Calculate the percentage of copper in copper oxide
Answers
Well, look up the atomic weights of copper and oxygen; add them appropriately; divide the total for the copper by the total for the molecule; then multiply by 100 to get it as a percentage.
Related Questions
Which is a characteristic of the part of the atom marked “A”?
•it contains most of the mass
•it is negatively charged
•it is mostly empty space
•it is composed of electrons
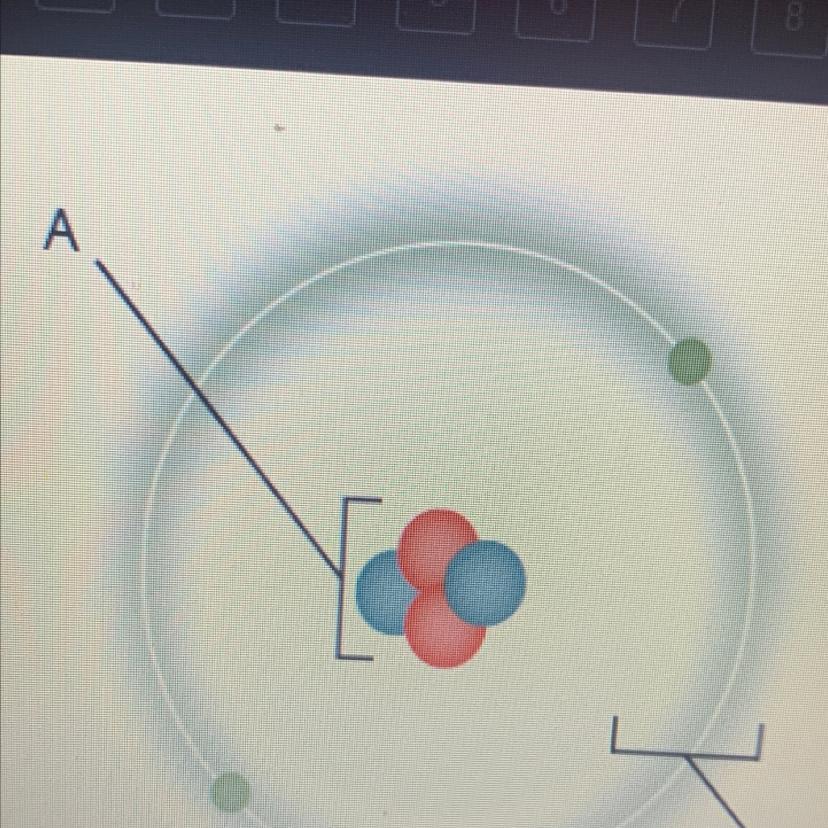
Answers
Answer:
it contains most of the mass
Using the dimensional Analysis
In the Above questions to convert 14,612 mL to DAL which of the following portrays to correct set up

Answers
Option A displays the accurate dimensional analysis for converting 14,612 mL to daL.
Determining the value of measurement across many units is crucial in many situations.This means that we must convert from one unit to another. The application of a conversion factor enables such a conversion.Now that we are aware of a conversion factor, we can utilize it to change mL into daL. In order for the units to cancel out, this conversion factor needs to be dimensionally precise in its arrangement.Option A displays the accurate dimensional analysis for converting 14,612 mL to daL.Learn more about unit conversion at:
brainly.com/question/11543684
#SPJ9
The half-life of a reaction of the first order completes in 10 minutes. How much time will be needed for the 80% completion of this reaction?
Answers
A first-order reaction refers to a reaction in which the rate of the reaction is directly proportional to the concentration of a single reactant raised to the first power and is expressed as it would take approximately 46.4 minutes for the 80% completion of this first-order reaction to occur. 46.4 minutes.
According to the given information:Rate = k[A]
In this equation, k is the reaction rate constant, and [A] represents the concentration of reactant A.
The half-life of a reaction of the first order completes in 10 minutes. We need to find out how much time will be needed for the 80% completion of this reaction.
To solve for the time needed for 80% completion of a reaction of the first order, we need to use the formula:
Time for 80% completion = 2.303/k x log ([A]₀/[A]t)
where k is the reaction rate constant, [A]₀ is the initial concentration of the reactant and [A]t is the concentration of the reactant after the given time t, and 2.303 is a conversion factor.
Let [A]₀ = 1 and [A]t = 0.2 (since 80% completion means 20% of the original concentration remains)
We know that, t1/2 = 10 min;
therefore, k = 0.693/t1/2
= 0.693/10
= 0.0693 (as 0.693 = ln2)Now,
Time for 80% completion
= 2.303/k x log ([A]₀/[A]t)
= 2.303/0.0693 x log(1/0.2)
= 46.4 minutes
Therefore, it would take approximately 46.4 minutes for the 80% completion of this first-order reaction to occur. 46.4 minutes.
To know more about first-order reactions visit:
https://brainly.com/question/15909753
#SPJ11
Dante loves taking care of the chickens on his farm. He feeds them rice everyday. His Grandma told him that he should feed them corn everyday instead of rice because it will make them grow fatter.
He decides to do an experiment to test what type of food will make his chickens fatter. Before the experiment, the chickens weigh an average of 5 pounds. David makes two groups of chickens. He feeds Group A rice everyday, and feeds Group B corn everyday. After one month, David weighs the chickens to see if they gained any weight. Group A weighs an average of 5 pounds and group B weighs an average of 7 pounds. What is the test variable (independent variable) in this experiment? *
Answers
Answer:
The independent variable in this experiment is what he feeds the chickens (rice or corn).
Explanation:
The independent variable is the variable the experimenter manipulates or changes.
When placing test tubes in the centrifuge, what are the best practices?a. Carefully stopper the test tubes to ensure the liquids do not spill out. b. Place test tubes containing approximately equal volumes in opposite positions on the rotor. c. Wait until all positions on the rotor contain a test tube before activating the centrifuge. d. Never put stoppers or other items in the centrifuge with your tubes.
Answers
When placing test tubes in the centrifuge, the best practices are the correct option are :
b. Place test tubes containing approximately equal volumes in opposite positions on the rotor.
d. Never put stoppers or other items in the centrifuge with your tubes.
The centrifuge is the device used in the laboratories to separate the materials according to the sedimentation coefficient. When we are operating the centrifuge, the students should careful that the lid is shut, and the safety knob is turned.
Thus, the best practices when placing test tubes in the centrifuge are to place the test tubes containing equal volumes in the opposite positions and never put the stoppers in the centrifuge.
To learn more about test tubes here
https://brainly.com/question/14865029
#SPJ4
Which of the following is true about tests we can perform on a mineral?
a) a softer mineral will scratch a harder mineral
b) many common minerals react (effervesce) with dilute hydrochloric acid
c) a streak plate is used to determine how hard a mineral is
d) many minerals are strongly magnetic
e) none of these
Answers
Answer:
The tests we can perform on a mineral are b) many common minerals react (effervesce) with dilute hydrochloric acid
Explanation:
This statement is true. Dilute hydrochloric acid is commonly used to test the reactivity of minerals. When certain minerals come into contact with hydrochloric acid, they can undergo a chemical reaction that produces effervescence, or the release of bubbles of gas. This reaction occurs due to the presence of carbonate minerals, such as calcite (CaCO3), which react with the acid to form carbon dioxide gas (CO2). The effervescence is a useful characteristic in identifying certain minerals.
It is important to note that not all minerals react with dilute hydrochloric acid. Minerals that lack carbonates will not show any effervescence when exposed to the acid. Therefore, the absence of effervescence does not necessarily indicate that a mineral is not present; it simply suggests that the mineral does not contain carbonates.
Learn more about minerals here, https://brainly.com/question/15844293
#SPJ11
Prediction is increasing the amount of reactant particles present increases the rate of a reaction then an increase in the concentration of reactants in a period. Which of the following best describes this prediction
Answers
The best description for the prediction that increasing the concentration of reactants increases the rate of a reaction is that an increase in the concentration of reactants leads to a higher reaction rate.
When the concentration of reactants is increased, there are more reactant particles available in the reaction mixture. This increases the frequency of collisions between the reactant particles, leading to a higher probability of successful collisions and therefore an increased rate of reaction.
According to the collision theory, for a reaction to occur, reactant particles must collide with sufficient energy and with the correct orientation. By increasing the concentration of reactants, the chances of effective collisions are increased, as there are more reactant particles in close proximity to each other. This results in a higher reaction rate. Therefore, the prediction states that increasing the concentration of reactants will increase the rate of the reaction.
To learn more about reactants click here : brainly.com/question/30129541
#SPJ11
what are the two ways in which the physical state of matter can be changed
Answers
The two ways in which the physical state of matter can be changed are melting and freezing.
Melting is the process by which a solid substance transitions to a liquid state. As a result, the energy added to the solid substance causes the molecules to vibrate at a higher rate. As a result, the heat breaks the bonds between the molecules, allowing them to flow freely.Freezing is the process by which a liquid substance transitions to a solid state. As a result, energy is removed from the liquid substance. The molecules in the substance are moving quickly, but when energy is removed, they slow down.Because of the decrease in energy, the molecules can no longer slide past one another and form a rigid structure, resulting in a solid state of matter.For such more questions on physical state
https://brainly.com/question/30214939
#SPJ8
Write method of manufacturing of oxygen gas from liquid air
Answers
Answer:
cryogenic distillation process
Explanation:
Cryogenic distinction between carbohydrates and harmon process.
how many molecules of HI are needed to produce 72.54g of BaL2? Equation: BaSO4+HI=Bal2+H2SO4
Answers
The number of molecules of HI that are needed to produce 72.54g of BaL2 is6.365 x 10^23.
How to find the molecules needed?To determine the number of molecules of HI needed to produce 72.54g of BaL2, we need to first find the moles of BaL2 and then use the balanced chemical equation to find the moles of HI.
First, we'll find the moles of BaL2:
72.54 g of BaL2 / 137.3 g/mol = 0.529 moles of BaL2
Next, we'll use the balanced chemical equation to find the moles of HI:
BaSO4 + 2HI -> BaL2 + H2SO4
From this equation, we can see that for every 2 moles of HI that react, 1 mole of BaL2 is produced. Therefore, to produce 0.529 moles of BaL2, we need 0.529 x 2 = 1.058 moles of HI.
Finally, we'll convert the moles of HI to the number of molecules:
1.058 moles of HI x 6.022 x 10^23 molecules/mole = 6.365 x 10^23 molecules of HI.
Therefore the number of molecules of HI needed to produce 72.54g of BaL2 is approximately 6.365 x 10^23.
Learn more about molecules here:https://brainly.com/question/26044300
#SPJ1
what is the ph of a calcium hydroxide solution obtained by dissolving 0.40 grams of calcium hydroxide in enough water to obtain 580. ml of solution?
Answers
The pH of a calcium hydroxide solution was obtained by dissolving 0.40 grams of calcium hydroxide in enough water to obtain 580. ml of solution is 11.8.
Calcium hydroxide is a strong base that is commonly used in various industries to neutralize acidic wastewater. Calcium hydroxide is commonly known as slaked lime, milk of lime, or hydrated lime. Calcium hydroxide is used in the following industries: steel, petroleum, water treatment, construction, and agriculture.
Here's how to solve the problem: First, find the number of moles of calcium hydroxide in the solution. A number of moles = (mass of solute) ÷ (molar mass)The molar mass of calcium hydroxide is 74.1 g/mol. The number of moles of calcium hydroxide = (0.40 g) ÷ (74.1 g/mol)= 0.00540 mol. Now, calculate the concentration of calcium hydroxide in the solution. Concentration (molarity) = (number of moles of solute) ÷ (volume of solution in L)The volume of the solution in liters is 580 mL or 0.580 L.Concentration (molarity) = (0.00540 mol) ÷ (0.580 L)= 0.00931 MFinally, calculate the pH of the solution using the pOH formula: pOH = -log[OH-]pOH = -log[0.00931]pOH = 2.03pH + pOH = 14pH + 2.03 = 14pH = 11.8Therefore, the pH of the calcium hydroxide solution is 11.8.
Read more about calcium:
https://brainly.com/question/26636816
#SPJ11
Which one is a single replacement reaction? (Whoever gets it correct first I’ll mark)

Answers
The equation that represents a single replacement reaction given the various options is 2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g) (option 2)
What is a single replacement reaction?A single replacement reaction, also known as single displacement reaction is a reaction in which elements higher in the electro-chemical series displace or replace elements lower in the electro-chemical series displace from a solution.
The following example illustrates single replacement reaction:
A + BC -> AC + B
From the above reaction, we can see that A has replace/displace B to from AC.
With the above information, we can determine the equation that represents single replacement reaction. Details below:
Equation from the questions:
2Al + 3Cl₂ -> 2AlCl₃2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g)2AlCl₃(aq) -> 2Al + 3Cl₂ AlCl₃ + 3KOH -> Al(OH)₃ + 3KClFrom the above, we can see that only 2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g) conform to single replacement reaction.
Thus, the correct answer to the question is: 2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g) (option 2)
Learn more about single replacement reaction:
https://brainly.com/question/29662825
#SPJ1
An object has a mass of 60 ounces and a volume of 30 cups. What is the density? _______ oz/cup (your answer should *only* be a number, round to one decimal place if needed) *
Answers
Answer:
2.0
Explanation:
Given data:
Mass of object = 60 ounce
Volume of object = 30 cups
Density = ?
Solution:
d = mass/ volume
d = 60 ounce/ 30 cup
d = 2.0 oz/cup
Thus, density of object is 2.0 oz/cup.
How many moles are represented by 3.01 x10^24 oxygen atoms?
Answers
5.00 mol O₂
General Formulas and Concepts:Math
Pre-Algebra
Order of Operations: BPEMDAS
Brackets Parenthesis Exponents Multiplication Division Addition Subtraction Left to RightChemistry
Atomic Structure
Avogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.Stoichiometry
Using Dimensional AnalysisExplanation:Step 1: Define
3.01 × 10²⁴ atoms O₂
Step 2: Identify Conversions
Avogadro's Number
Step 3: Convert
Set up: \(\displaystyle 3.01 \cdot 10^{24} \ atoms \ O_2(\frac{1 \ mol \ O_2}{6.022 \cdot 10^{23} \ atoms \ O_2})\)Multiply/Divide: \(\displaystyle 4.99834 \ mol \ O_2\)Step 4: Check
Follow sig fig rules and round. We are given 3 sig figs.
4.99834 mol O₂ ≈ 5.00 mol O₂
Avagadros Law..
Definition please..
Answers
Answer:
"Avogadro's law is an experimental gas law relating the volume of a gas to the amount of substance of gas present. The law is a specific case of the ideal gas law. A modern statement is: Avogadro's law states that "equal volumes of all gases, at the same temperature and pressure, have the same number of molecules."
Answer:
Avogadro's law states that the equal volume of all gases at the same temperature and pressure contain the same number of molecule
How many milliliters of 0. 250M NaOH is required to neutralize 30. 4mL of 0. 152M HCl?
Answers
Approximately 18.4832 mL of 0.250 M NaOH is required to neutralize 30.4 mL of 0.152 M HCl.
To determine the volume of 0.250 M NaOH required to neutralize 30.4 mL of 0.152 M HCl, we can use the concept of stoichiometry and the balanced chemical equation for the neutralization reaction between NaOH and HCl:
NaOH + HCl -> NaCl + H2O
From the balanced equation, we can see that the stoichiometric ratio between NaOH and HCl is 1:1. This means that for every mole of NaOH, we require an equal number of moles of HCl to neutralize.
First, let's calculate the number of moles of HCl present in the given volume:
Moles of HCl = concentration of HCl * volume of HCl
= 0.152 M * 30.4 mL
= 4.6208 mmol (millimoles)
Since the stoichiometric ratio is 1:1, the number of moles of NaOH required to neutralize the HCl is also 4.6208 mmol.
Now, let's calculate the volume of 0.250 M NaOH needed to contain 4.6208 mmol:
Volume of NaOH = (moles of NaOH) / (concentration of NaOH)
= 4.6208 mmol / 0.250 M
= 18.4832 mL
Therefore, approximately 18.4832 mL of 0.250 M NaOH is required to neutralize 30.4 mL of 0.152 M HCl.
learn more about NaOH here
https://brainly.com/question/20573731
#SPJ11
In the lab you take 50.0 mL of a 40.0 M NaOH solution and then add water to it until it reaches the 500.0 mL mark of the flask. What is the concentration of the new solution?
Answers
Answer:
4.00M NaOH
Explanation:
A 50-ml amount of 40.0M NaOH contains
(40.0 mol NaOH/1 L)×(0.0500L) = 2.00 mol NaOH
Adding enough water to make a 500.0 mL NaOH solution, the new concentration of this solution is
2.00 mol NaOH/0.5000 L = 4.00M NaOH
A car is traveling at 88 ft/sec. What is the car's speed in miles/hour?
Answers
Trinity conducted a science experiment using household products. She put a drop of a substance on a piece of litmus paper and it turned blue. What is the most probable substance that trinity tested?.
Answers
The most probable substance that trinity tested is an alkali.
How do you pass a litmus test?
To test at least nine different compounds, cut each piece of litmus paper into three smaller pieces. Take one tiny piece of red litmus paper. Dip it into a sample of the chemical being tested. Put each observation in a proper table. Then put the litmus paper scraps in the trash can.
When the pH is below 4.5 and above 8.3, the litmus paper becomes red. When the paper turns purple, the pH level is almost neutral. If the red paper doesn't change color, the sample is an acid. If the color of the blue paper does not change, the sample serves as a base.
To learn more about alkali use link below:
https://brainly.com/question/28745372
#SPJ4
How do the isotopes carbon-12 and carbon-13 differ?
Answers
Explanation:
Carbon exists in several isotopes. ... Carbon has the atomic number of 6 which means that all isotopes have the same proton number. However, the number of neutrons is different, thus giving different mass numbers. Carbon-12 has 6 neutrons, carbon-13 has 7 neutrons, and carbon-14 contains 8 neutrons.
Answer:
They have different number of neutrons.
Explanation:
Which of the following statements accurately describes a fission reaction?
A) More energy is required to split the nucleus of an atom than the energy actually released during a fission reaction.
B) A fission reaction occurs when two lighter nuclei combine to form a heavier nucleus.
C) The total mass of the products of a fission reaction is often slightly less than the total mass of the original nucleus.
Answers
The statement that accurately describes a fission reaction is that more energy is required to split the nucleus of an atom than the energy actually released during a fission reaction. That is option A.
What is a fission reaction?Fission reaction is defined as the disintegration of a heavy unstable nucleus into two or more smaller nuclei with the release of energy.
The energy required to split the unstable nucleus is greater than the one released because heavy elements are involved.
Therefore, the statement that accurately describes a fission reaction is that more energy is required to split the nucleus of an atom than the energy actually released during a fission reaction.
Learn more about nuclear reactions here:
https://brainly.com/question/984564
#SPJ 1
4. For a typical vertebrate cell with a membrane potential of 0.050 V (inside negative), what is the free-energy change for transporting 1 mol of Ca+2 from the cell into the blood at 37 °C? Assume the concentration of Ca+2 inside the cell is 145 mM and in blood plasma it is 25 mM. Does this transport take place spontaneously or not? (R= 8.315 J/mol.K)
Answers
Free energy change for transporting Ca2+ ions is calculated as follows:∆G = RT ln ([Ca2+]outside/[Ca2+]inside)∆G = 8.315 J/mol.K x 310 K x ln (25 mM/145 mM) = -15,400 J/mol.
Here, ∆G is negative, which implies that Ca2+ ions transport spontaneously from the cell to blood. This is because the free energy of the system decreases when Ca2+ ions move from high concentration to low concentration. Therefore, transporting Ca2+ ions is energetically favorable.
To know more about calculated visit:
https://brainly.com/question/30781060
#SPJ11
what volume of 0.500-m koh(aq) should be added to 100 ml of a buffer solution initially containing 0.13 mol hf (ka
Answers
The volume of 0.500-M KOH(aq) should be added to 100 ml of a buffer solution initially containing 0.13 mol HF and 0.16 mol of NaF is 100 mL of 0.500 M KOH. ka = 6.8 × 10⁴ and pH is 3.59
given that :
ka = 6.8 × 10⁴
and pH is 3.59
moles of HF = 0.13 mol
moles of NaF = 0.16 mol
the pH formula is given as :
pH = pka + log [base]/[acid]
pH = -log(6.8 × 10⁻⁴) + log (0.16 ) / 0.13
pH = 3.33 + 0.0899
pH = 3.5
Thus, the volume of 0.500 M KOH should be added to a buffer solution 0.500 M.
To learn more about buffer solution here
https://brainly.com/question/24262133
#SPJ4
what mass of propane could burn in 48.0 g of oxygen
Answers
Which of the following terms is a chemical substance made of a single type of atom that cannot be broken down into a simpler substance?
A- nucleus
B- molecule
C- Compound
D- Element
Answers
Explanation:
Elements are made of a single type of atom and cannot be broken down any smaller.
Which of the following statement is true about the following reaction?
3NaHCO3 ---> 3CO2+ 3H2O + Na3C6H5O7
A) 22.4 L of CO2 are produced for every liter of Na3C6H5O reacted
B) 3 moles of water is produced for every 3 moles of carbon dioxide
C) 51g of water is produced of every mole of Na3C6H5O7
Answers
The following statement is true about the given reaction:The statement that is true about the given reaction is:"51g of water is produced for every mole of Na3C6H5O7.
The reaction is given as:Na3C6H5O7 + 3HCl → 3NaCl + C6H5O7H2 + H2OIn the given reaction,Na3C6H5O7 and HCl react to give NaCl, C6H5O7H2, and H2O. To determine the mole of H2O formed, we need to balance the chemical reaction equation.The balanced equation for the given reaction is:Na3C6H5O7 + 3HCl → 3NaCl + C6H5O7H2 + 4H2OFrom the balanced equation, we can infer that 4 moles of H2O is produced for every mole of Na3C6H5O7.So, the correct statement is:"51g of water is produced for every mole of Na3C6H5O7."For such more question on mole
https://brainly.com/question/29367909
#SPJ8
A 5.25 gram sample of an unknown metal was placed in a water-bath at a temperature of 99.60 °C. Once the metal's temperature was the same as the water-bath, it was immediately transferred from the water-bath to a calorimeter containing 50.00 mL of distilled water at 11.25 °C. The final temperature of the water-metal mixture was 14.00 °C. Determine the specific heat of the unknown metal. Assume density the water is 1.000 grams/mL and the specific heat of water is 4.184 J/g °C
Answers
The specific heat of the unknown metal is approximately 1.22 J/g °C.
Given:
Mass of the metal (mmetal) = 5.25 g
Temperature change of the metal (ΔTmetal) = 14.00 °C - 99.60 °C = -85.60 °C
Mass of water (mwater) = 50.00 g
Specific heat of water (cwater) = 4.184 J/g °C
Temperature change of water (ΔTwater) = 14.00 °C - 11.25 °C = 2.75 °C
Using the equation:
mmetal × cmetal × ΔTmetal = -mwater × cwater × ΔTwater
We can rearrange the equation to solve for cmetal:
cmetal = (-mwater × cwater × ΔTwater) / (mmetal × ΔTmetal)
Substituting the given values:
cmetal = (-50.00 g × 4.184 J/g °C × 2.75 °C) / (5.25 g × (-85.60 °C))
Simplifying the expression:
cmetal = (-556.0 J) / (-454.20 J)
cmetal ≈ 1.22 J/g °C
Therefore, the specific heat of the unknown metal is approximately 1.22 J/g °C.
Know more about Specific Heat here:
https://brainly.com/question/31608647
#SPJ11
What type of intermolecular force is H3PO4?
Answers
The type of intermolecular force on Phosphoric Acid (H₃PO₄) is Hydrogen bonds.
Hydrogen bonds are a type of force between atoms that create a strong attraction between them. This attraction is due to the way the electrons of the hydrogen atoms orbit around the atoms with lone pairs.
Phosphoric acid (H3PO4) contains three H+ ions, which do not have the same acid strength as these ions. Phosphoric acid can form three types of salts according to the replacement of one, two, or three hydrogen atoms.
The phosphoric acid molecule has 7 bonds. There are 4 non-H bonds, 1 double bond, 1 double bond, 3 hydroxyl groups, and 1 phosphate/thiophosphate.
Learn more about intermolecular force at https://brainly.com/question/25920008
#SPJ4
balancing equations v 1) _____ al _____ zncl2 → _____ alcl3 _____ zn
Answers
The balanced equation for the reaction between aluminum and zinc chloride is 2 Al + 3 ZnCl2 → 2 AlCl3 + 3 Zn.The balanced equation is as follows:
2 Al + 3 ZnCl2 → 2 AlCl3 + 3 Zn
In the given unbalanced equation, we have aluminum (Al) reacting with zinc chloride (ZnCl2). To balance the equation, we need to ensure that the number of atoms on both sides of the equation is equal.
On the left side, we have one aluminum atom (Al), while on the right side, we have two aluminum atoms in aluminum chloride (AlCl3). To balance the aluminum atoms, we place a coefficient of 2 in front of Al on the left side.
Next, we move on to the chlorine atoms. On the left side, we have two chlorine atoms in zinc chloride (ZnCl2). On the right side, we have six chlorine atoms in aluminum chloride (AlCl3). To balance the chlorine atoms, we place a coefficient of 3 in front of ZnCl2 on the left side.
After balancing, we have 2 Al + 3 ZnCl2 → 2 AlCl3 + 3 Zn.
This balanced equation shows that two moles of aluminum react with three moles of zinc chloride to produce two moles of aluminum chloride and three moles of zinc.
In conclusion, the balanced equation for the reaction between aluminum and zinc chloride is 2 Al + 3 ZnCl2 → 2 AlCl3 + 3 Zn.
To know more about reaction, visit:
brainly.com/question/30464598
#SPJ11
give me the best pick up line
Answers
Answer:
There is something wrong with my cell phone. It doesn't have your number in it.
Explanation: