calculate the volume in liters of a 0.31 mol/l copper(ii) sulfate solution that contains 150. g of copper(ii) sulfate
Answers
The volume in liters of a 0.31 mol/l of copper(ii) sulfate solution that contains 150. g of copper(ii) sulfate is
Explanation:
A 0.31mol/L copper (II) sulfate solution, is the concentration in Molarity.
Let's convert the mass of solute into moles
Number of moles = (mass / molar mass)
150 g CuSO₄ / 159.61 g/m = 0.939 moles
Now we can apply this formula:
Molarity × Volume = Moles
0.31 mol/L × Volume = 0.939 moles
⇒ Volume = 0.939 moles / 0.31 mol/L = 3.02 L
Hence, the volume of the solution is 3.02 L.
Learn more about volume from the link given below.
https://brainly.com/question/17268822
#SPJ4
Related Questions
4.What type of energy is used to fuel the process of photosynthesis and what type is produced in
respiration?
A Light energy is used in photosynthesis and created by respiration.
B ATP is used in photosynthesis, which allows the plant to undergo respiration.
C Light energy is used in photosynthesis, whereas respiration creates the energetic product, ATP.
D Food energy is used in photosynthesis and ATP is produced in respiration.

Answers
Answer:
A. light energy is used in photosynthesis and created by respiration
What mass of HI should be present in 0.250 L of solution to obtain a solution with each of the following pH's?
1) pH=1.30
2)pH=1.70
3)pH=2.85
Answers
The mass of HI should be present in 0.250 L of solution to obtain a solution with each of the following pH's are given below:
1) pH=1.30: The pH of HI solution can be determined by the formula:
pH = -log [H+]
First, let's find the hydrogen ion concentration [H+]: pH = 1.30 => [H+] = 10^-1.30 = 5.01 × 10^-2M
The balanced equation for the ionization of HI is-H+ + I The number of moles of HI present in solution is found from the hydrogen ion concentration:
5.01 × 10^-2 M = moles of HI / 0.250 L moles of HI = 1.25 × 10^-2 L
The mass of HI in grams can be calculated as:
m = moles of HI × molar mass of HI = 1.25 × 10^-2 mol × 127.9 g/mol = 1.60 gSo, 1.60 g of HI should be present in 0.250 L of solution to obtain a solution with a pH of 1.30.
2) pH=1.70: The hydrogen ion concentration [H+] is calculated as:
pH = 1.70 => [H+] = 10^-1.70 = 1.99 × 10^-2 M
The moles of HI is calculated as:
1.99 × 10^-2 M = moles of HI / 0.250 L moles of HI = 4.97 × 10^-3 mol
The mass of HI in grams can be calculated as:
m = moles of HI × molar mass of HI = 4.97 × 1 HI →0^-3 mol × 127.9 g/mol = 0.635 g
So, 0.635 g of HI should be present in 0.250 L of solution to obtain a solution with a pH of 1.70.
3) pH=2.85: The hydrogen ion concentration [H+] is calculated as:
pH = 2.85 => [H+] = 10^-2.85 = 7.94 × 10^-3 M
The moles of HI is calculated as:
7.94 × 10^-3 M = moles of HI / 0.250 L moles of HI = 1.99 × 10^-3 mol
The mass of HI in grams can be calculated as:
m = moles of HI × molar mass of HI = 1.99 × 10^-3 mol × 127.9 g/mol = 0.255 g
So, 0.255 g of HI should be present in 0.250 L of solution to obtain a solution with a pH of 2.85.
#SPJ11
Learn more about pH equation :
https://brainly.com/question/26424076
the total energy gain of the ice cube and the resulting liquid water is blank joules
Answers
Answer:
bro
Explanation:
bro
Answer: yes
Explanation:
Because of the mass
The decomposition of N2O4 is studied at 20oC and 80oC. Which statement explains why the rate at 80oC is greater than at 20oC?
a.The activation energy is higher at 80oC.
b.The activation energy is lower at 80oC.
c.The concentration of a gas increases with increasing temperature.
d.The number of molecules with enough energy to react is greater at 80oC.
Answers
The correct statement is d. The number of molecules with enough energy to react is greater at 80oC.
This is due to the fact that at higher temperatures, the molecules of N2O4 possess more kinetic energy which increases the frequency of collisions and the proportion of molecules that exceed the activation energy required for the reaction to occur.
As a result, the rate of the reaction increases at higher temperatures. The activation energy of a reaction is the minimum energy required for a chemical reaction to occur, but it remains constant regardless of the temperature. Therefore, options a and b are incorrect.
Option c is also incorrect as the concentration of the gas does not increase with temperature, but its pressure does.
To know more about temperatures. please visit.....
brainly.com/question/31166060
#SPJ11
You plan to use the water displacement method to
determine if a ring is pure silver. Each of these tools Is
required, EXCEPT
A balance
A stopwatch
A measuring cup filled with water
A density of elements chart
Answers
Answer: A stopwatch
Explanation:
You don’t needed
A student requires all of them except a balance. The correct option is A.
What is the water displacement method?Students calculate the volume of various rods that have the same mass using the water displacement method. Each rod's density is calculated, and the distinctive densities of each material are used to distinguish the five rods.
Then, to explain why various rods have varied densities, students think about the connection between the mass, size, and arrangement of atoms. The periodic table will be briefly introduced to the class.
The volume displacement method is often used to determine the volume of an object with an irregular shape. This technique involves submerging an object into a known amount of water; as a result, the water level will rise.
Therefore, A student requires all of them except a balance. The correct option is A.
To learn more about displacement, refer to the link:
https://brainly.com/question/11934397
#SPJ5
a sample of ammonia gas occupies 20.0 ml at 585 torr and 20.0 °c. if the volume of the gas is 50.0 ml at 50.0 °c, what is the pressure?
Answers
To determine the pressure of the ammonia gas at a new volume and temperature, we can use the combined gas law, which states that the ratio of the initial pressure, volume, and temperature is equal to the ratio of the final pressure, volume, and temperature.
Using the combined gas law equation: (P1 * V1) / T1 = (P2 * V2) / T2
Given:
P1 = 585 torr (initial pressure)
V1 = 20.0 ml (initial volume)
T1 = 20.0 °C + 273.15 = 293.15 K (initial temperature)
V2 = 50.0 ml (final volume)
T2 = 50.0 °C + 273.15 = 323.15 K (final temperature)
We need to solve for P2 (final pressure).
Rearranging the equation, we have:
P2 = (P1 * V1 * T2) / (V2 * T1)
Substituting the given values into the equation:
P2 = (585 torr * 20.0 ml * 323.15 K) / (50.0 ml * 293.15 K)
Calculating this expression gives us the final pressure (P2) of the ammonia gas at the new volume and temperature.
In summary, using the combined gas law equation, we can determine the pressure of the ammonia gas at a new volume and temperature. By substituting the given values into the equation and performing the calculation, we can find the final pressure of the gas.
To learn more about ammonia click here:
/brainly.com/question/4143141
#SPJ11
(1. balancing chemical equations) why do we go through all this trouble to learn an algebraic method to balance chemical equations, instead of using the easy and simple trial-and-error method? group of answer choices it is general enough to balance any chemical equations, regardless of the complexity. this is programmable so that we can ask computer to help. we can analyze whether a reaction can actually be balanced. guess we just need to do some math anyway dr. chan just wants to make things more complicated so that he will be very happy when he sees us not knowing how to solve problems.
Answers
The reason we learn an algebraic method to balance chemical equations instead of using a trial-and-error method is because the algebraic method is more efficient and reliable.
Using trial and error can be time-consuming and may not always lead to the correct solution. Balancing chemical equations requires ensuring that the number of atoms of each element on both sides of the equation is equal. This can become complex when dealing with more complicated equations.
The algebraic method, on the other hand, provides a systematic approach that can be applied to any chemical equation, regardless of its complexity. By applying mathematical principles and using variables, we can determine the correct coefficients to balance the equation. This method is programmable, which means we can use computer algorithms to solve equations more quickly and accurately.
Additionally, the algebraic method allows us to analyze whether a reaction can actually be balanced. It helps us understand the stoichiometry of the reaction and determine the correct ratios of reactants and products.
Contrary to the belief that it is only meant to complicate things, learning this method is essential for a deeper understanding of chemistry and problem-solving skills. It enables us to apply logical thinking and mathematical concepts to chemical reactions, which is crucial in various scientific and industrial applications.
To know more about algebraic visit-
https://brainly.com/question/953809
#SPJ11
Areas near large bodies of water tend to have which type of climate a: moderate b: polar c: dry
Answers
Answer:
a
Explanation:
Answer:
a
Explanation:
How many grams of radioactive Cs-137 remain after 4 half-life periods 120.9years
Answers
Answer:
1
Explanation:
1
A rock dropped Into a glass of water ralses the water level
by 40 milliliters. What can you infer about the rock?
Answers
Answer:
You can infert that it has a mass of 40 grams.It also has desnity of 40 grams per millilieter.
Explanation:
The volume of water raised from the glass is equal to the density of the object which displaces water. Hence, 40 g/ml is the density of the rock.
What is Archimedes principle?The Greek mathematician and inventor Archimedes discovered the physical law of buoyancy, which states that any body completely or partially submerged in a fluid (gas or liquid) at rest is subject to an upward, or buoyant, force whose magnitude is equal to the weight of the fluid displaced by the body.
The volume of fluid that has been displaced is equal to the volume of an object that is completely submerged in the liquid or to the portion of the volume below the surface for an object that is only partially submerged.
Therefore, the density of the rock is equivalent to the volume of water level raised in the glass. Thus density of rock is 40 g/ml.
To find more on Archimedes principle, refer here:
http://brainly.com/question/13106989
#SPJ2
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11
What is the percent by mass of iron in FeCl 3 ?
Answers
Answer:
34.429%
Explanation:
Which of the following are cations? Check all that apply.
a
barium
b
calcium
c
oxygen
d
chlorine
e
aluminum
f
magnesium
g
copper
h
bromine
Answers
Answer:
a
barium
b
calcium
e
aluminum
f
magnesium
g
copper
Forms cation
Explanation:
Carbon dioxide is reduced by using electrons obtained from inorganic molecules, such as ammonia or hydrogen gas by ______________ since they do not use solar energy.
Answers
Answer:
chemoautotrophs
Explanation:
Answer is B
A metal conducts heat in a similar way to electricity so, as you touch it, it draws heat from your body and makes your hand feel cold.
Plastics don't have to conduct heat so they don't draw heat from your body as efficiently, that's why a piece of plastic feels
than a piece of metal even if it's at the same temperature.
A)
long-chain molecules; warmer
B)
free electrons, warmer
valence electrons; colder
D)
a crystalline lattice; colder
Answers
B)
free electrons, warmer
valence electrons; colder
What is AH and what type of equation is it used fo!? Why?
Answers
Answer:
I think it has to do with batteries 'n stuff
AH— Ampere Hour
4) The principle of ________ states that the physical, chemical, and biological processes at work shaping the Earth today have also operated in the geologic past.
A) catastrophism
B) plate tectonics
C) plutonism
D) Uniformitarianism
Answers
The principle of option D. Uniformitarianism states that the physical, chemical, and biological processes at work shaping the Earth today have also operated in the
Option D. Uniformitarianism is the principle stating that the physical, chemical, and biological processes at work shaping the Earth today have also operated in the geologic past. It is based on the idea that the present is the key to the past. In other words, the same natural laws that operate in the universe today have been operating since the beginning of time.
James Hutton was the first to propose this principle in the late 18th century. He suggested that the Earth was shaped by slow-acting geological forces such as erosion, sedimentation, and uplift over long periods of time. He believed that the same processes were still happening today and that they had operated in the past.
This principle is an important concept in geology because it allows scientists to interpret the Earth's history based on the processes that they observe today. By understanding how these processes work and how they have changed over time, scientists can reconstruct the history of the Earth and its environments.
Uniformitarianism has been tested and proven through many observations and experiments. For example, the study of sedimentary rocks has shown that they were formed in the past through the same processes that are observed today, such as deposition of sediment by water, wind, or ice.
Similarly, the study of volcanoes has shown that they are formed by the same processes as today, such as the movement of magma from deep within the Earth.
In conclusion, Uniformitarianism is the principle that allows us to interpret the Earth's history by observing the processes that shape it today. It is a fundamental concept in geology and has been tested and proven through many observations and experiments.
To know more about Uniformitarianism here
https://brainly.com/question/1324266
#SPJ11
I need help in this question pleaaaaase!
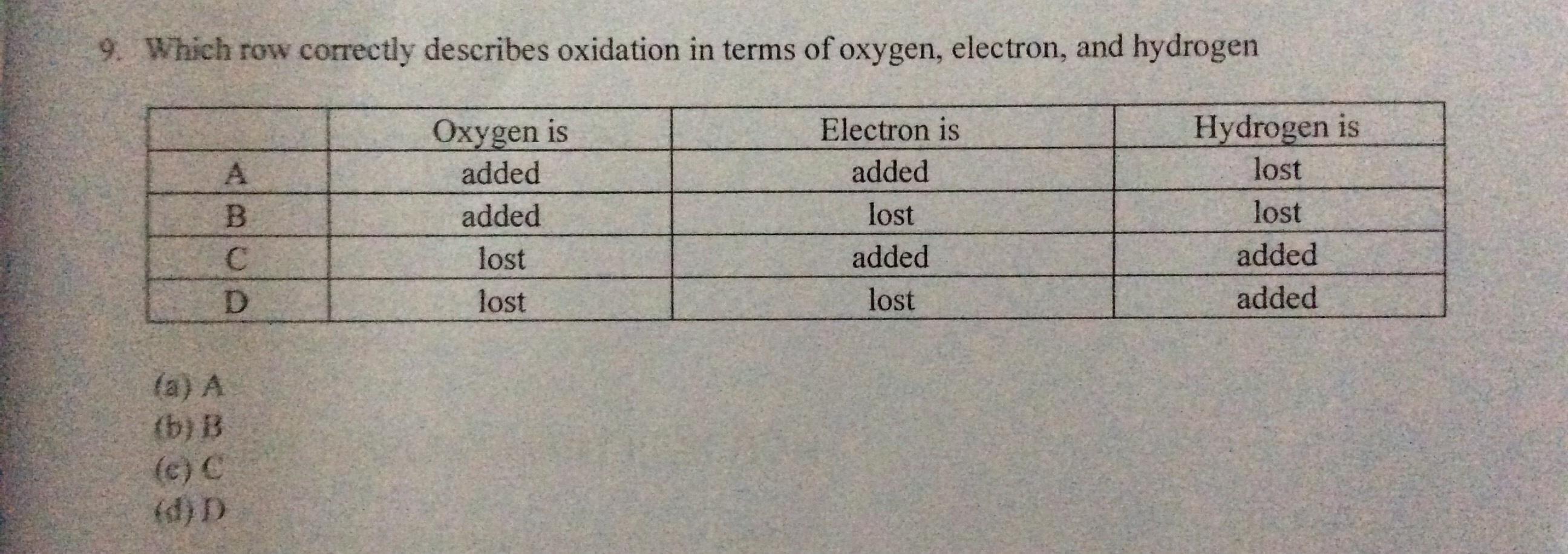
Answers
The correct definition of oxidation in terms of oxygen, electron and hydrogen is added, lost , lost, (Row B).
What is an oxidation reaction?An oxidation reaction is a reaction in which the oxidation number if the reacting species increases in a positive direction.
Oxidation can be defined in terms of oxygen as the addition of oxygen. Oxidation can be defined in terms of electron as removal of electron Oxidation can be defined in terms of hydrogen as removal of hydrogen.Therefore, the correct definition of oxidation in terms of oxygen, electron and hydrogen is added, lost , lost, (Row B).
Learn more about oxidation at: https://brainly.com/question/4222605
how would the acetic acid/acetate buffer system neutralize an added base?
Answers
The acetic acid/acetate buffer system consists of a weak acid (acetic acid, CH3COOH) and its conjugate base (acetate ion, CH3COO-). When a base is added to the buffer system, the following process occurs to neutralize it:
1. The base reacts with the weak acid (acetic acid) in the buffer system to form its conjugate base (acetate ion) and water. For example, if a hydroxide ion (OH-) is added, it reacts with acetic acid as follows:
OH- + CH3COOH → CH3COO- + H2O
2. The conjugate base (acetate ion) that is formed acts as a reservoir for hydrogen ions (H+). It can accept hydrogen ions from the solution if the pH increases. This helps to maintain the pH of the buffer system within a certain range.
3. The buffer system resists large changes in pH because the equilibrium between the weak acid and its conjugate base is shifted to maintain a relatively constant concentration of both species. This allows the system to neutralize the added base and maintain its acidic nature.
The acetic acid/acetate buffer system neutralizes an added base by reacting with it to form the conjugate base and water, and by utilizing the conjugate base to accept hydrogen ions and maintain the pH of the system.
To learn more about acetic acid, visit:
brainly.com/question/24586675
#SPJ11
What is the molarity of a 75.0 mL solution that contains 0.225 g of
potassium nitrate?
Answers
Answer:
M=0.0297M
Explanation:
we know that molarity equals number of mole of solute over the volume of solution.
M=n/V but n=m/M
n=0.225g÷101g/mol =0.00223mol
M=0.00223mol/0.075l
M=0.0297M
Answer:
0.0297M
Explanation:
We will use the formula M= moles of solute/lites of solution.
First we need to figure out the Moles of the solute, to get this we first need the molar mass of KNO3 (potassimum nitrate).
The Molar Mass of potassimum nitrate is 101.103 g/mol
K - 1 x 39.09
N - 1 x 14.006
O - 3 x 15.999
KNO3 - 101.103 g/mol
Now we get the moles of the solute by doing.
0225g/ 101.103g/mol = 0.00223 mol
Put the answer into the orginal equation to get 0.00223mol / 0.045 L
Which equals to 0.0297M. This is also the correct amount of significant figures.
About how old is the Sun?
1.2 billion years
4.6 billion years
6.4 billion years
8.2 billion years
Answers
Answer:
B : 4.6 billion years
Credits go to the person above me.
;)
Explanation:
EDGE 2021
The sun is about 4.6 billion years old. Therefore, option (B) is correct.
What is the sun made of?The Sun is made primarily of the elements hydrogen and helium. They account for 74.9% and 23.8% mass of the Sun in the photosphere. All heavier elements, called metals account for less than 2% of the mass, with oxygen (<1%), carbon (0.3%), neon (0.2%), and iron (0.2%) being the most abundant.
The hydrogen and helium in the Sun would have been produced by Big Bang nucleosynthesis and the heavier elements were produced by former generations of stars before the Sun was formed.
The amount of helium and its location within the Sun has gradually changed over the past 4.6 billion years. The proportion of helium has raised from about 24% to 60% due to fusion, and some of the He and heavy elements have settled from the photosphere toward the center due to gravity.
Therefore, the age of the sun is about 4.6 billion years.
Learn more about Sun, here:
https://brainly.com/question/2526507
#SPJ6
what is the pressure (in atm) on a surface 20.0 ft under water, if the atmospheric pressure is 1.023 atm, and the densities of water and mercury are 1.00 and 13.6 g/ml, respectively?
Answers
The pressure (in atm) on a surface 20.0 ft underwater, if the atmospheric pressure is 1.023 atm, and the densities of water and mercury are 1.00 and 13.6 g/ml, respectively is 1.067 atm
What is the pressure (in atm) on a surface 20.0 ft underwater?Generally, The pressure on a surface under water is equal to the atmospheric pressure plus the pressure due to the weight of the water above the surface.
To calculate the pressure due to the weight of the water, we need to know the depth of the surface under water and the density of the water.
Since the atmospheric pressure is 1.023 atm, and the density of water is 1.00 g/ml, the pressure on the surface 20.0 ft under water is given by:
Pressure = 1.023 atm + (depth * density of water * acceleration due to gravity)
= 1.023 atm + (20.0 ft * 1.00 g/ml * 32.2 ft/s^2)
= 1.023 atm + 644.4 lb/ft^2
= 1.023 atm + 44.6 psi
= 1.067 atm
So the total pressure on the surface 20.0 ft under water is 1.067 atm.
Read more about pressure
https://brainly.com/question/12971272
#SPJ1
When steel and zinc were connected, which one was the cathode?
Steel
Zinc
☐ neither
both
Answers
When steel and zinc were connected, zinc is the cathode. The term cathode refers to the electrode that is reduced during an electrochemical reaction.
The electrons are moved from the anode to the cathode during an electrochemical reaction in order to maintain a current in the wire that links the two electrodes.
According to the galvanic series, zinc is more active than iron, meaning that it is more likely to lose electrons and be oxidized. As a result, when steel and zinc are connected, zinc will act as the anode and lose electrons, whereas iron (steel) will act as the cathode and receive the electrons transferred by zinc.
To know more about electrochemical reaction visit:-
https://brainly.com/question/13062424
#SPJ11
9) Given the reaction: N2(g) + O2(g) + 182.6 kJ → 2 NO(g) What is the heat of formation of nitrogen (II) oxide in kJ/mole? A) AH = -182.6 B) AH = -91.3 C) AH = 91.3 Show D) AH = 182.6
PLEASE HELP please
Answers
The heat of formation of nitrogen (II) oxide : +91.3 kJ/mol
Further explanationGiven
Reaction
N2(g) + O2(g) + 182.6 kJ → 2 NO(g)
Required
The heat of formation
Solution
In the above reaction, the heat of the reaction is located on the reactant side which indicates that the formation of nitric oxide requires heat (endothermic reaction).
In the above reaction the heat required to form 2 moles of NO, so the heat required for each mole is:
+182.6 kJ : 2 = =+91.3 kJ/mol
Substance D - It has a high melting and boiling point. The arrangement of its particles are closely packed. It cannot conduct electricity unless molten or dissolved in water. What type of element is substance D?
Answers
Answer:
ionic
Explanation:
because when in molten or dissolved in water ions are free to move and carry a charge however when in solid the ions are fixed in position so can't carry a charge.
One drop of water evaporates every hour. If there are 20 drops in one milliliter of water and the density of water is 1. 00 g/mL, how many water molecules evaporate per second
Answers
Answer:
4.6466 x 10^17 molecule/s
Explanation:
1 drop/hr = 0.05mL/hr
0.05mL H2O x 1g/mL = 0.05g
0.05g/(18g/mol) = 2.778 x 10^-3 mol
2.778 x 10^-3 mol x Na(6.022x10^23) = 1.67 x 10^21 molecule
(1.67x 10^21 molecule / hr) / (3600s/hr) = 4.6466 x 10^17 molecule/s
PLEASE HELP ME QUICKLY (select all that apply) Enzymes made by extremophiles can be harvested and used in everyday applications. These uses include
making laundry detergents.
making dish detergents.
recycling old tires.
de-hairing hides.
making paper.
Answers
Answer:
A, B, D, E
Explanation:
did it on edg
Answer:
A. Making laundry detergent
B. Making dish detergent
D. De-hairing hides
E. Making paper
Explanation:
Gaseous carbon monoxide reacts with hydrogen gas to form gaseous methane (CH4) and oxygen gas. Express your answer as a chemical equation. Identify all of the phases in your answer. 0 ΑΣΦ ? * . x хь x A chemical reaction does not occur for this question
Answers
The states of the reactants and products are given as follows: CO (g) + 3 H2(g) → CH4(g) + H2O(g)Where (g) stands for the gaseous state, since all the reactants and products are in the gaseous state. Hence, the reaction is in the gaseous state. Therefore, the phases of all the components of the balanced chemical equation are gaseous.
The given reaction is: Gaseous carbon monoxide reacts with hydrogen gas to form gaseous methane (CH4) and oxygen gas. The balanced chemical equation is as follows: CO (g) + 3 H2(g) → CH4(g) + H2O(g)This reaction is an example of a reduction-oxidation (redox) reaction. In this reaction, carbon monoxide is oxidized to carbon dioxide, while hydrogen is reduced to methane. Water is formed as a byproduct of the reaction. Here, CO acts as an oxidizing agent, whereas hydrogen acts as a reducing agent. The states of the reactants and products are given as follows: CO (g) + 3 H2(g) → CH4(g) + H2O(g)Where (g) stands for the gaseous state, since all the reactants and products are in the gaseous state. Hence, the reaction is in the gaseous state. Therefore, the phases of all the components of the balanced chemical equation are gaseous.
To Know more about reduction-oxidation (redox) reaction visit:
brainly.com/question/31738793
#SPJ11
only Q4a, pls help i cant process this question
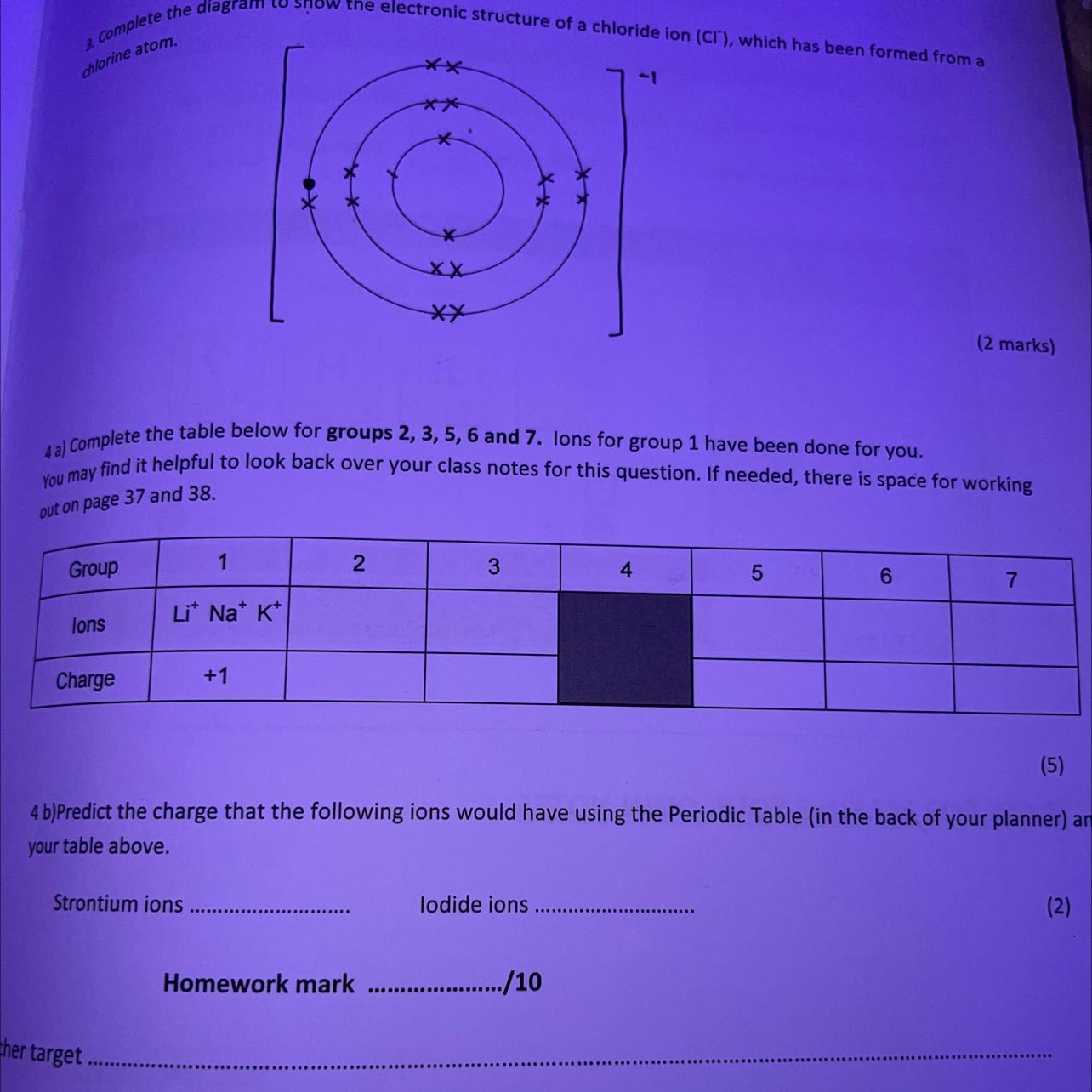
Answers
Explanation:
The group 2 ions are in the second column in the periodic table, for example Be, Mg and Ca
The group 3 ions are in the third column in the periodic table, for example B, Al and Ga
It's the same thing for the rest of the groups
Ions either lose or gain electrons to have a full outer shell, just like the noble gases
metal atoms lose electrons from their outer shell to form positively charged ions
From groups 1 to 3 the charge is the same as the group number
Non metals gain electrons on their outer shell to form negatively charged ions
From groups 5 to 7 the charge is 8 subtract the group number
Example: what is the charge for group 6 ions?
8 - 6 = 2, and the charge is negative so 2-

co2(g)+H20+148Kcal---->H2Co3 Endotérmica o exotérmica
Answers
endothermic
requires energy to occure