Calculate the nuclear binding energy per nucleon for tl205 which has a nuclear mass of 204.974 amu .
Answers
The nuclear binding energy per nucleon for tl205 which has a nuclear mass of 204.974 AMU is 7.68emv.
How do you compute binding energy per nucleon in MeV?If we have a nucleus with Z is for protons and N is for neutrons and mass as MA, where
A = Z+N
then its binding energy in MeV is provided by:
Eb (MeV) = ( Zmp + Nmn -MA) x 931.494 MeV/u
Operating in terms of the actual binding energy, we estimate as follows.
We get 7.68 emv.
Nuclear binding energy is described as the lowest amount of energy required to dismantle or break down an atom's nucleus into the subatomic particles that make it up (which are protons and neutrons).
To learn more about Nuclear binding energy visit the link
https://brainly.com/question/14287889
#SPJ4
Related Questions
Given+the+following+information,+calculate+the+molecular+formula:+c+=+40.00%;+h+=+6.71%;+o+=+53.28%;+molar+mass+=+90.08+g/mol
Answers
The molecular formula of the compound is C3H6O2, indicating that there are 3 carbon atoms, 6 hydrogen atoms, and 2 oxygen atoms in one molecule.
To calculate the molecular formula, we need to determine the ratio of each element present in the compound. Given the percentages of carbon (C), hydrogen (H), and oxygen (O) in the compound as 40.00%, 6.71%, and 53.28% respectively, we can assume a 100 gram sample.
Convert the percentages to grams:
C: 40.00% of 100 g = 40.00 g
H: 6.71% of 100 g = 6.71 g
O: 53.28% of 100 g = 53.28 g
Convert the grams to moles:
C: 40.00 g / 12.01 g/mol (molar mass of carbon) = 3.33 mol
H: 6.71 g / 1.01 g/mol (molar mass of hydrogen) = 6.64 mol
O: 53.28 g / 16.00 g/mol (molar mass of oxygen) = 3.33 mol
Divide the moles by the smallest number of moles:
C: 3.33 mol / 3.33 mol = 1
H: 6.64 mol / 3.33 mol = 2
O: 3.33 mol / 3.33 mol = 1
Therefore, the molecular formula of the compound is C3H6O2.
Learn more about Molecular formula
brainly.com/question/28647690
#SPJ11
All living organisms are composed of one or more cells.
A cell is the basic structural and functional unit of living organisms.
All cells arise from pre-existing cells.
Answers
Answer:
all cells contain nuclei.
Explanation:
prokaryotes dont have a nuclei
what is the molarity of an aqueous hcl solution which is 37.0% hcl by mass and has a density of 1.19 g/ml? report result to 3 significant figures.
Answers
The molarity of the aqueous HCl solution, which is 37.0% HCl by mass and has a density of 1.19 g/mL, is approximately 14.8 mol/L when rounded to three significant figures.
The molarity of an aqueous HCl solution can be calculated using the formula:
Molarity = (mass of solute (in g) / molar mass of solute (in g/mol)) / volume of solution (in L)
We are given that the solution is 37.0% HCl by mass and has a density of 1.19 g/mL. This means that in 100 g of solution, there are 37.0 g of HCl. We can calculate the volume of this solution using its density:
Volume of solution = mass of solution / density = 100 g / 1.19 g/mL = 84.03 mL = 0.08403 L
The molar mass of HCl is 36.46 g/mol. Now, we can substitute these values into the formula for molarity:
Molarity = (37.0 g / 36.46 g/mol) / 0.08403 L = 14.8 mol/L
Rounding the answer to three significant figures, the molarity of the HCl solution is approximately 14.8 mol/L.
To know more about molarity refer here:
https://brainly.com/question/31545539#
#SPJ11
13. Which compound reacts third fastest with fuming sulfuric acid? a. Toluene b. Chlorobenzene d. Nitrobenzene e. Benzene c. Aniline.
Answers
Toluene compound reacts third fastest with fuming sulfuric acid.
The reaction is attached in figure -
When nitration of toluene is carried out in presence of fuming nitric acid and fuming sulfuric acid under 333 K temperature , 2, 4, 6-trinitro toluene is the final product .This 2, 4, 6-trinitro toluene is also called TNT. TNT is used as explosive.What is toluene?
Toluene is a common ingredient in degreasers. It's a colorless liquid with a sweet smell and taste. It evaporates quickly. Toluene is found naturally in crude oil, and is used in oil refining and the manufacturing of paints, lacquers, explosives (TNT) and glues.What is a toluene used for?
Toluene is found naturally in crude oil, and is used in oil refining and the manufacturing of paints, lacquers, explosives (TNT) and glues. In homes, toluene may be found in paint thinners, paintbrush cleaners, nail polish, glues, inks and stain removers.Learn more about Toluene
brainly.com/question/9530946
#SPJ4

Pretend your friend was absent from class today...
Write what you would say if you had to explain how and why elements to
your friend.
Answers
Answer:
how and why elements what?? why they exist?? im gonna assume that's what you meant, so elements exist because we're all on earth and without elements, we don't have resources to live. the earth is made of elements and if there were none, we wouldn't even have a planet
Explanation:
^
How many ATOMS are in 5 grams of NaCl?
Answers
Answer:
2 atoms
Explanation:
Sodium Chloride or NaCl is made up of two elements, sodium (or Na) and chlorine (or Cl). A molecule of sodium chloride, NaCl, consists of one atom each of sodium and chlorine. Hence, each molecule of NaCl has 2 atoms total.
2原子。塩化ナトリウムまたはNaClは、ナトリウム(またはNa)と塩素(またはCl)の2つの元素で構成されています。
There are approximately 3.01 x 10²² atoms in 5 grams of NaCl.
To calculate the number of atoms, we first need to convert the mass of NaCl into moles. The molar mass of NaCl is 58.44 g/mol, which is the sum of the atomic masses of sodium (22.99 g/mol) and chlorine (35.45 g/mol).
Using the formula:
moles = mass / molar mass
we can calculate the number of moles of NaCl in 5 grams:
moles = 5 g / 58.44 g/mol ≈ 0.0857 mol
Next, we need to use Avogadro's number, which is approximately 6.022 x 10²³ atoms/mol, to convert moles into atoms.
Number of atoms = moles x Avogadro's number
Number of atoms = 0.0857 mol x 6.022 x 10²³ atoms/mol ≈ 5.16 x 10²² atoms
Therefore, there are approximately 3.01 x 10²² atoms in 5 grams of NaCl.
It's important to note that this calculation assumes that all the NaCl is fully dissociated into its constituent atoms, which may not be the case in all situations.
To learn more about ATOMS, here
https://brainly.com/question/33592727
#SPJ2
How many sig figs are there?
4000.
Answers
The number of significant figures in 4000 is 1
the half-life of a certain radioactive material is 4 days. the length of time it will take for the material to decay to 17 of its original mass is
Answers
If the half-life of a certain radioactive material is 4 days. the length of time it will take for the material to decay to 17 of its original mass is 11.06 days.
To solve this problem, we can use the formula for exponential decay:
N(t) = N0 * (1/2)²(t/T)
where:
N(t) is the amount of the radioactive material remaining after time t
N0 is the initial amount of the radioactive material
T is the half-life of the radioactive material
We want to find the time it will take for the material to decay to 1/7 of its original mass, which means that N(t) = N0/7.
Using the formula above and plugging in the given values, we get:
N0/7 = N0 * (1/2)²(t/4)
Dividing both sides by N0 and taking the natural logarithm of both sides, we get:
ln(1/7) = ln(1/2)²(t/4)
ln(1/7) = (t/4) * ln(1/2)
t = (4/ln(1/2)) * ln(1/7)
t ≈ 11.06 days
Therefore, it will take approximately 11.06 days for the radioactive material to decay to 1/7 of its original mass, given a half-life of 4 days.
Learn more about decay here:
https://brainly.com/question/30258090
#SPJ4
Which of the following tells you the number of molecules in 1 mole of a gas?
O A. The atomic mass
O B. Avogadro's number
C. The molar mass
O D. The ideal gas constant
SUBMIT
Answers
Answer:
Avogrado's number
Explanation:
It's unniversal measure of moles
HELP PLEESE HELP.....

Answers
Answer:
i'm sorry
Explanation:
One of the following equations is that of a parabola with x-intercepts -5 and +5 in the standard (x, y) coordinate plane. Which equation?
a. y = x² + 25
b. y = (x + 5)(x - 5)
c. y = x(x + 5)(x - 5)
d. y = (x + 5)² - 25
Answers
Given that the x-intercepts of the parabola are -5 and 5 and we need to find out which equation from the given options is that of the parabola. Option (b) y = (x + 5)(x - 5)
is the equation of a parabola with x-intercepts -5 and +5 in the standard (x, y) coordinate plane.
We know that the x-intercepts of a parabola are the points at which y is zero.
We have two x-intercepts: x = -5 and x = 5. Let's find which equation is correct.
a. y = x² + 25
For x = -5, we have
y = (-5)² + 25 = 50.
It does not satisfy the equation for the x-intercept of -5.
For x = 5, we have
y = (5)² + 25 = 50.
It does not satisfy the equation for the x-intercept of 5.
b. y = (x + 5)(x - 5)
For x = -5, we have
y = (0) (10) = 0.
This satisfies the equation for the x-intercept of -5.
For x = 5, we have
y = (10) (0) = 0.
This satisfies the equation for the x-intercept of 5.
c. y = x(x + 5)(x - 5)
For x = -5, we have
y = (-5) (0) (10) = 0.
This satisfies the equation for the x-intercept of -5.For x = 5, we have
y = (5) (10) (0) = 0.
This satisfies the equation for the x-intercept of 5.
d. y = (x + 5)² - 25
For x = -5, we have
y = (0) - 25 = -25.
It does not satisfy the equation for the x-intercept of -5.
For x = 5, we have
y = (10) - 25 = -15.
It does not satisfy the equation for the x-intercept of 5.
y = (x + 5)(x - 5)
is the equation of a parabola with x-intercepts -5 and +5 in the standard (x, y) coordinate plane.
Hence, the correct answer is option B.
To know more about x-intercepts visit:
https://brainly.com/question/32051056
#SPJ11
what are the configurations of the chiral carbon atoms in this compound? carbon atom number 1 is the carbon containing the aldehyde. (2s,3s,4r)(2s,3r,4s)(2r,3s,4r)(2r,3r,4r)(2s,3s,4s)(2r,3r,4s)(2s,3r,4r)(2r,3s,4s)
Answers
The compound has eight chiral carbon atoms, numbered 2 through 9. The configurations of each chiral carbon atom can be indicated by the stereochemical designations (R) or (S).
Using the numbering system provided, the configurations of the chiral carbon atoms are as follows:
- Carbon atom number 2: (2S)
- Carbon atom number 3: (3S) in configurations (1), (3), (5), and (7), and (3R) in configurations (2), (4), (6), and (8).
- Carbon atom number 4: (4R) in configurations (1), (3), (5), and (7), and (4S) in configurations (2), (4), (6), and (8).
- Carbon atom number 5: (5S) in configurations (1), (2), (5), and (6), and (5R) in configurations (3), (4), (7), and (8).
- Carbon atom number 6: (6R) in configurations (1), (2), (5), and (6), and (6S) in configurations (3), (4), (7), and (8).
- Carbon atom number 7: (7S) in configurations (1), (4), (5), and (8), and (7R) in configurations (2), (3), (6), and (7).
- Carbon atom number 8: (8R) in configurations (1), (4), (5), and (8), and (8S) in configurations (2), (3), (6), and (7).
- Carbon atom number 9: (9S) in configurations (1), (2), (3), and (4), and (9R) in configurations (5), (6), (7), and (8).
Therefore, the configurations of the chiral carbon atoms in this compound are:
(2S,3S,4R,5S,6R,7S,8R,9S) in configuration (1)
(2S,3R,4S,5R,6S,7R,8S,9R) in configuration (2)
(2R,3S,4R,5S,6S,7S,8R,9S) in configuration (3)
(2R,3R,4S,5R,6R,7R,8S,9R) in configuration (4)
(2S,3S,4S,5R,6S,7R,8R,9S) in configuration (5)
(2R,3R,4S,5S,6R,7S,8S,9R) in configuration (6)
(2S,3R,4R,5S,6R,7R,8R,9S) in configuration (7)
(2R,3S,4S,5R,6R,7S,8S,9R) in configuration (8)
To know more about chiral carbon atoms click here:
https://brainly.com/question/29131173
#SPJ11
Valence bond theory describes a single covalent bond as the _____ of orbitals from two atoms to form a shared space, which is occupied by _____ electron(s). multiple choice question. overlap; four overlap; two combination; four combination; two
Answers
Valence bond theory describes a single covalent bond as the overlap of orbitals from two atoms to form a shared space, which is occupied by two electron(s).
What is valence bond?
It is a chemical bonding hypothesis. According to VBT, the creation of a chemical bond between two atoms is caused by the overlap of incompletely filled atomic orbitals. Unpaired electrons are shared, resulting in the formation of a hybrid orbital.
What is covalent bond ?
Covalent bonds are formed by atoms exchanging electron pairs. Electron pairs shared by atoms with equal or very comparable electronegativity form a nonpolar covalent link (e.g., H-H or C-H), whereas electron pairs shared by atoms with uneven electronegativity form a polar covalent bond (e.g., H-H or C-H) (e.g., H–O).
Therefore, Valence bond theory describes a single covalent bond as the overlap of orbitals from two atoms to form a shared space, which is occupied by two electron(s).
Learn more about valence bond from the given link.
https://brainly.com/question/10729991
#SPJ4
Which one of the following is a strong base? A) Mg(OH)2 B) Ca(OH)2 C) NH3 D) CH3OH 21420
Answers
B) Ca(OH)2 is a strong base.
In chemistry, a base is a substance that can accept hydrogen ions (H+) from an acid and form a salt and water. The strength of a base refers to its ability to accept hydrogen ions and is often measured by the base dissociation constant (Kb).
Strong bases have a high dissociation constant and dissociate completely in aqueous solution, while weak bases dissociate only partially.
Among the options listed, Ca(OH)2 is a strong base as it dissociates completely in water to form hydroxide ions (OH-) and can accept hydrogen ions from an acid. In contrast, Mg(OH)2 is also a base, but it is weaker than Ca(OH)2.
NH3 is an amphoteric compound that can act as either a base or an acid depending on the conditions, and CH3OH is a neutral compound that does not act as a base.
To know more about dissociation constant click on below link:
https://brainly.com/question/28197409#
#SPJ11
I2(aq)+C6H8O6(aq)→C6H6O6(aq)+2I−(aq)+2H+(aq)
The compound C6H8O6 reacts with I2 according to the reaction represented by the equation above. The reaction is correctly classified as which of the following types?
Answers
The reaction is correctly classified as Oxidation-reduction, because I2 is reduced.
What is Oxidation?Redox reactions involve a change in the oxidation state of the substrate. When electrons are lost or the oxidation state increases, it is called oxidation; when they are gained or the oxidation state decreases, it is called reduction. When an object comes into contact with oxygen or another oxidizer, a chemical reaction occurs. Rust and the brown color of a cut apple are both examples of oxidation. Oxidation is the process in which a reactant loses electrons during a reaction. Reduction is the term used to describe a reaction in which a reactant picks up electrons. This happens frequently when acid and metals interact. Oxidation is the process in which a reactant loses electrons during a reaction.
Here,
Because I2 is reduced, the reaction is correctly categorized as an oxidation-reduction reaction.
To know more about Oxidation,
https://brainly.com/question/9496279
#SPJ4
1.A space shuttle's wings are useless as it travels around the exosphere. Explain, in your own words, why the wings are so useless and how the shuttles can move around.
2.Explain, in your own words, the thermosphere and the stratosphere are our hottest atmosphere layers.
3.Explain, in your own words, how and why “shooting stars” occur. For example, why and what causes meteors to burn up.
4.Explain, in your own words, the importance of our ozone layer in the stratosphere.
5.Explain, in your own words, why weather only occurs in the troposphere.
PLEASE HELP!!!!! Use your own words please! I will give the brainiest to the person who answers it!
Answers
Thrust acts on the spacecraft and propels in the space.
How space shuttles move?1. The force created by the shuttle's engines in expelling the burning fuel produces an equal thrust in the opposite direction. This thrust acts on the spacecraft and propels in the space.
2. The thermosphere and the stratosphere are our hottest atmosphere layers because these two layers are present near the sun as compared to other layers of atmosphere.
3. When the meteors hit the atmosphere, meteors rub against air particles and create friction which is heating the meteors. The heat vaporizes most meteors, creating what we call shooting stars.
Learn more about atmosphere here: https://brainly.com/question/24925283
How many bromide ions are there in 2.00g of MgBr2?
Answers
There are 1.31 x 1022 bromide ions in 2.00 g of \(MgBr_2\).
The chemical formula of magnesium bromide (\(MgBr_2\)) contains one magnesium ion (\(Mg2^+\)) and two bromide ions (Br-). To find the number of bromide ions in 2.00 g of \(MgBr_2\), we need to use the molar mass of \(MgBr_2\) to determine the number of moles of \(MgBr_2\) present in 2.00 g of the compound, then use the stoichiometry of the chemical formula to determine the number of bromide ions present. First, we need to calculate the molar mass of \(MgBr_2\). The molar mass of \(MgBr_2\) is equal to the sum of the atomic masses of magnesium (Mg) and two bromine (Br) atoms. The atomic mass of Mg is 24.31 g/mol, and the atomic mass of Br is 79.90 g/mol. Molar mass of \(MgBr_2\) = 24.31 g/mol + (2 x 79.90 g/mol) = 184.11 g/mol Next, we can use the molar mass to determine the number of moles of \(MgBr_2\) present in 2.00 g of the compound: Number of moles of \(MgBr_2\) = mass of \(MgBr_2\) / molar mass of \(MgBr_2\)= 2.00 g / 184.11 g/mol
= 0.0109 mol Finally, we can use the stoichiometry of the chemical formula to determine the number of bromide ions present: Number of bromide ions = 2 x number of moles of \(MgBr_2\)
= 2 x 0.0109 mol
= 0.0218 mol Therefore, there are 0.0218 moles of bromide ions in 2.00 g of \(MgBr_2\). To convert this to the number of bromide ions, we can multiply by Avogadro's number (6.02 x 1023): Number of bromide ions = 0.0218 mol x 6.02 x 1023 ions/mol = 1.31 x 1022 ions
For more questions on bromide ions
https://brainly.com/question/29228517
#SPJ8
OMG PLEASE HELP IM DOING SO WELL IN SCIENCE AND THIS IS GRADED AND MY PROFEESER WONT HELP
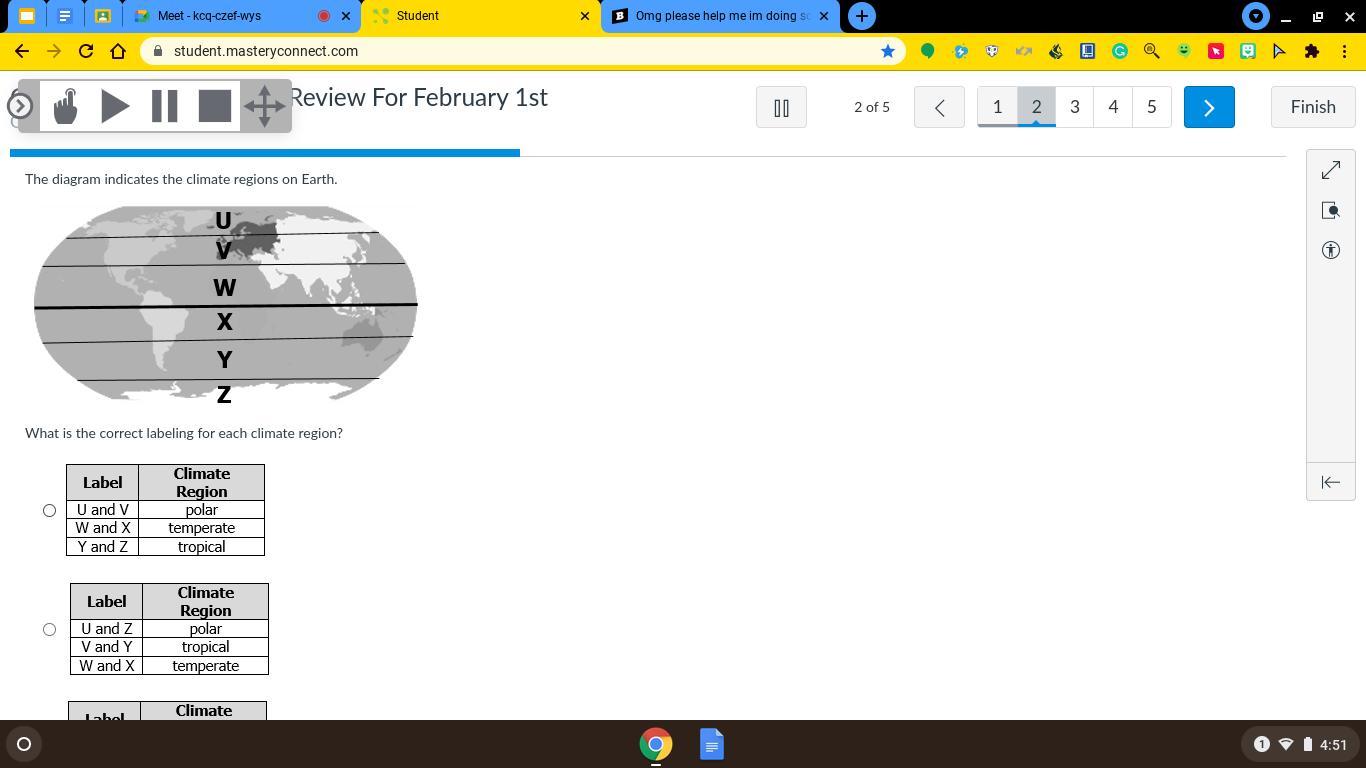

Answers
Answer:
bbbbbbbbbbbbbbbbbbb
Explanation:
Please help, photo attached.

Answers
220 g of propane produces 659.01 g of\(CO_{2}\) and 359.39 g of \(H_{2}O\)through combustion.
1. The balaced equation for this \(C_{5}H_{12}\) + \(O_{2}\) + \(CO_{2}\) + \(H_{2}O\) is
\(C_{5}H_{12}\) + \(8O_{2}\) -> \(5CO_{2}\) + \(6H_{2}O\)
Based on the balanced equation \(C_{5}H_{12}\)+ \(8O_{2}\)-> \(5CO_{2}\)+ \(6H_{2}O\), the table can be completed as follows:
Mol \(C_{5}H_{12}\)- 2 , 20 , 24 , 40
mol \(O_{2}\) - 16 , 2.5 , 12.5 , 15
Mol \(CO_{2}\)- 24 , 15 , 3 , 18
mol \(H_{2}O\)- 40 , 25 , 30 , 5.4
2. First, we need to balance the given equation:
\(C_{3}H_{8}\)(g) + \(5O_{2}\)(g) →\(3CO_{2}\)(g) + \(4H_{2}O\)(g)
The balanced equation shows that for every mole of propane (\(C_{3}H_{8}\)) combusted, we get 3 moles of carbon dioxide (\(CO_{2}\)) and 4 moles of water (\(H_{2}O\)) produced.
To calculate the grams of \(CO_{2}\)and \(H_{2}O\)produced from the combustion of 220 g of propane, we need to use stoichiometry. First, we calculate the number of moles of propane:
Molar mass of propane (\(C_{3}H_{8}\)) = 3(12.01 g/mol) + 8(1.01 g/mol) = 44.1 g/mol
Number of moles of propane = mass of propane / molar mass of propane
Number of moles of propane = 220 g / 44.1 g/mol
Number of moles of propane = 4.99 mol
Now, using the balanced equation, we can calculate the number of moles of carbon dioxide and water produced:
Number of moles of \(CO_{2}\)produced = 3 mol \(CO_{2}\)/ 1 mol \(C_{3}H_{8}\)* 4.99 mol \(C_{3}H_{8}\)
Number of moles of \(CO_{2}\)produced = 14.97 mol \(CO_{2}\)
Number of moles of \(H_{2}O\)produced = 4 mol \(H_{2}O\)/ 1 mol \(C_{3}H_{8}\)* 4.99 mol \(C_{3}H_{8}\)
Number of moles of \(H_{2}O\)produced = 19.96 mol \(H_{2}O\)
Finally, we can calculate the mass of \(CO_{2}\)and \(H_{2}O\)produced:
Mass of \(CO_{2}\)produced = number of moles of \(CO_{2}\)* molar mass of \(CO_{2}\)
Mass of \(CO_{2}\)produced = 14.97 mol * 44.01 g/mol
Mass of \(CO_{2}\)produced = 659.01 g
Mass of \(H_{2}O\)produced = number of moles of \(H_{2}O\)* molar mass of \(H_{2}O\)
Mass of \(H_{2}O\)produced = 19.96 mol * 18.02 g/mol
Mass of \(H_{2}O\)produced = 359.39 g
Therefore, 659.01 g of \(CO_{2}\)and 359.39 g of \(H_{2}O\)are produced from the combustion of 220 g of propane.
To learn more about balanced equation click on the given link brainly.com/question/11904811
#SPJ1
T or F: Lone pairs around the oxygen atom of a water molecule play no role in determining its molecular geometry?
Answers
Answer:
Explanation:
They play a very important part. The geometry is not a straight line. It is an angle over 90 which means that the molecule has the same general shape as a boomerang. The two hydrogens and the 2 lone electron pairs try to get away as far as possible from each other. The actual shape results in a tetrahedron shape. But the two hydrogens and 1 oxygen actually look like the aforementioned boomerang.
How many kinds of horses are there in the world? Give me a real number, plz its for a magazine article!
Answers
Answer:
784
Explanation:
Answer:
784 horse breeds
name given to atoms that have either gained or lost electrons is
Answers
An atom that has gained or lost electrons is considered to have experienced an ion if it now has a net charge.
What is the process of losing or gaining an electron known as?Reduction is the process of gaining electrons, whereas oxidation is the process of losing electrons. Oxidizing agents are any substances that make other substances lose electrons (become oxidized).
Why do atoms pick up or drop off electrons?Since it makes them more stable and requires less energy to sustain, atoms become ions by shedding or gaining electrons. An atom is in its most stable state when its outermost energy level is packed with as many electrons as is physically possible.
To know more about electrons visit :-
https://brainly.com/question/1255220
#SPJ4
where are noble gases located on the periodic table?
Answers
Hello! :)
Noble gases are located in group 8A in the periodic table.
Elements in this group contain 8 valence electrons, making them very stable elements. Some examples of elements in this group include Neon, Argon, and Krypton.
Engineers are looking for a new material to build an oil tanker. The material must be lightweight, strong, and durable. Several engineers have developed some initial plans. The engineers are in which stage of technological design? A) identifying a problem or need B) designing a solution C) implementing a solution D) evaluating a solution
Answers
Answer:
designing a solution
Explanation:
i found the answer on quizlet lol
Based on the given information, the engineers are in the "designing a solution" stage of technological design.The correct answer is option B.
Materials are substances or matter that are used to make objects, structures, or products. Materials possess physical and chemical properties that determine their behavior and suitability for various applications.
B. Designing a solution is an appropriate answer because after identifying the need for a new material to build an oil tanker, they are now supposed to develop plans and designs for the material that meets the desired characteristics of being lightweight, strong, and durable. The engineers are in second stage of technological design.
Therefore, based on the information provided, the engineers are currently in the "designing a solution" stage of technological design. Option B is the correct answer.
Learn more about materials here:
https://brainly.com/question/17185757
#SPJ2
how many moles of copper (ii) sulfate (cuso4) are in a 0.125g sample of cuso4?
Answers
The moles of the copper (ii) sulfate that is CuSO₄ are in the 0.125g sample of the CuSO₄ is 0.0007 g/mol.
The mass of the copper sulfate, CuSO₄ = 0.125 g
The molar mass of the copper sulfate, CuSO₄ = 159.6 g/mol
The number of moles of copper sulfate, CuSO₄ = mass / molar mass
Where,
The mass of CuSO₄ = 0.125 g
The molar mass of CuSO₄ 159.6 g/mol
The number of moles of copper sulfate, CuSO₄ = mass / molar mass
The number of moles of copper sulfate, CuSO₄ = 0.125 g / 159.6 g/mol
The number of moles of copper sulfate, CuSO₄ = 0.0007 mol
To learn more about moles here
https://brainly.com/question/30885025
#SPJ4
How to round the number 314.77 to 2 significant figures
Answers
Answer:
发你如果热客人看看如果
Explanation:
Answer:
result = 310
significant figures = 2
Explanation:
How to round significant figures:
look the first non-zero digit if rounding to one significant figure, look the digit after the first non-zero digit if rounding to two significant figures. draw a vertical line after the place value digit that is required.look at the next digit.
result = 310
significant figures = 2
Calculate the Empirical Formula for the following compound:
0.300 mol of S and 0.900 mole of O.
Answers
Answer:
\(\boxed {\boxed {\sf SO_3}}\)
Explanation:
An empirical formula shows the smallest whole-number ratio of the atoms in a compound.
So, we must calculate this ratio. Since we are given the amounts of the elements in moles, we can do this in just 2 steps.
1. DivideThe first step is division. We divide the amount of moles for both elements by the smallest amount of moles.
There are 0.300 moles of sulfur and 0.900 moles of oxygen. 0.300 is smaller, so we divide both amounts by 0.300
Sulfur: 0.300/0.300= 1 Oxygen: 0.900/0.300= 3 2. Write Empirical FormulaThe next step is writing the formula. We use the numbers we just found as the subscripts. These numbers go after the element's symbol in the formula. Remember sulfur is S and there is 1 mole and oxygen is O and there are 3 moles.
S₁O₃This formula is technically correct, but we typically remove subscripts of 1 because no subscript implies 1 representative unit.
SO₃\(\bold {The \ empirical \ formula \ for \ the \ compound \ is \ SO_3}}\)
the electrons used in bonding are known as
a. bonding electrons
b. proton
c. neutron
d.moron
Answers
Answer:
bonding electron
Explanation:
because they will bond together.
what ionic compound is gold found in
Answers
Gold is found in various ionic compounds, but one of the most well-known and commercially significant compounds is gold chloride, also known as auric chloride or gold(III) chloride.
The chemical formula for gold chloride is AuCl₃. Gold chloride is an ionic compound composed of gold cations (Au³⁺) and chloride anions (Cl-). It is a yellow-orange solid that is highly soluble in water. Gold chloride can be formed by reacting the gold metal with chlorine gas or by dissolving the gold metal in aqua regia, which is a mixture of concentrated nitric acid and hydrochloric acid.
Gold chloride has several uses and applications. It is commonly used in the field of nanotechnology for the synthesis of gold nanoparticles. These nanoparticles have unique optical, electronic, and catalytic properties, making them valuable in various fields such as medicine, electronics, and materials science.
In addition to gold chloride, gold can also form other ionic compounds with different anions, such as gold bromide (AuBr), gold iodide (AuI), gold sulfide (Au2S), and gold cyanide (AuCN). These compounds have their own unique properties and applications.
know more about anions here:
https://brainly.com/question/31485566
#SPJ8
PLEASE HELP ON EASY CHEMICAL PROPERTIES

Answers
Answer:
1, just I) color.
Explanation:
Physical properties are the properties that can be observed without changing the composition of a substance, such as color, temperature, density, and boiling point.
A physical change is a change in the substance that only modifies its aggregation state, such as solidification, and boiling.
Chemical properties are the properties that need a reaction to being observed, such as combustibility, which needs a combustion reaction to being quantified.
When a chemical reaction occurs, and the composition of the substance change, it's a chemical change.
So, heating copper with carbon is a chemical reaction, and purification by electrolysis is too. Color is the only physical property.