Calculate the grams of H2O produced when 9. 75 grams of NH3 reacts with excess oxygen.
4NH3 + 5O2 → 4NO + 6H2O
Molar Masses: NH3 = 17. 031 O2 = 31. 998 NO = 30. 006 H2O= 18. 015
A 11. 9 grams
B 10. 3 grams
C 61. 9 grams
D 15. 5 grams
Answers
The answer is D) 15.5 grams.
To solve this problem, we need to use stoichiometry and the given balanced chemical equation. First, we need to determine the limiting reagent by calculating the number of moles of NH3 and O2:
9.75 g \(NH_3\) x (1 mol \(NH_3\)/17.031 g \(NH_3\)) = 0.571 mol \(NH_3\)
Excess O2, so we do not need to calculate.
Now, we can use the mole ratio from the balanced equation to determine the moles of H2O produced:
0.571 mol \(NH_3\) x (6 mol H2O/4 mol \(NH_3\)) = 0.857 mol H2O
Finally, we can convert the moles of H2O to grams:
0.857 mol H2O x (18.015 g H2O/1 mol H2O) = 15.44 g H2O
Therefore, the answer is D) 15.5 grams.
To know more about balanced equations:
https://brainly.com/question/30485389
#SPJ11
Related Questions
∆E = −33 kJ/mol Ea = 20 kJ/mol What is E a′ ?
Answer in units of kJ/mol.
Answers
The value of Ea′ is -53 kJ/mol, and it represents the energy released during the chemical reaction.
The given values ∆E = −33 kJ/mol and Ea = 20 kJ/mol represent the activation energy and the change in energy, respectively, for a chemical reaction. The activation energy, Ea, is the minimum energy required for the reaction to occur, while the change in energy, ∆E, represents the difference between the energy of the reactants and the energy of the products.
The relationship between the activation energy, Ea, and the change in energy, ∆E, can be expressed using the equation: ∆E = Ea + Ea′ where Ea′ represents the energy released during the reaction. Since the change in energy and the activation energy are given, we can rearrange the equation to solve for Ea′: Ea′ = ∆E - Ea
Substituting the given values, we get: Ea′ = −33 kJ/mol - 20 kJ/mol = -53 kJ/mol. Therefore, the value of Ea′ is -53 kJ/mol. This negative value indicates that the reaction is exothermic, meaning that it releases energy as it proceeds. The magnitude of the value (-53 kJ/mol) indicates that the energy released during the reaction is significant.
In summary, the value of Ea′ is -53 kJ/mol, and it represents the energy released during the chemical reaction. This value can be calculated using the equation Ea′ = ∆E - Ea, where ∆E is the change in energy and Ea is the activation energy.
For more such on energy visit:
https://brainly.com/question/1634438
#SPJ11
How many atoms are in 4.3 moles of lithium?
Answers
A. Pure substance
B. homogeneous
C. distillation
D. mixture
E. 1502
F. intensive
G. decantation
H. extensive
I. solubility
J. 2 02
1. While preparing the ingredients she needs for cooking, Maria accidentally mixed cooking oil and water. In order to separate the two, she will have to perform __________________________.
2. Find the chemical formulas that will correctly balance the following chemical equations: CH4+
→CO2+2H2O
3. At sea level, the boiling point of water is 100°C regardless of how much water is present. This shows that boiling point is an _____________________ property.
4. Carbon dioxide is a gas that is important for photosynthesis. It is composed of the elements carbon and oxygen which cannot be separated through physical means. Carbon dioxide is a/an ___________________________.
5. Find the chemical formulas that will correctly balance the following chemical equations: 2C6H6+
→12CO2+6H2O
6. After adding 1 teaspoon of sugar to a cup of water and stirring, Denise noticed that the sugar completely dissolved into the water. This mixture is described as ________________________.
7. It has been found that at room temperature, a maximum of 35 g of salt can dissolve in 100 mL of water. This property of salt is known as _________________________.
8. The air we breathe is composed of 78% nitrogen, 21% oxygen, and 1% of other gases. This describes that the air we breathe is a _____________________.
9. The volume of a liquid such as water is measured using equipment like a graduated cylinder. In order to increase the volume, more of the liquid has to be added. This shows that volume is an ___________________ property.
10. Monique was given a homogeneous solution of acetone and water by her laboratory instructor. She was tasked to separate the two components from the mixture. Monique knows that acetone has a lower boiling point than water. She decides to perform _______________________.
Answers
1. decantation
2. 2O₂
3. intensive
4. Pure Substance
5. 15O₂
6. homogenous
7. Solubility
8. mixture
9. extensive
10. distillation
Of the following transitions in the Bohr hydrogen atom, which of the following
results in the emission of the lowest-energy photon.
n = 1 ® n= 6
n=5 ® n=1
n = 6® n=1
n=3® n=5
n=1 ® n=5
Answers
What is the advantages of generating I2 in situ, and doing so by using bleach instead of a more powerful oxidizing agent?
Answers
Generating I2 in situ, or on-site, has several advantages over purchasing and using pre-made iodine solutions. First, it is more cost-effective as it eliminates the need for expensive and hazardous iodine solutions.
It is more convenient as it can be prepared on-site as needed, rather than having to store and transport large quantities of iodine solutions.
Using bleach as an oxidizing agent to generate I2 in situ has additional advantages. Bleach is a readily available and inexpensive oxidizing agent, making it a more practical choice for smaller-scale reactions. Bleach also produces a lower concentration of iodine compared to more powerful oxidizing agents such as potassium permanganate or hydrogen peroxide, which can be advantageous in some reactions where a lower concentration of iodine is desired.
Furthermore, bleach is less hazardous and less reactive than other oxidizing agents, reducing the risk of accidents and making it safer to handle. This is especially important in laboratory settings where safety is a top priority.
Overall, generating I2 in situ using bleach as an oxidizing agent has several advantages including cost-effectiveness, convenience, and safety.
Learn more about oxidizing agents here:
brainly.com/question/10547418
#SPJ11
Which of the following statements correctly describe how formal charge is assigned to an atom in a Lewis structure?
A. ormal charge = (valence e-) - (unshared e- + 1/2shared e-)
B. The number of shared electrons equals twice the number of bonds in the structure.
C. The atom is considered to "own" half the shared electrons.
Answers
The amount of shared electrons is equivalent to twice as many bonds as there are in the structure. The phrase "is the correct assertion" indicates how an atom in a Lewis structure is given a formal charge.
The electron is a subatomic particle with an initial electric charge that is negatively one. Due to their lack of known components or substructure, electrons, which are part of the first generation of the lepton particle family, are typically considered to be elementary particles. A nucleus and one or more electrons attached to the nucleus make up every atom. One or more protons and many neutrons make up the nucleus. Neutrons are only absent from the most prevalent kind of hydrogen. Neutral or ionized atoms make up every solid, liquid, and form of plasma.
Learn more about electrons here
https://brainly.com/question/6283462
#SPJ4
What will increase the turgor pressure in a plant
Answers
Answer:
Cell expansion and an increase in turgor pressure is due to inward diffusion of water into the cell, and turgor pressure increases due to the increasing volume of vacuolar sap. A growing root cell's turgor pressure can be up to 0.6 MPa, which is over three times that of a car tire.
Explanation...read that and you will have a sucess
Draw the electron dot structure of the hydroxide ion (OH-).
Answers
Answer:
X = electrons from hydrogen
O (black) = electrons from oxygen
O (red) = electrons from when it was connected to a metal atom, hence why it has a negative charge
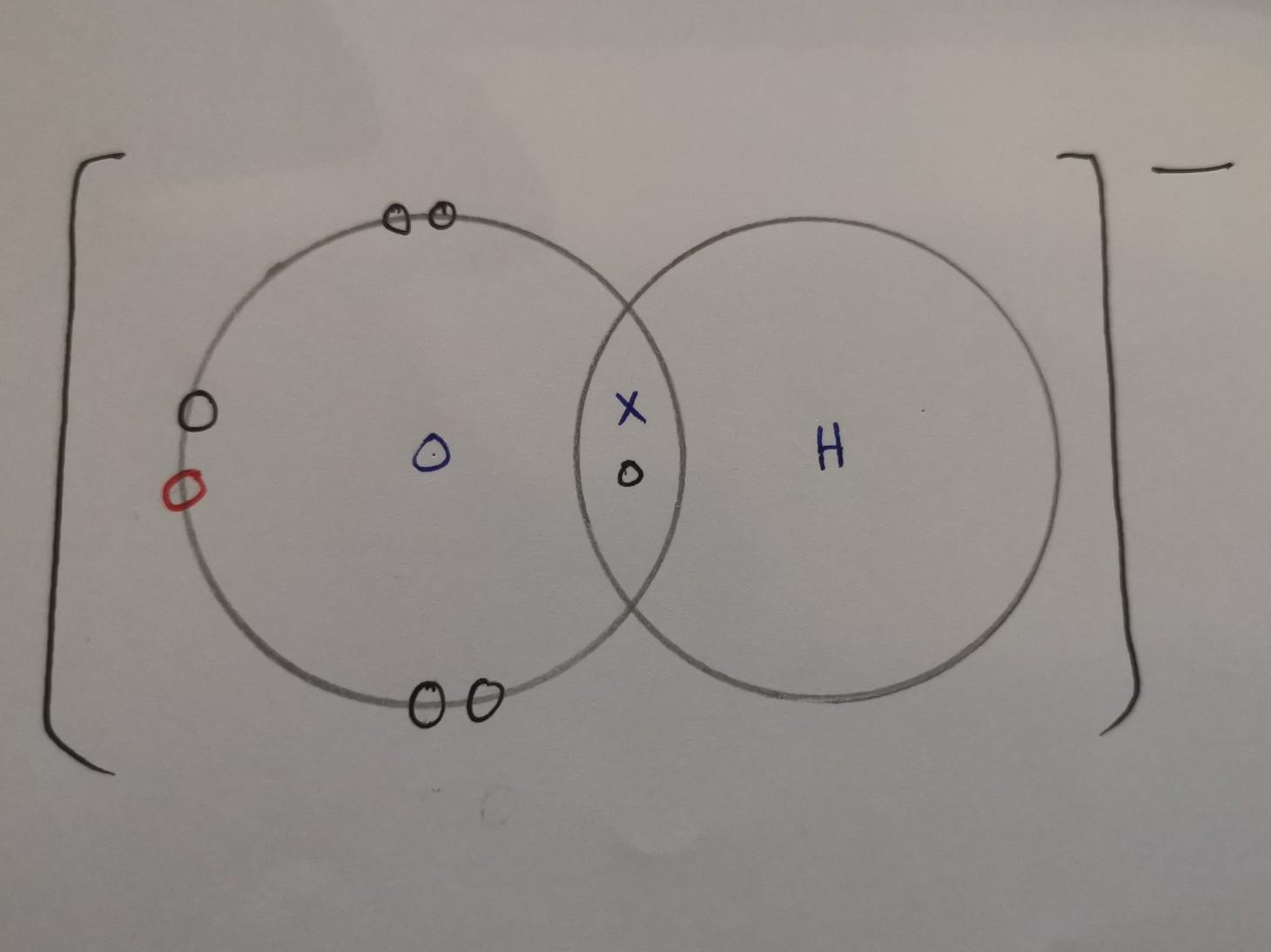
What is h2OO??????????
Answers
can someone help me with this question?!

Answers
Answer:
i think c
Explanation:
Answer:
Average atomic mass = 32 amu
Explanation:
Given data:
Abundance of X-30.00 amu = 30%
Abundance of X-32.00 amu = 50%
Abundance of X-35.00 amu = 20%
Average atomic mass = ?
Solution:
Average atomic mass = (abundance of 1st isotope × its atomic mass) +(abundance of 2nd isotope × its atomic mass) +(abundance of 3rd isotope × its atomic mass) / 100
Average atomic mass = (30×30)+(50×32)+(20×35) /100
Average atomic mass = 900 + 1600 +700 / 100
Average atomic mass = 3200 / 100
Average atomic mass = 32 amu.
A force is a push or a pull under the right circumstances anything can exert a force push a notebook or some other object so that it slides across a table or desktop what force causes the notebook to start moving what force causes the notebook to stop moving?
Answers
Answer:
the force that you applied caused the notebook to move.the force applied on the notebook by the table causes it to stop moving. this is because after sometime the book uses up the force and later the force you applied is less than that of the force by the table.
How is chemical equilibrium achieved?
Question 15 options:
When the concentrations of reactants and products are constantly changing.
When the concentrations of reactants and products are constant.
When the concentrations favor the reactants.
When the concentrations favor the products.
Answers
Answer:
Explanation:
Equilibrium is achieved in a chemical reaction when there is a steady state with no change in concentrations.
So the answer is "When the concentrations of reactants and products are constant."
How do rock transformations on Venus and Earth indicate the planetary similarities between the two planets?
Answers
Answer:
Both the Earth and Venus show the appearance of igneous rocks due to the cooling of the magma.
Explanation:
As we know, ignea rock is the first step in the transformation of rocks. This rock is formed by the overflow of terrestrial magma which, upon cooling, turns into ignea rock, which will undergo metamorphism and transform into metamorphic rock, which will continue to undergo metamorphosis until it becomes the other rocks that complete the rock cycle. This is a terrestrial process that can be found on Venus, which also presents the cooling of magma in the formation of ignea rock, which will undergo metamorphism, transforming into other rocks, the way it occurs on Earth.
How many elements are Al2O2
Answers
Answer:
i think 2?
Explanation:
AI2 O2
srry if wrong but
hope this helps
Draw a generic pH meter titration curve for the titration of a monoprotic weak acid, HA, with a strong base
Answers
The volume of the titrant, or in our example, the volume of the strong base, is graphed against the pH to create the titration curve. The titration curves of weak acids with strong bases have a number of properties.
The initial pH is more or less acidic than the titration of a strong acid (before the addition of any strong base).
At the start of the titration, the pH increases significantly. This occurs as a result of the weak acid's anion changing into a common ion, which lessens the acid's ability to ionize. Following the initial, abrupt increase, the titration curve only modifies gradually. This is a result of the solution's role as a buffer.
This will carry on until the base exceeds the capacity of the buffers. Half-neutralization takes place at the center of this progressively sloping curve. The concentration of the weak acid and the concentration of its conjugate base are now equal. As a result, pH=pKa. Because only half of the acid has been neutralized, this moment is known as the half-neutralization.
Since all of the acid (HA) has been changed to its conjugate base (A-) by the injection of NaOH, the pH is greater than 7 at this time. As the equilibrium shifts back towards HA, hydroxide is produced, which is represented by the formula: A+H2OAH+OH (2)Prior to the equivalence point, the curve has a brief steep section. It typically doesn't start until a pH of about 10.
Learn more about titration https://brainly.com/question/2728613
#SPJ4
3. The lowest temperature at which the oil gives off a vapor that can be readily ignited is the
O A. heating value.
O B. critical temperature.
O C. ignition point.
OD. flash point.
O Mark for review (Will be highlighted on the review page)
Answers
The lowest temperature at which the oil gives off a vapor which can be readily ignited is the flash point. Option D is correct.
The flash point of an oil or any other flammable liquid is the lowest temperature at which it gives off vapors that can ignite when exposed to an ignition source, such as a spark or a flame. It is the temperature at which the liquid produces enough vapor to form an ignitable mixture with air, but not necessarily sustain combustion.
The flash point is an important parameter in determining the flammability and safety of a liquid, as it indicates the temperature at which it can present a fire hazard. Once the flash point is reached, the liquid can release vapors that can ignite and result in a fire or explosion.
Hence, D. is the correct option.
To know more about flash point here
https://brainly.com/question/478199
#SPJ1
Imagine it's a cold winter night; you've turned the heater on to get toasty and warm. You open the door for a moment and someone yells to close it and says "you're letting the cold in!" Is this statement true or false? i need a paragraph explaining why and evidence.
Answers
The statement "you're letting the cold in" is technically false. When you open a door on a cold winter night, you are allowing the warm air inside to escape, which can cause a drop in temperature. However, you are not necessarily "letting the cold in." The cold air is already present outside, and it will continue to exist whether the door is open or closed. In fact, the temperature inside your home may only change slightly, and it will depend on many factors such as the size of the room, the amount of insulation, and the efficiency of the heating system.
The concept of heat transfer, which is the movement of heat from one place to another, helps explain why this statement is false. When you open the door, the warm air inside will move to the cold air outside, which is known as conduction. This transfer of heat will cause the temperature inside to drop until the heat source can warm up the air again. This process will continue as long as the door remains open. So, while opening the door on a cold winter night can cause a drop in temperature, it is not because you are "letting the cold in." Instead, it is because you are allowing the warm air inside to escape.
A sample of an ideal gas has a volume of 2.32 L at 285 K and 1.02 atm. Calculate the pressure when the volume is 1.76 L and the temperature is 308 K.
Answers
The pressure of the gas when the volume is 1.76 L and the temperature is 308 K is approximately 1.77 atm using the combined gas laws.
To calculate the pressure of an ideal gas using the combined gas law, we can use the formula:
P1 * V1 / T1 = P2 * V2 / T2
Where:
P1 = Initial pressure of the gas (1.02 atm)
V1 = Initial volume of the gas (2.32 L)
T1 = Initial temperature of the gas (285 K)
P2 = Final pressure of the gas (to be determined)
V2 = Final volume of the gas (1.76 L)
T2 = Final temperature of the gas (308 K)
Plugging in the values:
(1.02 atm) * (2.32 L) / (285 K) = P2 * (1.76 L) / (308 K)
Now, solve for P2:
P2 = (1.02 atm) * (2.32 L) * (308 K) / (1.76 L) / (285 K)
= 1.77 atm
Therefore, the pressure of the gas when the volume is 1.76 L and the temperature is 308 K is approximately 1.77 atm.
To know more about gas laws, refer here:
https://brainly.com/question/30458409#
#SPJ11
What element am I if I have 6 protons and
8 neutrons?
Answers
Answer:
The element you would have is Carbon-14.
Explanation:
Carbon-14 has 6 protons and 8 neutrons. To find the atomic mass, you add the protons to the neutrons, so the atomic mass of Carbon-14 is 14.
#teamtrees #WAP (Water And Plant)
What is the total pressure of a mixture of he and h2 if the partial pressures are 320 mm hg and 800 mm hg respectively
Answers
Answer:
1120 mm Hg
plus give brainliest
Match the following terms describing phase changes with their definitions.
Liquid to gas Solid to gas Solid to liquid Liquid to solid boiling
freezing
melting sublimation
Answers
Liquid to gas - Boiling: The phase change from a liquid to a gas that occurs when the substance reaches its boiling point, resulting in the formation of vapor.
Solid to gas - Sublimation: The phase change from a solid directly to a gas without going through the liquid state.
Solid to liquid - Melting: The phase change from a solid to a liquid when heat is applied, causing the substance to transition from a rigid to a more fluid state.
Liquid to solid - Freezing: The phase change from a liquid to a solid when the substance loses heat, resulting in the formation of a solid crystal lattice.
Therefore, the correct match would be:
Liquid to gas - Boiling
Solid to gas - Sublimation
Solid to liquid - Melting
Liquid to solid - Freezing
To learn more about phase change of matter, visit:
https://brainly.com/question/30720253
#SPJ11
what is the chief function of the circulatory system
Answers
Answer:
Explanation:
The circulatory system is made up of blood vessels that carry blood away from and towards the heart The circulatory system carries oxygen, nutrients, and hormones to cells, and removes waste products, like carbon dioxide.....etc
The most important thing in the circulatory system is The heart.
It pumps blood around the body. It sits inside the chest, in front of the lungs, and slightly to the left side. The heart is a double pump made up of four chambers, with the flow of blood going in one direction due to the presence of the heart valves.
The chief function of the circulatory system is,
Oxygen and Carbon Dioxide Transport.
(Circulates OXYGEN and removes Carbon Dioxide)
Other functions of the circulatory system are:
Nutrient and Waste Product Transport.
Disease Protection and Healing.
Hormone Delivery.
Body Temperature Regulation.
Which two are isotopes of one another?
a. Atoms 1 & 2
b. Atoms 2 & 3
c. Atoms 1 & 3
d. Atoms 1 & 4
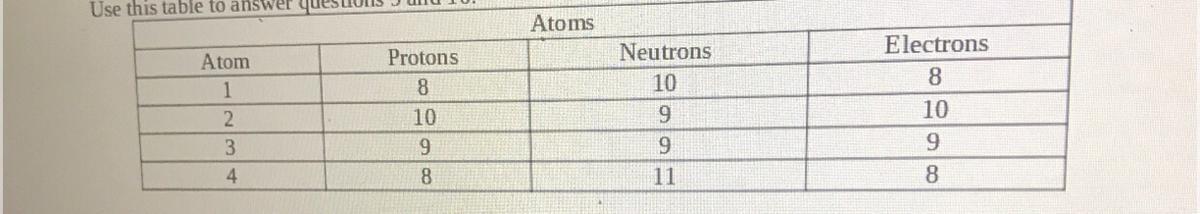
Answers
Your team collaborated with the National Aeronautics and Space (NASA) to send the Curiosity rover to the newly discovered planet in order to investigate the chemical makeup, take exclusive photos, and look for possible signs of microbial life.
The Curiosity rover was able to trace substances concluding that the planet consists mostly of materials having melting points above 700C. What does this tell you about the interior makeup of the planet?
Answers
The Curiosity rover found that ancient Mars had the right chemistry to support living microbes.
What is Microbes?These are organisms which can be seen with microscope and not with the nak ed eyes.
Curiosity rover was sent to mars to study life forms in which it was discovered that they were present.
Read more about Curiosity rover here https://brainly.com/question/11277933
#SPJ1
explain why ply(ethene) can become a pollutant?
Answers
Answer:
ipotseeeeeeeeeeeeeeee
What is nebular theory
Answers
Answer: The nebular theory is an explanation for the formation of solar systems.
Explanation:
3)Which of the following is an example of a subatomic particle?
A)Carbon incorrect answer
B)Oxygen incorrect answer
C)Electron incorrect answer
D)Hydrogen
Answers
Answer:
hydrogen
Explanation:
its the only one that doesn't say incorrect answer
The following is an example of a subatomic particle is hydrogen. Hence option D is correct.
What is subatomic particle?Subatomic particle is defined as any of the different self-contained particles of matter or energy that make up the building blocks of all matter. Protons, neutrons, and electrons are the three subatomic particles that make up a normal atom. The neutron is the subatomic particle with the most mass.
The chemical element hydrogen is represented by the letter H and atomic number 1. Three subatomic particles make up a hydrogen atom. They include the heavier constituents of the small but extremely dense atom's nucleus, the positively charged protons and the electrically neutral neutrons, as well as the electrons, the negatively charged, nearly massless particles that still make up the majority of the atom's size.
Thus, the following is an example of a subatomic particle is hydrogen. Hence option D is correct.
To learn more about subatomic particle, refer to the link below:
https://brainly.com/question/13303285
#SPJ6
what type of energy transfer occurs as a coiled spring is released
Answers
Answer:
When a spring is coiled up or a rubber band is stretched, mechanical energy is stored in it. When the spring uncoils or the rubber band snaps back, this energy is released. This stored mechanical energy is called potential energy because it has the potential to make things happen.Explanation:
Hope it helps you :)
Keep Learning :)
the isotope carbon-14 decays over time into nitrogen-14 with a half life of 5,730 years. suppose you find a fossil that contains 1.25 grams of carbon-14 and 3.75 grams of nitrogen 14. how much carbon-14 was present in the organism at the time of death?
Answers
At the time of death, the creature contained 5 g of carbon-14.
How can carbon-14 become radioactive?Although the nucleus of carbon-14 is unstable due to the two extra neutrons, the substance is still carbon because it has six protons. Carbon-14 transforms one of its neutrons into a proton by releasing a negatively charged particle from its nuclei in order to attain a more stable state.
In this case, N-14 is the daughter isotope and C-14 is the parent isotope.
in present time the fossil contain, amount of parent isotope is = 1.25 gm
amount of daughter isotope = 3.75 gm .
As we amount of C-14 is constant until the death of organism or body . After the death of plants or animals C-14 starts decay in to N-14 .
so at initial time or at the time of decay , the amount of C-14 was = 1.25 gm + 3.75 gm = 5 gm .
so answer is 5 gm.
To know more about carbon-14 visit :
https://brainly.com/question/4206267
#SPJ4
at 4.00 l , an expandable vessel contains 0.864 mol of oxygen gas. how many liters of oxygen gas must be added at constant temperature and pressure if you need a total
Answers
First, we'll look at the ideal gas equation,
PV = nRT
The temperature and pressure are said to be constant; Additionally, R is a constant already. Along these lines, we get:
V = constant * n
The direct proportional equation is as follows: As a result, we get:
V/n = constant
V₁/n₁ = V₂/n₂
Replace V₂ with the qualities and address.
V₂ = (4 * 1.48) / 0.864
V₂ = 6.85
In the end, 6.85 Liters of gas must be present, so we must add:
6.85 - 4 = 2.85 liters
The volume of a gas is directly proportional to its mole volume at a fixed temperature and pressure.
To learn more about ideal gas here
https://brainly.com/question/28257995
#SPJ4
Q- At 4.00 L, an expandable vessel contains 0.864 mol of oxygen gas. How many liters of oxygen gas must be added at constant temperature and pressure if you need a total of 1.48mol of oxygen gas in the vessel?