occur spontaneously?
Answers
The temperature in kelvin (K) in which the reaction occurs spontaneously is given as 349 K
What is entropy?Entropy is simply defined as the measure of the degree of the disorderliness of a system. Mathematically, it can be expressed as:
ΔS = ΔH / T
Where
ΔS is the change in entropy of system ΔH is the enthalpy change T is the temperature How to determine the temperature Enthalpy change (ΔH) = +573 KJ/molChange in entropy (ΔS = +1.64 KJ/mol・K Temperature (T) =?ΔS = ΔH / T
1.64 = 573 m T
Cross multiply
1.64 × T = 573
Divide both side by 1.64
T = 573 / 1.64
T = 349 K
Learn more about entropy:
https://brainly.com/question/13834300
Michelle is trying to find the average atomic mass of a sample of an unknown
element. She finds that her sample contains 59.34% of an isotope with a mass of
113.6459, while the rest of the sample is an isotope with a mass of 115.8488. What
is the average atomic mass of her sample? Please round your answer to 0.01 amu.
Answers
The average atomic mass of her sample is 114.54 amu
Let the 1st isotope be A
Let the 2nd isotope be B
From the question given above, the following data were obtained:
Abundance of isotope A (A%) = 59.34% Mass of isotope A = 113.6459 amuMass of isotope B = 115.8488 amuAbundance of isotope B (B%) = 100 – 59.34 = 40.66%Average atomic mass =?The average atomic mass of the sample can be obtained as follow:
\(Average \: atomic \: mass \: = \frac{mass \: of \: A \times A\%}{100} + \frac{mass \: of \: B \times B\%}{100} \\ \\ Average \: atomic \: mass \: = \frac{113.6459\times 59.34}{100} + \frac{115.8488\times 40.66}{100} \\ \\ Average \: atomic \: mass \: = 114.54 \: amu \\ \\ \)
Thus, the average atomic mass of the sample is 114.54 amu
Learn more about isotope: https://brainly.com/question/25868336
Carbon doesn't have a charge because it doesn't generally share or donate electrons.
*
True
O
False
Answers
Answer:
Correct me if i'm wrong but i'm pretty sure its FALSEExplanation:
Its because In a single bond, two carbon atoms share one pair of electrons. In a double bond, they share two pairs of electrons, and in a triple bond they share three pairs of electrons.