Answers
Answer:
10.328 m
Explanation:
normal atmospheric pressure = 101325 Pa
density of water at 25 °C = 1.0 g/cm^3 = 1000 kg/m^3
pressure = pgh
where p = density
g = acceleration due to gravity = 9.81 m/s^2
h = height of column
imputing values, we have
101325 = 1000 x 9.81 x h
height of column h = 101325/9810 = 10.328 m
Related Questions
The temperature above which a substance cannot exist as a liquid but behaves as a gas regardless of the pressure upon it is the CRITICAL TEMPERATURE.
Answers
The temperatures and pressures when the liquid and gaseous phases are equally stable and in equilibrium.
The temperature range and pressure range at which a supercritical fluid can exist. The physical states of a substance at various pressure and temperature levels are depicted in a phase diagram. In this diagram, the pressure is often depicted on the y-axis while the temperature is displayed on the x-axis. The critical point, which is where the line separating the liquid and gaseous phases disappears, is depicted graphically. A substance that has passed the critical point is referred to as a supercritical fluid. Based on the description provided above, all responses other than A are false. The flexibility to alter fluid density-directly affecting extraction parameters like temperature and pressure.
Learn more about The temperature range and pressure range here:
https://brainly.com/question/14769510
#SPJ4
1. If you place 30.0 L of ethyl acetate (C4H8O2) in a sealed room that is 7.25 m long, 2.75 m wide, and 2.75 m high, will all the ethyl acetate evaporate? If some liquid remains, how much will there be? The vapor pressure of ethyl acetate is 94.9 torr at 25 °C, and the density of the liquid at this temperature is 0.901 g/mL. Treat the room dimensions as exact numbers.
Answers
There will be 0.4589 mL of ethyl acetate left in the space after evaporation.
What is evaporation?The conversion of a liquid substance into a gas is known as evaporation. As a result of the liquid absorbing energy from its surroundings, molecules begin to travel faster and faster until they finally become a vapour and escape into the environment. Usually, the energy is absorbed as heat, but it can also be in the form of light or electricity.
No, the ethyl acetate won't all evaporate. The amount of ethyl acetate that will stay in the space after evaporation can be determined using the ideal gas law. As per the ideal gas law, PV = nRT
P is the overall system pressure, V is the room's volume, n is the amount of ethyl acetate in moles, R is the ideal gas constant, and T is the temperature.
To solve for n, the quantity of moles of ethyl acetate, we can rearrange the equation as follows: n = PV/RT
When the values are plugged in, we get:
n = (94.9 torr)(7.25 m x 2.75 m x 2.75 m)/(8.314 J/K mol)(298 K)
\(n = 4.666 \times 10^{-3} mol\)
The molar mass of ethyl acetate (88.11 g/mol) can then be used to compute the mass of ethyl acetate:
Mass = \(n \times M = (4.666 x 10^{-3} mol)(88.11 g/mol)\) = 0.4125 g
Using the density of ethyl acetate (0.901 g/mL), it is possible to determine the volume of the liquid that is still present:
Volume = mass/density = (0.4125 g)/(0.901 g/mL) = 0.4589 mL
As a result, there will be 0.4589 mL of ethyl acetate left in the space after evaporation.
To learn more about evaporation, visit:
brainly.com/question/24258
#SPJ1
Calculate the amount of copper in moles in a 27.5g pure copper sheet
Answers
The amount of copper in moles in the 27.5 g pure copper sheet is approximately 0.433 moles.
To calculate the amount of copper in moles in a pure copper sheet, we need to use the molar mass of copper and the given mass of the sheet.
The molar mass of copper (Cu) is approximately 63.55 g/mol. This value represents the mass of one mole of copper atoms.
Given that the mass of the pure copper sheet is 27.5 g, we can calculate the number of moles using the following formula:
moles = mass / molar mass
Substituting the values:
moles = 27.5 g / 63.55 g/mol
moles ≈ 0.433 mol
Therefore, the amount of copper in moles in the 27.5 g pure copper sheet is approximately 0.433 moles.
To arrive at this result, we divided the given mass of the sheet (27.5 g) by the molar mass of copper (63.55 g/mol). This calculation allows us to convert the mass of the sheet into the corresponding number of moles of copper.
The result tells us that the 27.5 g pure copper sheet contains approximately 0.433 moles of copper atoms. This conversion to moles is useful in various chemical calculations and allows for easier comparison and analysis of quantities on a molecular scale.
for more such question on copper visit
https://brainly.com/question/29176517
#SPJ8
if the body is moving with uniform acceleration then, eng of motion are given as s = u+v/2+t
Answers
Yes, s = u+v/2+t, where s is the displacement, u is the beginning velocity, v is the end velocity, and t is the time required, is the equation of motion for a body travelling with uniform acceleration.
The basic law of motion, which states that a body's rate of change in displacement is directly proportional to its velocity, provides the basis for this equation. The equation of motion for a body travelling with constant acceleration, s = ut + 1/2at2, may be used to derive it.
The equation of motion for a body travelling with uniform acceleration is given by replacing the value of an as (v-u)/t and getting s = u+v/2+t. This formula is only accurate when the body's acceleration is constant and uniform.
Learn more about acceleration at:
https://brainly.com/question/12550364
#SPJ1
A sample of ammonia has a mass of 18.2g. How many molecules are in this sample?
Answers
Answer:
1.8x10_24 moleclues
Explanation:
because you have to divide the numbers and then times them together
A chemist decomposes samples of several compounds; the masses of their constituent elements are shown. Calculate the empirical formula for each compound. a. 1.651 g Ag, 0.1224 g O b. 0.672 g Co, 0.569 g As, 0.486 g O c. 1.443 g Se, 5.841 g Br
Answers
The empirical formula for each compound are as follow,
a)\(Ag_2 O\)
b)\(Co_3As_2O_8\)
c)\(SeBr_4\)
As per the data share in the above question are as follow,
The mass of their constituent elements are as follow,
a) Ag=1.651 g
O=0.1224 g
b)Co =0.672 g
As =0.569 g
O=0.486 g
c) Se =1.443 g
Br =5.841 g
We have to calculate the empirical formula for each compound.
a)Now Moles of (given mass upon Molar mass) \(Ag=\frac{1.651}{108} =0.015 \:moles\)
Moles of \(O=\frac{0.1224}{8\times 2} =0.0077 \:moles\)
Smallest mole value \(0.0077 \:moles\)
Divide all component by smallest mass.
Therefore \(Ag \Rightarrow\frac{0.015}{0.0077} \rightarrow2\\\\O \Rightarrow\frac{0.0077}{0.0077} \rightarrow1\)
Combine to get empirical formula,
\(Ag_2 O\)
b)Now Moles of (given mass upon Molar mass) \(Co=\frac{0672}{59} =0.011 \:moles\)
Moles of \(As=\frac{0.569}{75} =0.0076\: moles\)
Moles of \(O=\frac{0.486}{16} =0.0304 \:moles\)
Smallest mole value \(0.0076 \:moles\)
Divide all component by smallest mass.
Therefore
\(Co \Rightarrow\frac{0.011}{0.0076} \rightarrow1.5\rightarrow1.5 \times 2\rightarrow3\\\\As \Rightarrow\frac{0.0076}{0.0076} \rightarrow1\rightarrow1 \times 2\rightarrow2\\\\O \Rightarrow\frac{0.0304}{0.0076} \rightarrow4\rightarrow4 \times 2\rightarrow8\)
Combine to get empirical formula,
\(Co_3As_2O_8\)
c)Now Moles of (given mass upon Molar mass) \(Se=\frac{1.443}{79} =0.018 \:moles\)
Moles of \(Br=\frac{5.841}{80} =0.073 \:moles\)
Smallest mole value \(0.018 \:moles\)
Divide all component by smallest mass.
Therefore
\(Se \Rightarrow\frac{0.018}{0.018} \rightarrow1\\\\Br \Rightarrow\frac{0.073}{0.018} \rightarrow4\)
Combine to get empirical formula,
\(SeBr_4\)
For such more question on empirical formula.
https://brainly.com/question/1603500
#SPJ4
How many oxygen atoms are in 3.30 g of quartz?
Answers
Answer:
3.30 gSiO2 / 60.085g SiO2 x 2 mol 0 / 1 mol SiO2 x (6.022*1023 ) = 6.61*1022 O atoms
Explanation:
an enclosed gas is at a pressure of 143,95kpa .if the volume is increased by 40% at constant temperature. what is the new pressure in kilopascals ?
Answers
All processes for which the temperature stays constant are described by Boyle's law.
Boyle's law is expressed as p1 * V1 = p2 * V2, where p1 and V1 are starting pressure and volume, respectively. The ultimate values of these gas parameters are p2 and V2.
Boyle's law formula can be stated in a variety of ways, depending on the parameter we want to estimate. Let's imagine we wish to determine the pressure that results from changing a gas' volume under isothermal conditions. The Boyle's law equation thus reads: p2 = p1 * V1 / V2 or p2 / p1 = V1 / V2.
As we can see, the final-to-initial pressure ratio is the opposite of the volume ratio.
Learn more about Boyle's law here-
https://brainly.com/question/1437490
#SPJ9
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
A student is studying the rate of the following reaction: C2H4O NaOH --> H20 NaC2H3O Knowing that this is an exothermic reaction, he is measuring the rate of the reaction by timing how quickly the reaction vessel heats up. He notices that if he adds HCl to this reaction, the rate increases dramatically. He also determines that the HCl is being used up during the reaction. Is the HCl a catalyst for this reaction.
Answers
Answer:
HCl is not a catalyst because these are not used up during the chemical reactions.
Explanation:
Hello there!
In this case, according to the performed experiments, it is possible for us to realize that HCl cannot be a catalyst for this reaction because it is used up during the reaction. This is explained by the fact that catalyst are able to return to the original form once the reaction has gone to completion; this is the example of palladium in the hydrogenation or dehydrogenation of hydrocarbons depending on the case. Moreover, we know that the catalysts increase the reaction rate because they decrease the activation energy of the reaction and therefore the student observed such increase.
Best regards!
Liquid octane (CH3(CH2)6CH3) will react with gaseous oxygen (O2) to produce gaseous carbon dioxide (CO2) and gaseous water (H2O). Suppose 17. g of octane is mixed with 112. g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to significant digits.
Answers
The maximum mass of water that could be produced by the chemical reaction is 162 g.
The given chemical equation is: 2 C8H18(l) + 25 O2(g) → 16 CO2(g) + 18 H2O(l)In the chemical reaction of liquid octane with gaseous oxygen, the products are gaseous carbon dioxide and gaseous water.According to the balanced chemical equation, 2 moles of C8H18 react with 25 moles of O2 to form 18 moles of H2O.So, 1 mole of C8H18 react with 25/2 = 12.5 moles of O2 to form 9 moles of H2O.The molar mass of C8H18 is 114 g/mol. So, the number of moles in 17 g of C8H18 is:17 g / 114 g/mol = 0.149 molThe molar mass of O2 is 32 g/mol. So, the number of moles in 112 g of O2 is:112 g / 32 g/mol = 3.5 molFrom the balanced chemical equation, 1 mole of C8H18 react with 12.5 moles of O2 to form 9 moles of H2O.So, the number of moles of O2 required to react with 0.149 mol of C8H18 to form H2O is:(12.5 mol / 1 mol) × (0.149 mol / 2 mol) = 0.935 molThe maximum number of moles of H2O that can be produced from 0.149 mol of C8H18 and 0.935 mol of O2 is 9 mol.So, the mass of water produced from 17 g of C8H18 and 112 g of O2 is:9 mol × 18 g/mol = 162 g
for more questions on chemical reaction
https://brainly.com/question/25769000
#SPJ8
10.What is the name of the smallest atom?
Answers
Answer:
The name of the smallest atom is Hydrogen
Explanation:
It's the first atom in the periodic table, meaning it has the least protons or electrions, I forgot.
How radioactive decay affects the atomic number and mass number of an atom?
Answers
Answer:
Nuclear decay changes the number of protons in the nucleus of an atom, and in doing so changes the element. ... If the same element were to undergo beta decay, the mass number would stay the same, and the atomic number would increase by 1, giving neptunium-235. Also Radioactive decay involves the emission of a particle and/or energy as one atom changes into another. In most instances, the atom changes its identity to become a new element.
Hope this helps you.
What would this mechanism look like?

Answers
Answer:
Not sure sorry :(
Explanation:
In your own words, explain why electronegativity increases from left to right across a period and increases from the bottom to the top of a group.
Answers
When an atom goes through alpha decay,
a. Only the atomic number changes
b. Only the mass number changes
c. Both the mass and atomic numbers change
d. Neither the mass nor atomic number changes, as only energy is emitted.
Answers
I need help!!!!!!!!!!!!!!!!
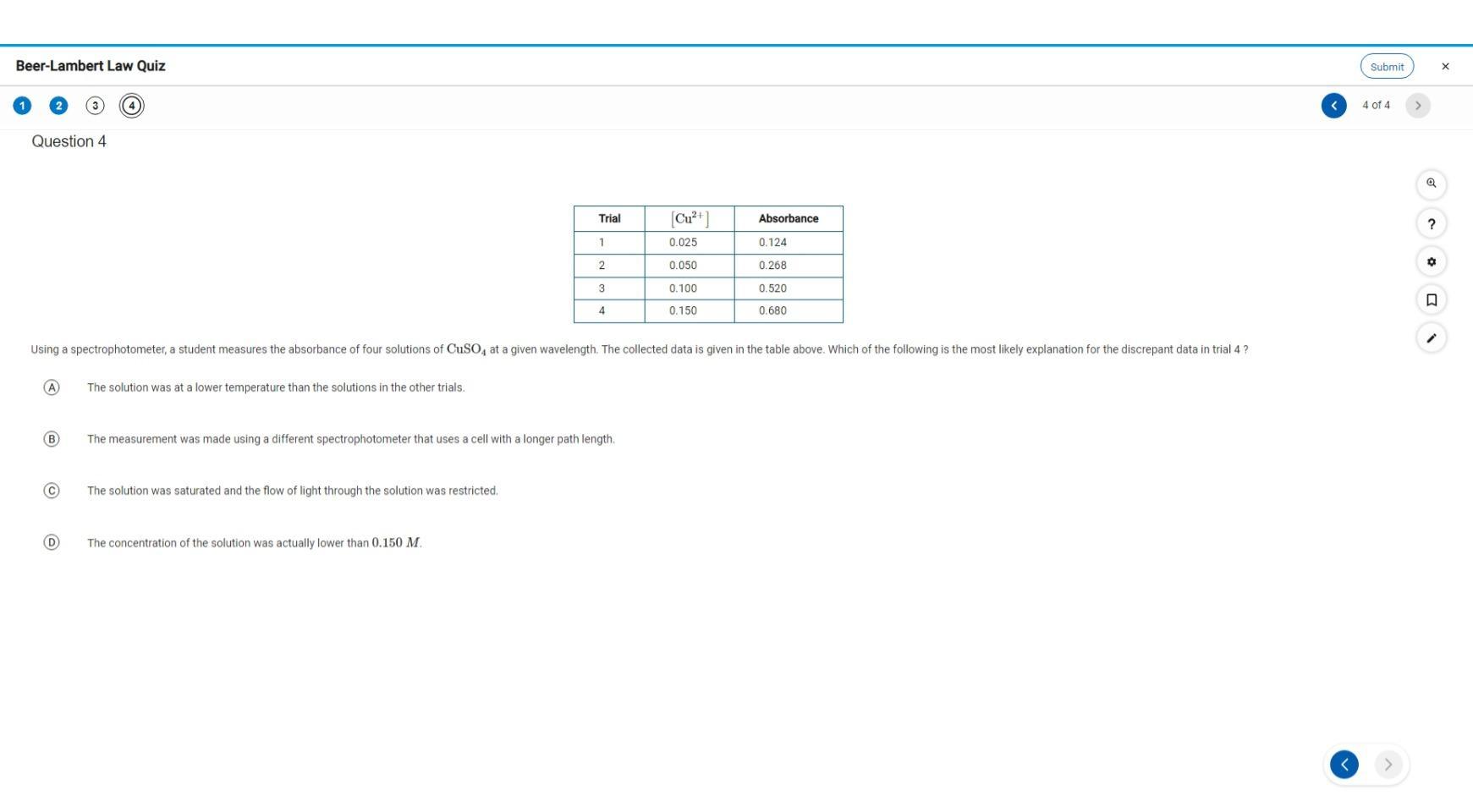
Answers
The reason for the fourth measurement is that the solution is saturated. Option C in the question.
What is the Beer Lambert's law?According to the Beer Lambert's law, the absorbance of the solution is found to the proportional to the concentration of the solution. This implies that as the concentration of the solution increases, the absorbance of the solution also increases.
The absorbance is proportional to the concentration, and the pathlength of the cell where the measurement is taken.
We can now see from the table that the absorbance of the solution is largely affected by the concentration of the solution and not really the pathlength.
Learn more about Beer Lambert's law:https://brainly.com/question/8831959
#SPJ1
A gas occupying 50.0 ml volume in a confined space at 20.0 dc at 50.0 kpa is heated to 40.0 dc. What is the pressure exerted by the gas in the container?
Answers
Answer:The pressure exerted by the gas is 100kPa
Explanation:Let's apply the Charles Gay Lussac law, to solve the question.
At constant volume, the pressure varies proportionally with the temperature.
P initial / T° initial = P final / T° final
50kPa / 20°C = P final / 40°C
Temperature has increased the double, so the pressure will be increased, the double too.
100 kPa
If you need more help go to this link https://brainly.com/question/14378507
During a chemical reaction, how do the substances that form differ from the substances that react?
Answers
what is the percent by mass of nitrogen in the following fertilizers? NH3
Answers
The percent by mass of nitrogen in ammonia (NH3) is approximately 82.15%
Calculating the mass of nitrogen to the total mass of the compound and then expressing the result as a percentage will allow us to determine the percent by mass of nitrogen in NH3 (ammonia).
Ammonia's molecular structure, NH3, indicates that it is made up of one nitrogen atom (N) and three hydrogen atoms (H). We must take both the molar masses of nitrogen and ammonia into account when calculating the percent by mass of nitrogen.
Nitrogen's (N) molar mass is roughly 14.01 g/mol. The molar masses of nitrogen and hydrogen are added to determine the molar mass of ammonia (NH3). Since hydrogen's molar mass is around 1.01 g/mol, ammonia's molar mass is:
(3 mol H 1.01 g/mol) + (1 mol N 14.01 g/mol) = 17.03 g/mol = NH3.
Now, we can use the following formula to get the nitrogen content of ammonia in percent by mass:
(Mass of nitrogen / Mass of ammonia) / 100% is the percentage of nitrogen by mass.
Ammonia weighs 17.03 g/mol and contains 14.01 g/mol of nitrogen by mass. By entering these values, we obtain:
(14.01 g/mol / 17.03 g/mol) 100% 82.15 % of nitrogen by mass
Ammonia (NH3) has a nitrogen content that is roughly 82.15 percent by mass.
For more questions on mass
https://brainly.com/question/24191825
#SPJ8
why does humans are afraid of the dark
Answers
Answer:
Because in Dark it will be fully dark and we cannot see any thing
At what temperature does uranium hexafluoride have a density of 0.5820 g/L at 0.5073 atm?
Answers
Answer:
4204 K
Explanation:
Step 1: Data
Given data
Density of uranium hexafluoride (ρ): 0.5820 g/LPressure of uranium hexafluoride (P): 0.5073 atmRequired data
Universal gas constant (R): 0.08206 atm.L/mol.KMolar mass of uranium hexafluoride (M): 352.02 g/molStep 2: Calculate the temperature of the gas
We will use the following expression derived from the ideal gas equation.
P × M = ρ × R × T
T = P × M/ρ × R
T = 0.5073 atm × (352.02 g/mol)/(0.5820 g/L) × (0.08206 atm.L/mol.K)
T = 4204 K
The temperature of the uranium hexafluoride has been 3,737.36 K.
The uranium hexafluoride has been the gas. Assuming the gas to follow the ideal equation:
Pressure × Volume = Moles × R × Temperature
Moles can be defined as:
Moles = \(\rm \dfrac{mass}{molecular\;mass}\)
The ideal gas equation can be written in terms of mass as:
Pressure × Volume = \(\rm \dfrac{mass}{molecular\;mass}\) × R × Temperature
Pressure = \(\rm \dfrac{mass}{volume}\) × \(\rm \dfrac{1}{molecular\;mass}\) × R × Temperature
Density can be defined as:
Density = \(\rm \dfrac{mass}{volume}\)
The equation in terms of density can be written as:
Pressure = Density × \(\rm \dfrac{1}{molecular\;mass}\) × R × Temperature
The molecular mass of uranium hexafluoride = 352.02 g/mol
R = 0.0816 L.atm/K.mol
Pressure = 0.5073 atm
Density = 0.5820 g/L
Substituting the values:
0.5073 atm = 0.5820 g/L × \(\rm \dfrac{1}{352.02\;g/mol}\) × 0.0821 L.atm/K.mol × Temperature
0.5073 = 0.000135 × Temperature
Temperature = 3,737.36 K.
The temperature of the uranium hexafluoride has been 3,737.36 K.
For more information about the temperature of the gas, refer to the link:
https://brainly.com/question/16957585
Identify the substance that has formula mass of 133.5amu.
(a) MgCI
b)SCI
c)BCI
D) AICI
Answers
The calculated formula masses to 133.5 amu, we find that the substance with a formula mass closest to 133.5 amu is (d) AlCl3. Therefore, the answer is option D.
To identify the substance with a formula mass of 133.5 amu, we need to calculate the formula mass of each given substance and compare it to 133.5 amu.
(a) MgCl2:
The formula mass of MgCl2 can be calculated by adding the atomic masses of magnesium (Mg) and chlorine (Cl).
Mg: atomic mass = 24.31 amu
Cl: atomic mass = 35.45 amu
Formula mass of MgCl2 = (24.31 amu) + 2(35.45 amu) = 95.21 amu
(b) SCl:
The formula mass of SCl can be calculated by adding the atomic masses of sulfur (S) and chlorine (Cl).
S: atomic mass = 32.07 amu
Cl: atomic mass = 35.45 amu
Formula mass of SCl = 32.07 amu + 35.45 amu = 67.52 amu
(c) BCl:
The formula mass of BCl can be calculated by adding the atomic mass of boron (B) and chlorine (Cl).
B: atomic mass = 10.81 amu
Cl: atomic mass = 35.45 amu
Formula mass of BCl = 10.81 amu + 35.45 amu = 46.26 amu
(d) AlCl3:
The formula mass of AlCl3 can be calculated by adding the atomic mass of aluminum (Al) and 3 times the atomic mass of chlorine (Cl).
Al: atomic mass = 26.98 amu
Cl: atomic mass = 35.45 amu
Formula mass of AlCl3 = 26.98 amu + 3(35.45 amu) = 133.78 amu. Option D
For more such questions on masses visit:
https://brainly.com/question/24191825
#SPJ8
In the hot climate, human bodies had to adapt by increasing the number of ____________ on the skin to cool off the body.
A. Body hair
B. Sweat Glands
C. UV rays
D. None of the above
Answers
Answer:
body hair
Explanation:
in the hot climate,human bodies had to adapt inscrasing the number of bodyhair on the skin to coll off the body
A block has 73 kg is being pushed and accelerated at rate of 10 m/s. what force is being applied to the block?
730 N
7.3 N
7300 N
730 Kg
Answers
Answer:
730 N
Explanation:
force =mass ×acceleration
force= 73kg×10m/s
F=730N
75 POINTS!!!
Describe the plate movements in a Divergent(Constructive), Convergent (Destructive) and a Transform (Conservative) Plate Margin. (these are also called plate boundaries). Your answer should define these THREE types of margins or boundaries by explaining the type of movement that occurs.
Answers
The type of movement that occurs in the plate movement listed above include the following:
A divergent boundary occurs when two tectonic plates move away from each other.A convergent boundary occurs when lithospheric plates are moving towards one another.Transform boundaries are created when tectonic plates slide past each other horizontally.What is a Tectonic plate?These are gigantic pieces of the Earth's crust and uppermost mantle and are made up of oceanic crust and continental crust.
A convergent boundary as the name implies occurs when lithospheric plates are moving towards one another.
Read more about Tectonic plate here https://brainly.com/question/1162125
#SPJ1
An isotope of hydrogen, known as Tritium (hydrogen-3), has a half-life of 12 years. If a sample of tritium was prepared 60 years ago, what was its original mass if its current mass is 0.42 micrograms?
Options for answers:
a.) 1.7mg b.) 13.4mg c.) 6.7mg d.) 26.8mg e.) 3.4mg
Answers
The original mass of Tritium (hydrogen-3) was 13.4mg if its current mass is 0.42 micrograms.
The formula for radioactive decay is given by:
N = N0 x (1/2)^(t/T)
where,
N = final number of radioactive atoms
N0 = initial number of radioactive atoms
t = time elapsed
T = half-life of the radioactive substance
Let's substitute the given values into the formula:
0.42 μg = N0 x (1/2)^(60/12)
0.42 μg = N0 x (1/2)^5
0.42 μg = N0 x 1/32
N0 = 0.42 μg x 32
N0 = 13.44 μg
Therefore, the original mass of the tritium sample was 13.44 micrograms.
What is radioactive decay?
Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting ionizing radiation, such as alpha particles, beta particles, and gamma rays. This process can result in a change in the number of protons and/or neutrons in the nucleus, leading to the transformation of one element into another. The rate of decay is typically characterized by a half-life, which is the time required for half of the atoms in a sample to decay.
To know more about radioactive decay, visit:
https://brainly.com/question/1770619
#SPJ1
What is the change in enthalpy in joules when 5.44 x 10- mol of AgCl dissolves in water according to the following chemical equation:
AgCl(s) rightarrow Ag+(aq)+ Cl-(aq) AH=65.5 kj
Answers
Answer:
\(\Delta H=35.3J\)
Explanation:
Hello,
In this case, since the dissolution of silver chloride involves a change in enthalpy of 65.5kJ per 1 mole when undergone, for 5.44x10⁻⁴ moles, the enthalpy change is:
\(\Delta H=65.5\frac{kJ}{1mol}*5.44x10^{-4}mol\\ \\\Delta H=0.0353kJ*\frac{1000J}{1kJ}\\ \\\Delta H=35.3J\)
Best regards.
The change in enthalpy (in joules) when AgCl dissolves in water is 35.362 Joules.
Given the following data:
Number of moles (\(A_gC_l_{(s)}\) ) = \(5.44\) × \(10^{-4}\) mol.
To find the change in enthalpy (in joules) when AgCl dissolves in water according to the following chemical equation:
\(A_gC_l_{(s)}\) -----> \(Ag^{+}_{(aq)} + Cl^{-}_{(aq)}\) \(\Delta H = 65.5 \;kJ/mol\)
1 mole of silver chloride = \(65.5\) × \(10^{3}\) J/mol
\(5.44\) × \(10^{-4}\) mole of silver chloride = X J/mol
Cross-multiplying, we have:
\(X =\) \(5.44\) × \(10^{-4}\) × \(65.5\) × \(10^{3}\)
\(X = 0.544\) × \(65.5\)
X = 35.362 Joules.
Read more: https://brainly.com/question/13197037
A sea turtle is 3feet below the surface of the sea ??? Help me ple

Answers
A 32.65-g sample of a solid is placed in a flask. Toluene, in which the solid is insoluble, is added to the fla sk so that the total volume of solid and liquid together is 50.00 mL. The solid and toluene together weigh 58.58 g. The density of toluene at the temperature of the experiment is 0.864 g/ mL. What is the density of the solid?
Answers
Answer:
Density of the solid is 1.63g/mL
Explanation:
Hello,
Data;
Mass of solid = 32.65g
Mass of toluene = ?
Mass of solid + toluene = 58.58g
Density of toluene = 0.864g/mL
Mass of toluene = (mass of solid + toluene) - mass of solid
Mass of toluene = 58.58 - 32.65
Mass of toluene = 25.93g
But density = mass / volume
Density of toluene = mass of toluene / volume of toluene
Volume of toluene = mass of toluene / density of toluene
Volume of toluene = 25.93 / 0.864
Volume of toluene = 30.01mL
Volume of solid = total volume of the mixture (solid + toluene) - volume of toluene
Volume of solid = 50 - 30.01
Volume of solid = 19.99mL
Density of the solid = mass of solid / volume of solid
Density of the solid = 32.65 / 19.99
Density of the solid = 1.63g/mL
Therefore the density of the solid is 1.63g/mL