Calculate the percent ionization of formic acid in a solution that is 0.010 M HCOOH and 0.005 M HCOONa. Ka (HCOOH) = 1.7x10-4 ?
Answers
The percent ionization of formic acid in a 0.010 M HCOOH and 0.005 M HCOONa solution is approximately 2.57%.
To calculate the percent ionization, follow these steps:
1. Write the ionization equation: HCOOH + H2O ↔ H₃O⁺ + HCOO⁻
2. Identify the initial concentrations: [HCOOH] = 0.010 M, [HCOONa] = 0.005 M
3. Recognize that HCOONa is the sodium salt of formic acid and contributes [HCOO⁻] = 0.005 M
4. Create an ICE table to determine the change in concentrations during ionization.
5. Use the Ka expression: Ka = ([H₃O⁺][HCOO⁻])/([HCOOH])
6. Substitute the values from the ICE table into the Ka expression: (1.7x10⁻⁴) = (x²)/(0.010-x)
7. Solve for x, which represents the concentration of [H₃O⁺]: x ≈ 0.000257 M
8. Calculate percent ionization: (0.000257 M / 0.010 M) * 100% ≈ 2.57%
To know more about percent ionization click on below link:
https://brainly.com/question/31358773#
#SPJ11
Related Questions
Light travel in __________ lines until reflected or refracted .

Answers
Answer:
Light travel in _____straight_____ lines until reflected or refracted
is italian salad dressing homogeneous or heterogeneous
Answers
Answer:
Heterogeneous
Explanation:
what special equipment did niels bohr use to develop his atomic model?
Answers
Neil Bohr used the fluorescent screen and an alpha particle detector to study the structure of an atom.
What is an atom?
An atom is defined as the smallest unit of matter which forms an element. Every form of matter whether solid,liquid , gas consists of atoms . Each atom has a nucleus which is composed of protons and neutrons and shells in which the electrons revolve.
Atoms of the same element are similar as they have number of sub- atomic particles which on combination do not alter the chemical properties of the substances.
The atom as a whole is neutral and stable due to presence of oppositely charged particles.
Learn more about atom,here:
https://brainly.com/question/13654549
#SPJ1
A 360. g iron rod is placed into 750.0 g of water at 22.5°C. The water temperature rises to 46.7°C. What was the initial temperature of the iron rod?
Answers
Answer: The initial temperature of the iron was \(515^0C\)
Explanation:
\(heat_{absorbed}=heat_{released}\)
As we know that,
\(Q=m\times c\times \Delta T=m\times c\times (T_{final}-T_{initial})\)
\(m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]\) .................(1)
where,
q = heat absorbed or released
\(m_1\) = mass of iron = 360 g
\(m_2\) = mass of water = 750 g
\(T_{final}\) = final temperature = \(46.7^0C\)
\(T_1\) = temperature of iron = ?
\(T_2\) = temperature of water = \(22.5^oC\)
\(c_1\) = specific heat of iron = \(0.450J/g^0C\)
\(c_2\) = specific heat of water= \(4.184J/g^0C\)
Now put all the given values in equation (1), we get
\(-360\times 0.450\times (46.7-x)=[750\times 4.184\times (46.7-22.5)]\)
\(T_i=515^0C\)
Therefore, the initial temperature of the iron was \(515^0C\)
A radiosotope emits a positron to form titanium -48. Express your answer as a nuclear equation.
Answers
The nuclear equation for the formation of titanium-48 from a radiosotope emitting a positron is: Radiosotope → Titanium-48 + Positron.
To express the formation of titanium-48 from a radiosotope emitting a positron, we can write the nuclear equation as follows:
Reactant Nucleus: Radiosotope
Product Nucleus: Titanium-48
Particle Emitted: Positron
The nuclear equation can be written as:
Radiosotope → Titanium-48 + Positron
This equation represents the decay of the radiosotope, where it emits a positron and forms titanium-48 as the product nucleus.
Learn more:About radiosotope here:
https://brainly.com/question/11913067
#SPJ11
The process in which substances are changed into different substances is called
A. Chemical property
B. Physical change
C. Physical property
D. Chemical change
Two elements in the same group may have the same ion charge.
A. True
B. False
A polar covalent bond involves _______________ of valence electrons?
A. an equal sharing
B. a transfer
C. an electrostatic attraction
D an unequal sharing
Answers
Answer:
1. chemical change
2. true
3. an unequal sharing
Explanation:
what is the speed and appearance of most cold fronts
Answers
Answer:
25mph
Explanation:
cold fronts generally Advance at an average speed of 20 to 25 miles per hour. Towards the east faster in the winter than summer and are usually oriented along the northeast to Southwest line
acid rain is made up mainly of two acids. they are
Answers
Sulfuric acid is formed when sulfur dioxide (SO2) and other sulfur compounds are released
The atmosphere from the combustion of fossil fuels, such as coal and oil, and industrial processes, including the burning of sulfur-containing materials. These sulfur compounds undergo chemical reactions in the atmosphere, combining with oxygen and water vapor to form sulfuric acid.Nitric acid is produced when nitrogen oxides (NOx), primarily nitrogen dioxide (NO2), react with water vapor in the atmosphere. Nitrogen oxides are released from various sources, including vehicle emissions, power plants, and industrial processes. These nitrogen oxides undergo atmospheric reactions, leading to the formation of nitric acid.
To know more about reactions visit :
https://brainly.com/question/16737295
#SPJ11
Pls Help! one multiple-choice question for 13 points and brainliest!
Where does the energy from sunlight go in photosynthesis?
a) covalent bonds in carbon dioxide
b) covalent bonds in glucose
c) covalent bonds in oxygen
d) polar covalent bonds in water
Answers
Answer:
B
Explanation:
They use energy from sunlight to synthesise glucose from carbon dioxide and water.
If the volume of a gas is decreased by changing the shape of the container, the number of collisions per area between the molecules and the container walls will increase. This corresponds to an increase in the gas pressure, which is the force exerted per area. What gas law is kinetic molecular theory of gases (KMT) explaining in this case
Answers
Answer:
If the volume of a gas is decreased by changing the shape of the container, the number of collisions per area between the molecules and the container walls will increase. This corresponds to an increase in the gas pressure, which is the force exerted per area.
PLEASE HELP!
Which of the following has greater entropy?
A) NaCI (s) or NaCI (aq)
B) MgS (s) or MgF2 (s)
C) Ne (g) in a 1L container or Ne (g) in a 2L container
Answers
Answer:
c
Explanation:
i think soo. but not sure.
How many grams are in 9.05 x 1023 atoms of silicon
Answers
Answer:
42.2075 grams
Explanation:
which chemical equation represents a redox reaction?

Answers
Answer:
B if i'm not mistaken.
Explanation:
What evidence do we have that atoms have nuclei with a relatively small size compared to the entire atom
Answers
The evidence for atoms having nuclei with a relatively small size compared to the entire atom comes from a variety of sources.
One of the most important is the fact that atoms are largely empty space. If the nucleus were a significant fraction of the size of the entire atom, we would expect to see much less empty space in the structure of matter than we actually observe.
Additionally, experiments using X-rays and other high-energy particles have shown that these particles are scattered by the electrons in atoms, indicating that the electrons occupy a relatively large space compared to the nucleus.
Finally, studies of atomic spectra have revealed that certain lines in the spectra correspond to transitions between energy levels in the nucleus, suggesting that the nucleus is indeed a small, highly concentrated region within the atom.
To know more about atoms click on below link :
https://brainly.com/question/31625450#
#SPJ11
draw the major organic product of the bimolecular substitution and use curved‑arrow notation to draw the mechanism. be sure to draw any non‑bonding electrons.
Answers
Here, we have to draw the main organic products of a given bimolecular substitution reaction and the curved arrow mechanism of the reaction.
(Answer is attached in the picture)
Substitution reactions involve the replacement of one functional group in a molecule by another functional group. Nucleophilic substitution reactions involve a nucleophilic reagent with a substrate that has a positively or partially positively charged portion of the molecule (electrophile).
An electrophile is an atom or molecule that lacks electrons. In organic reactions, electrophiles act as electron acceptors (Lewis's acids). These reagents can be cations or neutral molecules that have relatively positively charged atoms.
Meanwhile, a nucleophile is an atom or molecule that is rich in electrons. The nucleophile has an electron pair that can be donated (a Lewis base). Some nucleophiles are neutral molecules that have a PEB and some are negatively charged. In a chemical reaction, electrons from the nucleophile strike the electrophile center to form new bonds as a result of the reaction.
On the question:
Use of curved arrows to indicate: A broken or formed bond.
Electron layer protective layer arrow
In the reaction of this problem, I replaced Br (Substitution) and Br bonded with Na⁺.
The nucleophile "replaces" the leaving group.
Called a substitution reaction: I replace Br (change places).
Learn more about substitution reactions at https://brainly.com/question/10143438.
#SPJ4


If 2.2 moles of calcium oxide are decomposed, how many moles of oxygen are produced?
2CaO --> 2Ca + O2
Answers
Answer: Since the reaction is 2Ca(NO3)2 = 2CaO + 4NO2 + O2
1) - given the stoichiometric coefficients, we know that 2 moles of Ca(NO3)2 will produce 4 moles of NO2, hence, 1 mole will produce 2 moles of NO2
2) - 328 g produces 22.4 L, since One mole of any gas at S.T.P. occupies the same volume which is 22.4 L.
Hence, 65.6 g produces (65.6*22.4)/328 = 4.48 L
3) - 328 g produces 112 g CaO. Therefore, 65.6 g produces = (65.6*112)/328 = 22.4 g CaO.
4) - Given the stoichiometric coefficients, we know 5 moles of gaseous products are already being produces ( 4+1) by 2 moles of reactant.
5) - 44.8 L at STP = 2 moles of NO2, since One mole of any gas at S.T.P. occupies the same volume which is 22.4 L.
Hence, to produce 2 moles of NO2, we need 1 mole of reactant = 164 g
Hope this helps :)
Explanation:
Imagine you are looking at a bottle of salad dressing containing oil, vinegar, and water. Why are there two layers in the salad dressing? Water and vinegar are both hydrophobic and mix, whereas the oil is hydrophilic. Vinegar and oil are both hydrophobic and mix, whereas the water is hydrophilic. Water and vinegar are both hydrophilic and mix, whereas the oil is hydrophobic. Vinegar dissolves into both the oil and the water in this container.
Answers
Answer:
Oil and vinegar separate because it is a suspension
Explanation:
When you let suspensions sit the particles begin to layer out, in the salad dressing the oil layers out on top of the vinegar. The two layers of oil and vinegar don't actually dissolve in each other. The layer with the lower density (oil) floats on top of the layer with a higher density (vinegar)
Water and vinegar are both hydrophilic and mix, whereas the oil is hydrophobic. Oil and vinegar separate because it is a suspension
What is the difference between hydrophilic and hydrophobic molecules ?Hydrophilic molecule are water-loving polar molecule.
They interact with water or they are dissolved in water or water like polar solvent.
For instance, the phospholipid molecules of the plasma membrane has a hydrophilic phosphate group.
Hydrophobic is a water hating substance which can not dissolve in water, but other non-polar solvent.
Thus hydrophobic substances are lipophilic in nature and the hydrophobic solvents are used to separate water-immiscible substances from water.
The layers of oil and vinegar can not dissolve with each other. The oil layer has the lower density floats on top of the layer with a higher density product called vinegar.
Learn more about hydrophilic molecule, here:
https://brainly.com/question/4692308
#SPJ2
Mass = 35g
Density = 5 g/cm3
∙What is the Volume?
Answers
Answer:
7cm3
.........
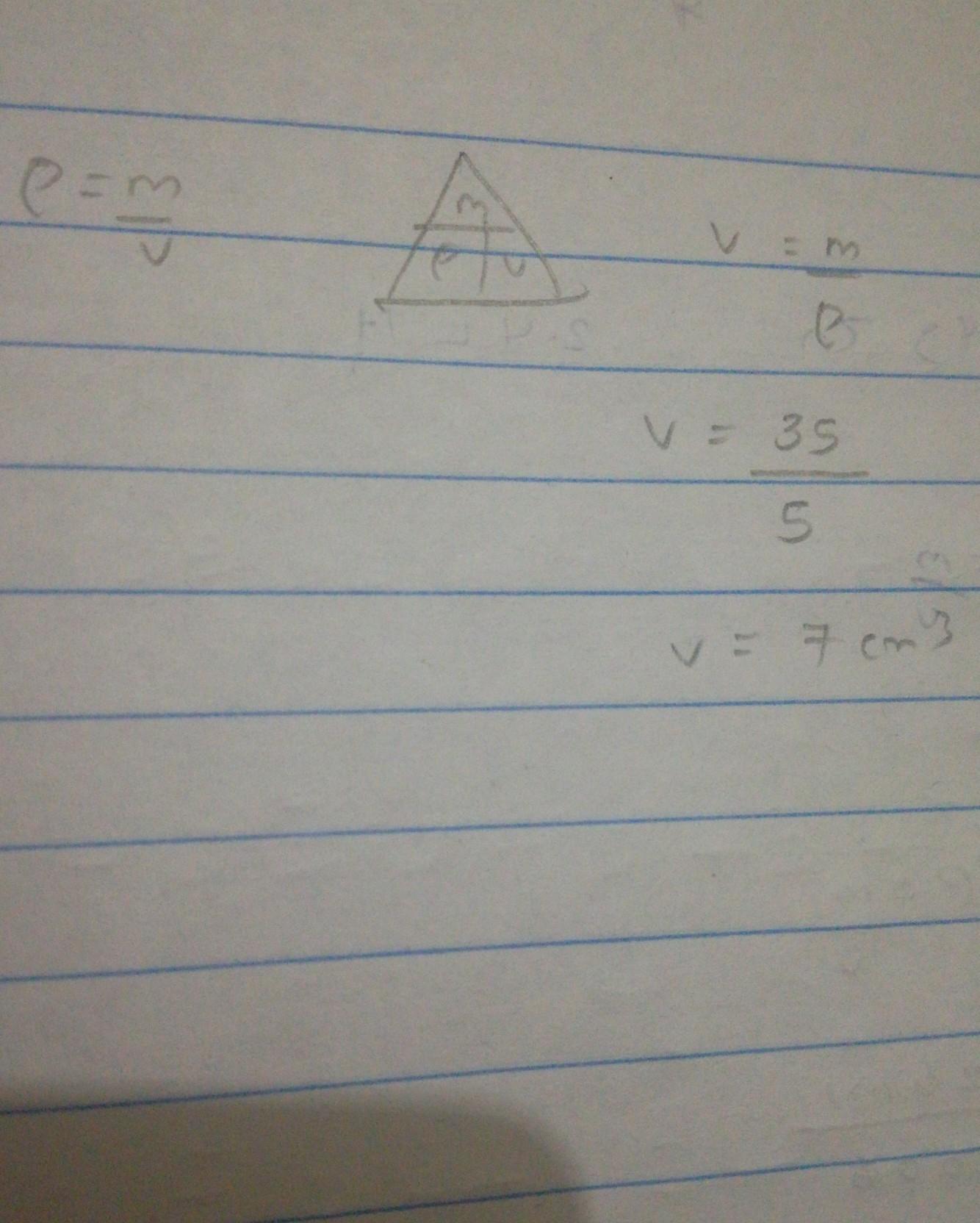
how often a wave passes a single point per second is called the ____.
A trough
B. frequency
C. crest
D. amplitude
Answers
Which of the following is a compound?
A. Zinc
B. S8
C. CaCl2

Answers
C. Calcium is your best bet. Calcium chloride is amongst inorganic compounds.
What is the change in temperature if an object goes from 273 k to 243 k?
Answers
Since 273 k – 243 k equals 30, the temperature change for an object going from 273 k to 243 k is 30 k.
We use 273 in Kelvin because?Colors and temperatures can both be measured in Kelvin: A temperature unit commonly used in science is the Kelvin scale. Lord Kelvin created the scale after realizing the need for one with absolute zero as the zero point, which translates to 0 K as the lowest attainable temperature, or -273,15 °C.
Can Kelvin scale be negative?On the Kelvin scale, a body's temperature cannot be negative. Since the Kelvin scale's absolute zero represents the lowest temperature that may exist, it is impossible for it to be negative.
To learn more about Kelvin scale visit:
brainly.com/question/29151720
#SPJ4
students of different ages were given the same puzzle to assemble. The puzzle assembly time was measured.
what is the independent variable?
what is thw dependent variable?
what is the constant?
Answers
Answer:
1. Age
2. Time
3. Same Puzzle
Explanation: Independent variable are what changes in the experiment and allow you to get a result. Dependent variables measure what you are trying to find from an experiment. Constants are what remain the same throughout all trials.
why is dilute hydrochloric acid added into distilled water?
Answers
Answer:
Explanation:
dilute hydrochloric acid is added to distilled water to increase the ionization of water.
A wind up toy that is wound up and moving
Answers
Answer:
A wind-up toy is wound up and moving across level ground. The toy is speeding up.
Explanation:
this will or should helpyou
When an organism eats food, energy becomes available for metabolic activities. In the process, some energy enters the environment as heat. According to the Law of Conservation of energy …a) energy which is given off as heat is not part of the total energy budget of the system b) some energy is lost; as no metabolic process is 100% efficient c) no energy is lost; the total amount of energy remains the samed) energy which is not immediately used by the organism cannot be stored and is lost
Answers
Answer
C) no energy is lost; the total amount of energy remains the same.
Explanation
The law of conservation of energy states that "The energy can neither be created nor destroyed - only converted from one form of energy to another." Therefore some energy will be used for metabolism while the other can be used for other properties and some can be stored.
A sample of gas at 53.0 oC and 1.19 atm occupies a volume of 2.3 L. What volume would this gas occupy at 107 oC and 0.60 atm?
Answers
Answer:
\(V_2=5.32L\)
Explanation:
Hello there!
In this case, according to the given pressure, temperature and volume, it is possible for us to calculate the final volume via the combined ideal gas law:
\(\frac{P_2V_2}{T_2}=\frac{P_1V_1}{T_1}\)
In such a way, we solve for the final volume, V2, to obtain:
\(V_2=\frac{P_1V_1T_2}{T_1P_2}\)
Then, we plug in the given data to obtain:
\(V_2=\frac{1.19atm*2.3L*380K}{326K*0.60atm}\\\\V_2=5.32L\)
Regards!
how many mililiters is 0.55 L
Answers
Answer:
It's 550 milliliters.
Explanation:
I hope this helps.
liquidus line separates which of the following combinations of phase fields? a) alpha and alpha+beta b) Liquid and Liquid + alpha c) alpha and Liquid + alpha d) Liquid +alpha and alpha+beta
Answers
The liquidus line separates the following combinations of phase fields: Liquid and Liquid + alpha. The correct option is b.
What is a phase field? A phase field is a technique for representing the microstructure of materials. It is used in materials science, mathematics, and computer science to simulate and study the behavior of materials in the solid and liquid phases. It is a multi-component field that contains information on the concentration of various components, their phase, and the local temperature, as well as other relevant variables.
The liquidus line is defined as the boundary between the liquid phase field and the field that includes both the liquid and the alpha phase. As a result, the liquidus line separates the following combinations of phase fields: Liquid and Liquid + alpha.
So, the correct option is b) Liquid and Liquid + alpha.
Learn more about Liquidus at https://brainly.com/question/31486571
#SPJ11
Where v stands for __________________________________ and the units are meters per second (m/s).
Answers
Answer:
Velocity
Explanation:
Answer: velocity I’m sure
Explanation:
please help!!!!!!!!!

Answers
Answer:
H2O (2x1.008 + 1x15.999) = 18.015 g/mol
CO2 (1x12.011 + 2x15.999) = 44.01 g/mol
BF3 (1x10.81 + 3x18.998) = 67.81 g/mol
K2O (2x39.098 + 1x15.999) = 94.20 g/mol
BaCO3 (1x137.33 + 1x12.011 + 3x15.999) = 197.34 g/mol