Answers
50.3 J of heat have been released.
How are heat energies released calculated?The equation q = mcT, where m is the mass of the sample, c is the specific heat, and T is the temperature change, can be used to determine how much heat is gained or lost by a sample (q).
Having said that,
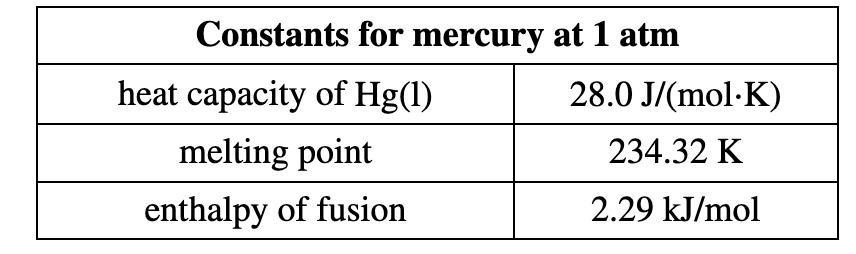
Mercury has a mass of 17.7 g and a starting temperature of 25.00 °C.
Mercury's melting point is -39 °C.
Mercury has a specific heat capacity of 0.14 J/g/°C.
Mercury fusion heat equals 11.8 J/g
Obtaining heat from the transformation of liquid mercury into solid mercury;
H = mcθ + mL
H = m(cθ + L)
H = 17.7 g[(0.14 × -64) + 11.8 )
H = 50.3 J
To know more about heat energy visit:-
https://brainly.com/question/7496871
#SPJ1
Related Questions
What is the de Broglie wavelength of an electron traveling at 1.59×105m/s ?
Answers
The de Broglie wavelength of an electron traveling at 1.59×105m/s is 0.4547 x 10⁻⁹ m.
What is an electron ?The elementary electric charge of the electron is a negative one, making it a subatomic particle. Due to their lack of known components or substructure, electrons, which are part of the first generation of the lepton particle family, are typically regarded to be elementary particles.
The length scale at which a particle's wave-like characteristics are significant is indicated by its de Broglie wavelength. The symbol or dB is typically used to indicate the De Broglie wavelength. The de Broglie wavelength for a particle with momentum p is given by dB = hp.
λ = h/mv
Where,
λ = wavelength of electron
m = mass of electron = 9.11e-31 kg
v = speed of electron = 1.59 × 10⁵ m/s
h = constant
Therefore,
λ = (6.626x10⁻³⁴J-s) ÷ [(9.11e-31 kg) (1.59 x 10⁵ m/s)]
λ = 0.4547 x 10⁻⁹ m
Thus, The de Broglie wavelength of an electron traveling at 1.59×105m/s is 0.4547 x 10⁻⁹ m.
To learn more about an electron, follow the link;
https://brainly.com/question/1255220
#SPJ1
what is the frequency in herts of an xray having wavelength of 4.73 x 10-14 hectometers to 3 significant figures?
Answers
The frequency (in hertz) of the x-ray having a wavelength of 4.73×10⁻¹⁴ hectometers is 6.34×10¹⁹ Hertz
How do I determine the frequency of the x-ray?Frequency is defined as the number of complete oscillations made in 1 second.
However, the frequency of a wave is related to wavelength according to the following formula:
Velocity (v) = wavelength (λ) × frequency (f)
v = λf
With the above formula, we can determine the frequency of the x-ray. Details below:
Wavelength (λ) = 4.73×10⁻¹⁴ hectometers = 4.73×10⁻¹⁴ × 10² = 4.73×10⁻¹² metersSpeed of x-ray (v) = 3×10⁸ m/sFrequency (f) =?Velocity (v) = wavelength (λ) × frequency (f)
3×10⁸ = 4.73×10⁻¹² × frequency
Divide both sides by 4.73×10⁻¹²
Frequency = 3×10⁸ / 4.73×10⁻¹²
Frequency = 6.34×10¹⁹ Hertz
Thus, we can conclude, the frequency is 6.34×10¹⁹ Hertz
Learn more about frequency:
https://brainly.com/question/15326129
#SPJ1
What is the name of Na
Answers
mdmsjdkskdkskdjxjxjjz
Among nonmetals,which nonmetal is most likely to form a covalent bond?
Answers
Answer:
most common non metal is chlorine
2CuCl + H2S → Cu2S + 2HCl
How many moles of copper I chloride, CuCl, are necessary to react completely with 4.4 moles of hydrogen sulfide, H2S?
Answers
8.8 moles of CuCl are required for 4.4 moles of H2S to totally react.
What is mole?The unit of measurement known as a mole (mol) is used to represent the quantity of a substance. The amount of a substance that has the same number of particles (atoms, molecules, or ions) as there are in 12 grams of carbon-12 is referred to as a mole. This number, which is roughly 6.022 x 1023, is referred to as Avogadro's number.
How do you determine it?The reaction between copper I chloride and hydrogen sulfide has the following balanced chemical equation:
2CuCl + H2S → Cu2S + 2HCl
The balanced equation shows that the reaction between 2 moles of CuCl and 1 mole of H2S results in 2 moles of HCl and 1 mole of Cu2S.
So, we must use stoichiometry to calculate the amount of CuCl needed to thoroughly react with 4.4 moles of H2S:
4.4 moles of H2S × (2 moles of CuCl / 1 mole of H2S) = 8.8 moles of CuCl
As a result, 8.8 moles of CuCl are required for 4.4 moles of H2S to totally react.
To know more about mole, visit:
https://brainly.com/question/26416088
#SPJ1
Why is there something blocking the answer?
Answers
Answer: the answer you are looking for does not exist
Explanation:
a graduated cylinder which contains 47.3 mL, weighs 89.62 grams. when a solid submerged in the water, the volume level rises to 55.4 ml and the mass to 123.79 grams. a) what is the volume of the solid? b) what is the mass of the solid? c) what is the density of the solid?
Answers
Explanation:
the mass of the object is 123.97-89.62=34.35g
the volume is 55.4-47.3=8.1ml
the density is 34.35/8.1=4.2g/ml
how many sulfur atoms are there in 5.5 moles?
Answers
Answer:
3.31x10^24 atoms
Explanation:
1 mole of S atom = 6.023^23 atoms
5.5 moles = 5.5 x 6.023^23
= 3.31x10^24 atoms
Task:
For each "station", click on the link. You should describe the initial appearances and observations of the
reaction during and after. Using your observations, determine if the change is a physical or chemical change.
Station #1: Lead Nitrate and Potassium lodide solutions. Shower of yellow
QUESTION/OBSERVATION
INITIAL APPEARANCE (what does the
substance look like in the beginning)
Answers
The expected observations for the chemical reaction involving lead nitrate and potassium iodide are as follows as per theory.
INITIAL APPEARANCE:Before the reaction, you'd have two separate solutions:
Lead Nitrate solution: This is typically a clear, colorless solution.
Potassium Iodide solution: This is also usually a clear, colorless solution.
REACTION OBSERVATIONS:
As soon as you combine these two solutions, a chemical response takes place, resulting in the almost instantaneous development of a yellow precipitate. Lead iodide is a substance that cannot be dissolved in water.
FINAL APPEARANCE:
The final mixture would have a yellow precipitate (lead iodide) suspended in the solution.
The reaction leads to the formation of lead iodide, a substance with distinctive properties, suggesting a chemical change. The presence of this novel compound is indicated by the yellow hue of the precipitate.
Read more about chemical change here:
https://brainly.com/question/1222323
#SPJ1
The table describes a gas stored in four different containers. Properties of Stored Gas Container Properties 1 · Low number of collisions with container walls · Medium average kinetic energy · Large number of particles 2 · Large number of collisions with container walls · Medium average kinetic energy · Small number of particles with little spaces between them 3 · Large number of collisions with container walls · High average kinetic energy · Large number of particles with large spaces between them 4 · Few collisions with container walls · Low average kinetic energy · Small number of particles Which container has gas stored at the highest temperature? 1 2 3 4
Answers
Container 3 has the gas stored at the highest temperature.
Temperature is a measure of the average kinetic energy of the particles in a substance. In the given table, it is stated that container 3 has a large number of collisions with container walls, high average kinetic energy, and large number of particles with large spaces between them.
These properties indicate that the gas in container 3 has higher kinetic energy and more vigorous movement compared to the other containers.
Container 1 has a low number of collisions with container walls and a medium average kinetic energy. This suggests that the gas in container 1 has lower energy and less movement than the gas in container 3.
Container 2 has a large number of collisions with container walls, but it also has a small number of particles with little spaces between them. While the collisions may be frequent, the limited number of particles and the lack of space between them may result in lower overall kinetic energy compared to container 3.
Container 4 has few collisions with container walls, low average kinetic energy, and a small number of particles. These properties indicate that the gas in container 4 has the lowest energy and least movement among all the containers.
Container 3
For more such questions on temperature visit;
https://brainly.com/question/4735135
#SPJ8
If you want there to be less of you tomorrow than today... you need to lose
Answers
Answer:
rise your words, not your voice rain grows flowers thunder does not.
Explanation:
off the top of my head
If a penguin has to walk 70 miles at a rate of .75 meters per secound how many days does it take to make the trip
Answers
If a penguin has to walk 70 miles at a rate of .75 meters per second, 66 days do it take to make the trip.
What do you mean by speed ?The term speed is defined as the rate of change of position of an object in any direction. Speed is calculated as follows: speed = distance × time.
The distance traveled in relation to the time it took to travel that distance is how speed is defined. Since speed simply has a direction and no magnitude, it is a scalar quantity.
If you know how far something has traveled and how long it took to get there, you can calculate its average speed.
Time = distance / speed
distance = 0.75 meters
speed = 70 miles = 112.654 kilometres
Substitute this value in above formula
Time = 0.75 / 112.654
= 0.66
Thus, If a penguin has to walk 70 miles at a rate of .75 meters per second, 66 days do it take to make the trip.
To learn more about the speed, follow the link;
https://brainly.com/question/28224010
#SPJ1
What water system is part of the hydrological cycle and generally collects water from precipitation through a drainage basin from
surface runoff and other sources such as groundwater recharge and springs? This water system is usually freshwater flowing towards
an ocean, sea, or lake. In a few cases, it simply dries up completely at the end of its course, and does not reach another body of water.
es 0))
A)
tidal area
B)
watershed
09
wetland
D)
river
Answers
Rivers are part of the hydrological cycle. Water generally collects in a river from precipitation through a drainage basin from surface runoff and other sources such as groundwater recharge, springs, and the release of stored water in natural ice and snowpacks (e.g., from glaciers).
Lesson one review:
Copy and fill in the graphic organizer below with details about substances and mixtures
Answers
Mixtures are impure substances composed of two or more substances physically combined.
What are mixtures?Mixtures are substances that are composed of two or more substances physically combined.
Mixtures are one of the different types of substances which are composed of matter.
The classification of matter by type of substance is as follows:
Matter - Pure and impure substances or mixturesPure substances - elements and compoundsMixtures: homogenous and heterogenousIn conclusion, substances can be classified into either pure substances or mixtures.
Learn more about substances and mixtures at: https://brainly.com/question/1867365
#SPJ1

What happens to the amount of solution when we add food colour to it?
Answers
Answer:
We need more? What else is in the question? This is unanswerable.
Explanation:
as the ionic compound decreases in size, the melting point __
A. increases
B. decreases
c stays the same
Answers
Answer:
A. increase
Explanation:
the smaller the size of the ionic compounds, the more closer they get packed together. This means electrostatic attraction also increases, which means the ionic bonds gets stronger...therefore increases the melting point
When did China's earliest civilization originate?
Answers
Answer:
The Chinese civilization really has an exact chronology, and the historical period began in the first year of the Western Zhou Dynasty in 841 BC. Before that, only the lineage records of the kings were recorded. Therefore, the earliest origin of Chinese civilization has not yet been finalized. Although there are many records about the Xia Dynasty in the traditional literature, due to the late completion of the book, there is no recognized evidence of the existence of the Xia Dynasty and its previous history. Therefore, some people in the modern and modern historical circles have always questioned the existence of the Xia Dynasty. sex.
Explanation:
5. If the reaction of carbon monoxide and oxygen gas in question 2
produced 7.03E24 molecules of CO2, what is the mass in grams? Use
44g/mol for M.M. of CO2 and round your answer to the nearest whole
number. *
Answers
Answer: The mass in grams is 514.8.
Explanation:
According to avogadro's law, 1 mole of every substance weighs equal to molecular mass and contains avogadro's number \(6.023\times 10^{23}\) of particles.
To calculate the number of moles, we use the equation:
\(\text{Number of moles}=\frac{\text{Given molecules}}{\text{Avogadros number}}\)
given molecules = \(7.03\times 10^{24}\)
Putting in the values we get:
\(\text{Number of moles}=\frac{7.03\times 10^{24}}{6.023\times 10^{23}}=11.7moles\)
mass of \(CO_2=moles\times {\text {Molar mass}}=11.7mol\times 44g/mol=514.8g\)
The mass in grams is 514.8.
How many grams are there in 5.699 moles of copper
Answers
To convert moles of copper to grams, we need to use the molar mass of copper, which is 63.55 g/mol.
Grams of copper = moles of copper x molar mass of copper
Grams of copper = 5.699 mol x 63.55 g/mol
Grams of copper = 362.071 g
Therefore, there are 362.071 grams of copper in 5.699 moles of copper.
So there are 361.28 grams in 5.699 moles of copper.
How many moles of CO are needed to produce 209.7 moles Fe?
Answers
We must utilise the chemical formula for the reaction of iron and carbon monoxide to make iron oxide in order to respond to this question. It goes like this: CO + 2 Fe = Fe2O3. Therefore, 1 mole of CO is required for every 2 moles of Fe.
We must multiply the necessary 209.7 moles of Fe by 1/2 to determine the necessary moles of CO. We now have 104.85 moles of CO overall. We may utilise the stoichiometric coefficient for CO, which is 1, to verify our response. It is 1.
Accordingly, 1 mole of Fe2O3 is created for every mole of CO. As a result, 104.85 moles of Fe2O3 are created for every 104.85 moles of CO. because 2 moles If 104.85 moles of Fe2O3 are needed to make 1 mole of Fe2O3, then multiply that figure by two to find the amount of Fe that was created. This provides us the same quantity of Fe that we started with, or 209.7 moles, which supports our conclusion.
Learn more about moles at:
https://brainly.com/question/26416088
#SPJ1
Identify the Brønsted acid in the following equation:
H2SO4(aq) + 2NH3(aq)
(NH4)2SO4aq
Answers
Answer:
H₂SO₄
Explanation:
A Brønsted acid is a proton donor. It loses protons.
This equation may be easier to understand if we write it ionically.
\(\underbrace{\hbox{H$_{2}$SO$_{4}$}}_{\hbox{Br$\o{}$nsted acid}} + 2 \text{NH}_{3} \longrightarrow \, \underbrace{\hbox{SO$_{4}^{2-}$}}_{\hbox{Br$\o{}$nsted base}} + \text{2 NH}_{4}^{+}\)
We see that the H₂SO₄ has lost two protons to become SO₄²⁻, so it is a Brønsted acid.
PLS help I will give 100 points

Answers
Answer:
3700000000
Explanation: Move decimal to the right of the amount the exponent is for 10 Give brainliest pls
why are divergent boundaries are also called a constructive boudary
Answers
Answer: Divergent boundaries are where two of those plates are moving away from each other. They are called constructive plates because when they move apart, magma rises up in the gap- this forms volcanoes and eventually new crust.
Explanation:
CuBr2 percent composition
Answers
The percent composition of CuBr₂ is approximately 28.46% of Cu and 71.54% of Br.
To determine the percent composition of CuBr₂ (copper(II) bromide), we need to calculate the mass of each element in the compound and then divide it by the molar mass of the entire compound.
The molar mass of CuBr₂ can be calculated by adding up the atomic masses of copper (Cu) and bromine (Br) in the compound. The atomic masses of Cu and Br are approximately 63.55 g/mol and 79.90 g/mol, respectively.
Molar mass of CuBr₂ = (63.55 g/mol) + 2(79.90 g/mol) = 223.35 g/mol
Now, let's calculate the percent composition of each element in CuBr₂:
Percent composition of copper (Cu):
Mass of Cu = (63.55 g/mol) / 223.35 g/mol × 100% ≈ 28.46%
Percent composition of bromine (Br):
Mass of Br = 2(79.90 g/mol) / 223.35 g/mol × 100% ≈ 71.54%
Therefore, the percent composition of CuBr₂ is approximately:
- Copper (Cu): 28.46%
- Bromine (Br): 71.54%
These values represent the relative mass percentages of copper and bromine in the compound CuBr₂.
for more such questions on composition
https://brainly.com/question/28250237
#SPJ8
Electron Identify the type of charge of the following particles in an
atom
Electron
Neutron
Proton
Answers
Answer:
Electron charge: -1
Neutron charge: 0
Proton charge: +1
accelerating hydrogen absorption and desorption rates in palladium nanocubes with an ultrathin surface modification
Answers
An incredibly intriguing Palladium approach to high-rate energy storage and distribution is to take advantage of the high surface-area-to-volume ratio of nanomaterials by storing energy in the form of electrochemical alloys.
Palladium hydride at the nanoscale is a great model system for learning how characteristics at the nanoscale influence the absorption and desorption of energy-carrying equivalents. In shape-controlled Pd nanostructures, hydrogen absorption and desorption do not take place uniformly throughout the surface of the nanoparticles. Instead, high-activity spots at the corners and edges are used to selectively absorb and desorb hydrogen. With such a process, the benefits of shrinking the palladium's size to the nanoscale are significantly diminished. We alter the palladium surface with an incredibly thin platinum shell to resolve this. This alteration allows diffusion to occur across the entire Pd/Pt surface and practically eliminates the barrier to hydrogen absorption (89 kJ/mol without a Pt shell and 1.8 kJ/mol with a Pt shell).
To learn more about Palladium please click on below link
https://brainly.com/question/28597992
#SPJ4
How many ways can you recall to synthesize
Answers
there are an infinite number of ways to synthesize an answer to a question, including the following:
Summarize the key points in a concise manner.
Provide a detailed explanation of the topic.
Use examples or analogies to illustrate the concept.
Break down the answer into smaller, more digestible pieces.
Address potential counterarguments or alternative perspectives.
Incorporate relevant statistics or data to support the answer.
Compare and contrast different aspects of the topic.
Provide historical context or a timeline of events.
Use a storytelling approach to engage the reader.
Use a Q&A format to organize the information.
To know more about answer synthesize, visit:
https://brainly.com/question/30029537
#SPJ1
A gas is at 273.9 K and 99.6 atm and its volume changed to 429.1 mL at STP. Find the original volume of the gas in mL
Answers
We are given a sample of gas and we know the initial temperature and pressure. The original volume is our unknown.
P₁ = 99.6 atm T₁ = 273.9 K V₁ = ?
We are told that the gas at the end of the process is at STP (standard temperature and pressure). The standard pressure is 1 atm and the standard temperature is 273.15 K. We are also given the final volume.
P₂ = 1 atm T₂ = 273.15 K V₂ = 429.1 mL
Since the temperature is not constant, we can use this formula to solve our problem:
P₁ * V₁ / T₁ = P₂ * V₂ / T₂
We can replace the given values and solve the equation for V1 to get the answer to our problem.
P₁ * V₁ / T₁ = P₂ * V₂ / T₂
V₁ = P₂ * V₂ * T₁/ (T₂ * P₁)
V₁ = 1 atm * 429.1 mL * 273.9 K/(273.15 K * 99.6 atm)
V₁ = 4.32 mL
Answer: the original volume of the gas was 4.32 mL.
It is not safe to put an aerosol canister in a campfire, because the pressure Inside the can gets very
high at the temperature rises....it can explode! If you have a 1500 milliliter canister that holds 3 moles
of gas, and the campfire temperature reaches 1500 °C, what is the pressure in atmospheres inside the
canister?

Answers
The pressure inside the canister at a campfire temperature of 1500°C is 0.227 atm
Given that the temperature of the campfire is 1500°C, the volume of the aerosol canister is 1500 milliliters, and the number of moles of gas in the canister is 3 moles.The ideal gas equation is given as PV = nRT, where P is pressure, V is volume, n is the number of moles of gas, R is the ideal gas constant, and T is the absolute temperature of the gas in Kelvin.To convert 1500°C to Kelvin, we use the equation: T(K) = T(°C) + 273T(K) = 1500 + 273T(K) = 1773 KSubstitute the values given in the ideal gas equation:P = (nRT) / VWe are given n, R, V, and T, hence we substitute and solve for P as follows:P = (3 × 0.082 × 1773) / 1500P = 0.227 atm.It is not safe to put an aerosol canister in a campfire because the pressure inside the canister will increase at higher temperatures. If the pressure in the canister continues to increase beyond the safe limit, it can explode. Therefore, always keep aerosol canisters away from sources of high temperatures.
for such more questions on pressure
https://brainly.com/question/24719118
#SPJ8
How does the mean free path in a sample of gas vary with temperature in a constant-volume container ?
Answers
Answer:
The mean free path in a sample of gas is the average distance traveled by a gas molecule between successive collisions with other molecules. In a constant-volume container, the mean free path is influenced by temperature.
As temperature increases, the kinetic energy of gas molecules also increases. This leads to higher molecular speeds and more frequent collisions between molecules. Consequently, the average distance traveled by a gas molecule between collisions decreases, resulting in a shorter mean free path.
Therefore, in a constant-volume container, as temperature increases, the mean free path of gas decreases.
brainlest pleos :)