Answers
Answer:
On Question 3
Explanation:
try using any type or liquid like water, milk, soda, juice, coffee, tea, beer, or wine or any type and use the following questions by using a measuring cup from the kitchen tooling shelf. Use that to answer the question and see if that helps. your welcome btw
Related Questions
pOH of the 0.001M NaOH solution is
Answers
The pOH of the 0.001 M NaOH solution is approximately 3.
To determine the pOH of a solution, we need to know the concentration of hydroxide ions (OH-) in the solution.
In the case of a 0.001 M NaOH solution, we can assume that all of the NaOH dissociates completely in water to form Na+ and OH- ions. Therefore, the concentration of hydroxide ions in the solution is also 0.001 M.
The pOH is calculated using the equation:
pOH = -log[OH-]
Substituting the concentration of hydroxide ions, we have:
pOH = -log(0.001)
Using a calculator, we can evaluate the logarithm:
pOH ≈ 3
Therefore, the pOH of the 0.001 M NaOH solution is approximately 3.
Know more about hydroxide ions here:
https://brainly.com/question/28464162
#SPJ8
Sugar dissolves in water. Which statement best describes what is happening? (1 point) Sugar stays together but in a liquid form that can no longer be seen in the water. Sugar stays together but in a liquid form that can no longer be seen in the water. Sugar slowly melts, turning from a solid to a liquid and combining with the water. Sugar slowly melts, turning from a solid to a liquid and combining with the water. The water molecules in sugar will separate from each other and combine with the water, releasing the sweetness into the water. The water molecules in sugar will separate from each other and combine with the water, releasing the sweetness into the water. A large collection of sugar molecules break into individual molecules and disperse in the water.
Answers
Yes sir solid gas and liquid
What is the specific heat capacity of gold if it requires 48.8 J to raise the temperature of 15 grams of gold 25oC?
Answers
Answer:
0.13 J/gC i believe.
Explanation:
Answer:
130.133 J/(kg·K)
Explanation:
Result in other units:
0.130133 J/(g·K)
31.0876675695 cal/(kg·K)
0.0310876675695 kcal/(kg·K)
0.130133 J/(g·°C)
5. Consider the diprotic acid H2A with K1=1.00x104 and K2=1.00x108. Find the pH and concentrations of H2A, HA, and A-2 in 0.20 M of the acid solution.
Answers
In a 0.20 M solution of H2A with \(K_1=1.00*10^4\) and \(K_2=1.00*10^8\), the pH is 1.88, and the concentrations of \(H_2A\),\(HA^-\), and\(A^2-\) are approximately \(0.197 M, 2.54*10^{-3} M\), and \(1.95*10^{-15} M\).
To solve this problem, we need to use expressions for the equilibrium concentrations of the species in a diprotic acid:
\([H_2A] = [H_2A]0/(1 + K_1/[H+] + K_1K_2/[H+]^2) \\\)
\([HA^-] = K_1[H_2A]/(1 + K_1/[H+] + K_1K_2/[H+]^2) \\\)
\([A^2-] = K_1K_2[H_2A]/(1 + K_1/[H+] + K_1K_2/[H+]^2)\)
Given \(K_1\) and \(K_2\) values, we can assume that \(K_2\) is much larger than \(K_1\), and thus we can approximate the concentration of \(H_2A\) to be equal to its initial concentration. Therefore, we can simplify the above expressions to:
\([H_2A]\) ≈\([H_2A]0/(1 + K_1/[H+])\)
[HA^-] ≈ \(K_1[H_2A]/(1 + K_1/[H+])\)
\([A^2-]\)≈\(K_1K_2[H_2A]/[H+]^2\)
Substituting the given values:
\([H_2A]\) ≈\(0.20 M/(1 + 1.00*10^4/[H+])\)
[HA^-] ≈ \(1.00*10^4 * 0.20 M/[H+]/(1 + 1.00*10^4/[H+])\)
\([A^2-]\)≈ \(1.00*10^8 * 0.20 M/[H+]^2\)
To find the pH, we can use the equation:
\(pH = -log[H+]\)
After solving the problem iteratively, we get:
pH ≈ 1.88
\([H_2A]\)≈ 0.197 M
[HA^-] ≈ \(2.54*10^{-3} M\)
\([A^2-]\) ≈ \(1.95*10^{-15} M\)
To know more about equilibrium concentrations, here
brainly.com/question/13043707
#SPJ1
--The complete Question is, Consider the diprotic acid H2A with K1=1.00x10^4 and K2=1.00x10^8. If you have a 0.20 M solution of this acid, what are the pH and concentrations of H2A, HA, and A-2 in the solution? --
Kinetic energy is described as - The answers are stored energy - destroyed energy movement or created energy if you answer the question I will give you the brainiest.

Answers
1Touch and hold a clip to pin it. Unpinned clips will be deleted after 1 hour.
1. A cell must continuously sort useful materials from its waste products and then remove or recycle the waste. One way a cell does this is to first put tiny tags on proteins that are no longer needed. These tags, which are small polypeptide chains 76 amino acids long, are called ubiquitin.
2. Ubiquitin tags direct proteins to compartments of the cell called proteasomes. Within each proteasome, ubiquitin-tagged proteins are deconstructed into their component amino acids. These pieces can be repurposed to build new proteins.
3. Sometimes organs stop processing waste effectively, allowing the waste to pile up and contributing to many diseases. Alzheimer's disease, for example, includes piles of waste proteins in the brain called tangles, while Parkinson's disease involves the accumulation of recyclable material into Lewy bodies. Mallory bodies are piles of waste proteins common in liver disease. These piles, known generically as inclusions, can be identified under the microscope because they are covered in tiny ubiquitin tags.
4. A problem in the breakdown of ubiquitin-tagged proteins may be one cause of spinocerebellar ataxia type 1. In this disease, a protein called ataxin-1 has an abnormal genetic mutation, and ubiquitin is able to attach to the mutant protein. Scientists believe that the mutated protein cannot be chopped up by the proteasome because of its shape, so ubiquitin-tagged ataxin-1 starts to accumulate, which leads to cell death. The cells most affected by this genetic mutation are neurons in a part of the brain associated with motor control and the spinal cord. So, a person with mutant ataxin-1 progressively loses muscle control as these cells die.
Which phrase describes the purpose of ubiguitin
A. to tag waste proteins for recycling in the proteasomes
B. to create small polypeptide chains that are 76 amino acids long
C. to create inclusions that can be identified under the microscope
D. to attach to mutant proteins so that they can accumulate In the cell
If proteins contain mutations, the proteins often do not maintain the proper shape and are unable to perform their functions. Properly functioning proteins are essential to maintaining healthy organisms.
Which statement describes the overall consequence of proteins containing mutations like those described in the passage?
A. Wastes will build up and will be reassembled to build new proteins.
B. Wastes will build up, and Mallory bodies will accumulate in the liver.
C. Wastes will build up in cells and will eventually be excreted from each cell.
D. Wastes will build up in vital organs, and the organism will experience disease.
Why do individuals with spinocerebellar ataxia experience loss of muscle control, even though the ataxin-1 protein builds up only In the brain and spinal cord?
A. Inclusions can build up in all body systems and can cause debilitating effects.
B. Genetic mutations in the ataxin-1 protein cause muscular proteins to change shape.
C. When the ubiquitin tagging system does not work effectively, cellular waste can build up in the muscles.
D. The neurons affected by the ataxin-1 protein buildup are in a region of the brain that manages muscle control.
Answers
Based on the role of ubiquitin in protein deconstruction as stated in the passage, the correct options are as follows:
to tag waste proteins for recycling in the proteasomes; option A.Wastes will build up in vital organs, and the organism will experience disease; option Dthe neurons affected by the ataxin-1 protein buildup are in a region of the brain that manages muscle control; option D.What are phrases?
Phrases refers to a group of words which contains a verb but which on their own do not make complete sense.
In the description of the role of Ubiquitin, it is described as a small polypeptide chain containing 76 amino acids. The main tole of ubiquitin is to tag proteins for deconstruction in the proteasomes.
Therefore, the phrase describes the purpose of ubiquitin is to tag waste proteins for recycling in the proteasomes; option A.
Based on the passage, the statement that describes the overall consequence of proteins containing mutations is: Wastes will build up in vital organs, and the organism will experience disease; option D
Individuals with spinocerebellar ataxia experience loss of muscle control, even though the ataxin-1 protein builds up only in the brain and spinal cord because the neurons affected by the ataxin-1 protein buildup are in a region of the brain that manages muscle control; option D.
In conclusion, mutations in ubiquitin-tagged proteins will result in accumulation in the body since they cannot be deconstructed in the proteasomes.
Learn more about ubiquitin at: https://brainly.com/question/27951044
#SPJ1
Which reaction would occur at the anode in a voltaic cell?
Cd2+(aq)+Zn(s)→Zn2+(aq)+Cd(s)
K+(aq)+e−→K(s)
Cu2+(aq)+2e−→Cu(s)
Ca(s)→Ca2++2e−
Answers
In a voltaic cell, the anode is the electrode where oxidation takes place. So, among the given reactions, the reaction that involves oxidation at the anode is: Ca(s) → Ca2+ + 2e-.
In a voltaic cell, the reaction at the anode is an oxidation reaction, wherein a substance loses electrons. From the provided reactions, the reaction of calcium (Ca) transforming to a calcium ion by losing two electrons (Ca(s)→Ca2+ + 2e−) occurs at the anode.
Explanation:In a voltaic cell, oxidation occurs at the anode. Therefore, the reaction that would take place at the anode would be the one in which the substance loses electrons, transforming from a neutral state to an ion. From the provided reactions, Ca(s)→Ca2+ + 2e− represents this process as calcium (Ca) loses two electrons (2e−) and becomes a cation (Ca2+). This reaction is called an oxidation reaction, characteristic of what occurs at the anode in a voltaic cell.
This equation represents the oxidation of calcium (Ca) as it loses two electrons (2e⁻) and becomes a calcium ion (Ca²⁺). This process is indeed an oxidation reaction, and it accurately represents what occurs at the anode in a voltaic cell.
The anode is where electrons are generated and flow through an external circuit to the cathode, where reduction reactions take place. In this electrochemical setup, the anode and cathode are essential components that drive the flow of electrons and generate electrical energy.
Therefore, the given reaction involving the oxidation of calcium (Ca) at the anode accurately reflects the principles of electrochemical cells, including voltaic cells, where oxidation reactions occur at the anode.
Learn more about Voltaic Cell here:https://brainly.com/question/34404030
#SPJ2
Make a
prediction about how a lack
of resources in an ecosystem
might impact the levels of
organization.
Answers
Answer:
Limiting factors of an ecosystem include disease, severe climate and weather changes, predator-prey relationships, commercial development, environmental pollution and more. An excess or depletion of any one of these limiting factors can degrade and even destroy a habitat.
Explanation:
The limiting factor of an ecosystem involve disease, weather change, climate change, environment pollution and more. An excess or depletion of any of these factor can destroy over habitat.
What is Ecosystem?An ecosystem is define as a community or a group of living organisms that live together and are dependent on each other.
There are two main types of ecosystem.
Terrestrial ecosystem-Terrestrial ecosystem are land based ecosystem and interaction of biotic and abiotic component in the specific area.
Example- forest, grassland desert etc.
Aquatic ecosystem-Aquatic ecosystem are ecosystem formed by surrounding water bodies. They are dependent on each other and their environment .
Example- lake, pond, river etc.
Thus ecosystem and their limiting factor have great impact on the level of organization.
To learn more about ecosystem, refer to the link below:
https://brainly.com/question/13979184
#SPJ2
The cylinder of a car's engine has a volume of 0.6250 L when the piston is at the bottom of the cylinder. When the piston is at the top of the cylinder the volume is 0.0600 L. If the cylinder is filled with air at an atmospheric pressure of 765.1 mm Hg the piston is at the bottom, what is the pressure in units of kPa when the piston is at the top of the cylinder?
Answers
The new pressure in the cylinder of the car engine as the piston is moved to the top is 1062.55kPa
Boyle's lawBoyle's law states that "volume of a given quantity of gas is inversely proportional to its pressure as long as temperature remains constant.
It is expressed as;
P₁V₁ = P₂V₂
Given the data in the question;
Initial volume V₁ = 0.6250LNew volume V₂ = 0.0600LInitial pressure P₁ = 765.1mmHg = 1.0067107atmNew pressure P₂ = ?To determine the new pressure as the piston is at the top of the cylinder, we substitute our given values into the expression above.
P₁V₁ = P₂V₂
P₂ = P₁V₁ / V₂
P₂ = ( 1.0067107atm × 0.6250L ) / 0.0600L
P₂ = 0.629194188Latm / 0.0600L
P₂ = 10.4865698atm
P₂ = 1062.55kPa
Therefore, the new pressure in the cylinder of the car engine as the piston is moved to the top is 1062.55kPa
Learn more about Boyle's law here: brainly.com/question/1437490
Adding thermal energy to a gas at constant pressure will cause...
A. the volume of the gas to decrease.
B. the volume of the gas to increase.
C. the temperature of the gas to decrease.
D. the pressure of the gas to decrease.
Answers
B. the volume of the gas to increase.
What is thermal energy to a gas at constant pressure?Thermal energy is the energy associated with the random motion of the particles in a substance, such as a gas. When thermal energy is added to a gas at constant pressure, the increased kinetic energy of the gas particles causes them to move apart, increasing the volume of the gas. This relationship is described by Gay-Lussac's Law, which states that at a constant pressure, the volume of a gas is directly proportional to its temperature.
Learn more about Thermal energy in brainly.com/question/18989562
#SPJ1
why do Aluminium oxide have these properties
Answers
The main reason for these properties is that aluminum oxide forms a protective coating when formed, which prevents further oxidation. Also, it is a covalent crystal, which means its intermolecular forces are strong, this is why its melting point is so high.
Barium nitrate (Ba(NO3)2) reacts with sodium chloride (NaCl) in a double replacement (displacement) reaction, shown below.
Ba(NO3)2(aq)+NaCl(aq)-->???
How many grams of barium salt are produced when a solution containing 21.7 g of Barium nitrate is mixed with a solution containing excess sodium chloride?
Use 261.34 as the molar mass for barium nitrate. Round to three significant digits.
Answers
Answer:
you know that they will be a displacement reaction that will form a barium salt:
Ba(NO3)2+ 2NaCl--> BaCl2 + 2NaNO3
So now that we have that formula and the molecular weight we can determine how much salt will be made. So here we convert the grams to moles
(42.3g Ba(NO3)2)*(1 mole/261.34g) = 0.16185 mol
In the molecular formula we know that 1 mole of Barium nitrate will create 1 mole of Barium chloride, so in this case (in a perfect world) you should get 0.16185 mole of barium chloride (208.23 g/mol) that we then have to convert to grams.
(0.16185 mol BaCl2) * ( 208.23 g/mol) = 33.7037 g of Barium Chloride (rounded to 3 significant digits = 33.7g)
What element is the electron configuration representing?
1s2 2s2 2p6 3s2 3p6 4s2.
Answers
Answer:
calcium
Explanation:
1s2 2s2 2p6 3s2 3p6 4s2
total number of electrons: 2+2+6+2+6+2=20
the atomic number of the element is 20
the element is calcium
The electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 represents the element calcium (Ca).
The electron configuration notation describes the distribution of electrons in the energy levels or orbitals of an atom. In this case, the numbers and letters indicate the specific orbitals and the number of electrons in each orbital.
Breaking it down:
1s2 represents the 1s orbital with 2 electrons.
2s2 represents the 2s orbital with 2 electrons.
2p6 represents the 2p orbital with 6 electrons.
3s2 represents the 3s orbital with 2 electrons.
3p6 represents the 3p orbital with 6 electrons.
4s2 represents the 4s orbital with 2 electrons.
Considering the filling order of orbitals and the number of electrons, the element with this electron configuration is calcium (Ca).
Learn more about electron configuration, here:
https://brainly.com/question/29157546
#SPJ6
Describe how you can figure out how many grams of a COMPOUND if you know how many moles there
are.
Answers
2K + MgCh2 -> 2KCI + Mg
Which of the following statements best describes the reaction?
O This reaction is a double replacement reaction because K replaced Mg and a redox reaction because the ion charges of the elements changed from a 0 charge on K to +1 charge, and a +2 charge on Mg to a 0 charge.
• This reaction is a double replacement reaction because K replaced Mg and not a redox reaction because the ion charges of the elements remained the same.
This reaction is a single replacement reaction because K replaced Mg and a redox reaction because the ion charges of the elements changed from a 0 charge on K to +1 charge, and a +2 charge on Mg to a 0 charge.
O This reaction is a single replacement reaction because K replaced Mg and not a redox reaction
because the ion charges of the elements remained the same.
Answers
Answer:
The third option
Explanation:
This reaction is a single replacement reaction because K replaced Mg and a redox reaction because the ion charges of the elements changed from a 0 charge on K to +1 charge, and a +2 charge on Mg to a 0 charge.
why is it preferred to use calcium oxide rather than calcium chloride in preparation of iron (III) chloride
Answers
Answer:
Calcium Oxide is a drying agent, hence it dehydrates the reaction to give pure solid Iron ( III ) chloride, which cannot be done by calcium chloride.
It preferred to use calcium oxide rather than calcium chloride in preparation of iron (III) chloride because Calcium Oxide is a drying agent, hence it dehydrates the reaction to give pure solid Iron ( III ) chloride, which cannot be done by calcium chloride.
What is Dehydration ?A process such as a chemical reaction that removes water.The atoms which constitute the molecule of water that is removed.
Hence,It preferred to use calcium oxide rather than calcium chloride in preparation of iron (III) chloride because Calcium Oxide is a drying agent,
Thus, it dehydrates the reaction to give pure solid Iron ( III ) chloride, which cannot be done by calcium chloride.
Learn more about reactions here ;
https://brainly.com/question/17434463
#SPJ2
What type of reaction is C3H6O + O2 --> CO2 + 3 H2O
Answers
Answer:
Combustion of propanone I believe
Explanation:
Which of the following statements is a hypothesis?
A Why do I see more rats at a smoker's house than
a non-smoker's house
B I think that cigarette smoke increases the
appetite of rats
C
I will place three rats in three separate cages and
expose them to varying amounts of cigarette smoke
D The rat which exposed to the most smoke, ate a
larger amount of cheese compared to the other rats
Answers
Correct hypothesis is 'i will place three rats in three separate cages and
expose them to varying amounts of cigarette smoke'
Here given statement C is correct in hypothesis because when we place three rats in three separate cages and expose them to varying amounts of cigarette smoke then 50% rat are kill because nicotine concentration in the mainstream smoke particulate matter of reference cigarettes are 70%-8% and it take 20 min to kill the rats
Know more about rats
https://brainly.com/question/14313260
#SPJ1
what's the definition of sublimation according to kinetic particle theory?
Answers
Answer:
Sublimation is the term for when matter undergoes a phase transition directly from a solid to gaseous form, or vapor, without passing through the more common liquid phase between the two. It is a specific case of vaporization.
Explanation:
Identify the activated complex in the following reaction.
a. CuFeSO
b. FeFe
c. FeCuSO4
d. FeSO4
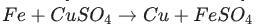
Answers
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. Option C)
An activated complex is a structure that exists temporarily during a chemical reaction and corresponds to the top of the energy barrier that must be overcome for the reaction to proceed to completion.
The activated complex in the following reaction is: FeCuSO4. The activated complex is a transition state that is an intermediate structure in a chemical reaction. It is the structure with the greatest energy within the reaction process and is used to determine the rate at which the reaction occurs. An activated complex exists when the energy required to break the old bonds and form new ones has been absorbed. It has a specific configuration and energy content that is precisely defined.
A chemical reaction is the process by which atoms or groups of atoms in molecules interact to form new molecules. A chemical reaction is caused by the motion of electrons, which are negatively charged particles that surround atomic nuclei. The reaction proceeds through the formation of an intermediate species known as the transition state or activated complex. Reaction mechanisms are the sequence of steps involved in a chemical reaction. These steps describe the intermediate species formed as the reactants are converted to products. Hence option C) is correct.
for more questions on reaction
https://brainly.com/question/25769000
#SPJ8
Three electrolytic cells A,B,C containing solutions of ZnSO4, AgNO3 and CuSO4, respectively are connected in series. A steady current of 1.5 amperes was passed through them until 1.45 g of silver deposited at the cathode of cell B. How long did the current flow?
don't spam ;-;
Answers
The current flowed for 864 seconds or 14.4 minutes.
What is the time of current flow?
The amount of silver deposited at the cathode of cell B can be used to calculate the total amount of charge that flowed through the circuit. Once we know the total charge, we can use the current to calculate the time required for that amount of charge to flow.
First, let's calculate the total amount of charge that flowed through the circuit. The amount of silver deposited at the cathode of cell B is 1.45 g. The molar mass of silver is 107.87 g/mol, so the number of moles of silver deposited is:
1.45 g / 107.87 g/mol = 0.0134 mol
Each mole of silver ions (Ag+) requires one mole of electrons (e-) to form one mole of silver metal, so the number of electrons involved in the reduction of Ag+ ions is also 0.0134 mol. The charge carried by one mole of electrons is given by the Faraday constant, which is 96,485 coulombs/mol.
Therefore, the total charge that flowed through the circuit is:
0.0134 mol × 96,485 C/mol = 1296 C
Now that we know the total charge, we can use the current to calculate the time required for that amount of charge to flow. The current is 1.5 amperes, so the time required is:
time = total charge / current = 1296 C / 1.5 A = 864 seconds
Therefore, the current flowed for 864 seconds, or 14.4 minutes.
Learn more about electrolysis here: https://brainly.com/question/12994141
#SPJ1
Please help me
A proton is approximately 2000 times heavier than an electron
How would the speeds of these particles compare if their corresponding wavelengths were equal
Answers
The speed of the proton would be 1/2000th that of the electron for their corresponding wavelengths to be equal.
Speed of atomic particlesThe de Broglie wavelength of a particle is given by:
λ = h/p
where λ is the wavelengthh is the Planck's constantp is the momentum of the particle.If the wavelengths of a proton and an electron are equal, their momenta must also be equal, since h is a constant.
The momentum of a particle is given by:
p = MV
m is the mass of the particlev is its velocity.Thus: MV proton = MV electron
Vp = Ve x (Me/Mp)
Given that a proton is approximately 2000 times heavier than an electron, we can substitute the mass ratio into the equation to get:
Vp = Vp x (1/2000)
This tells us that the speed of the proton would be 1/2000th that of the electron for their corresponding wavelengths to be equal.
More on the speed of atomic particles can be found here: https://brainly.com/question/15315577
#SPJ1
Chlorophyll is found in
A. neither plant
nor animal cells.
B. animal cells.
C. plant cells.
D. plant and animal cells.
Answers
Answer:
Only plant cells
Explanation:
This organelle helps plants photosynthesize. Humans do not do that.
When the equation
Ca3(PO4)2+HNO3→Ca(NO3)2+H3PO4
is balanced, the smallest whole number coefficient for HNO3 is
Answers
Answer:
The least whole number coefficient for HNO₃ is 6
Explanation:
The chemical equation above is the reaction between calcium orthophosphate and nitric acid.
To balance a chemical equation, we have to consider law of conservation of matter which states that matter can neither be created nor destroyed.
What this law implies is that, whatever we have at the reactant side must be equal to whatever is obtainable at the product side.
The above equation is
Ca₃(PO₄)₂ + HNO₃ → Ca(NO₃)₂ + H₃PO₄
To balance the equation, we'll have to check the number of atoms at each side and possibly balance the equation with the number of moles.
The balanced equation is
Ca₃(PO₄)₂ + 6HNO₃ → 3Ca(NO₃)₂ + 2H₃PO₄
From the balanced equation above, we can see that the number of calcium (Ca), Phosphorus (P), Oxygen(O), Nitrogen(N) and hydrogen (H) are balanced at both sides of the equation.
The least number coefficient for HNO₃ is 6
is lithium batteries a limited quantity item
Answers
Lithium metal (non-rechargeable) batteries are limited to two grams of lithium per battery.
Lithium-ion is the most popular rechargeable battery chemistry used these days. Lithium-ion batteries power the devices we use every day, like our mobile phones and electric cars. Lithium-ion batteries encompass a single or more than one lithium-ion cell, along with a defensive circuit board.
Lithium-ion batteries energy the lives of tens of millions of human beings each day. From laptops and cell phones to hybrids and electric vehicles, this generation is growing in popularity due to its lightweight, high electricity density, and capability to recharge.
No matter its typical advantages, lithium-ion has its drawbacks. It's far fragile and requires a safety circuit to maintain secure operation.
Learn more about Lithium battery here:-https://brainly.com/question/18670702
#SPJ4
The following reaction is investigated: 2N2O(g) N2H4(g) <---> 3N2(g) 2H2O(g) Initially there are 0.100 mol of N2O and 0.25 mol of N2H4, in a 10.0-L container. If there are 0.059 mol of N2O at equilibrium, how many moles of N2 are present at equilibrium
Answers
Answer:
6.2 × 10^-2
Explanation:
Given the reaction:
------------2N2O(g) N2H4(g) <---> 3N2(g) 2H2O(g)
Initial: N2O = 0.1, N2H4 = 0.25-- 0 - - - - - 0
Then: N20 = -2x, N2H4= - x - - - +3x, +2x
Equ : 0.1 - 2x ; 0.25 - x ; +3x ; +2x
At equilibrium :
Add both:
N2O = 0.1 - 2x ;
N2H4 = 0.25 - x;
3N2 = 3x
2H2O = 2x
Moles of N2O at equilibrium = 0.059
Then;
0.1 - 2x = 0.059
-2x = 0.059 - 0.1
-2x = - 0.041
x = 0.041 / 2
x = 0.0205
Moles of N2 present at equilibrium ;
3N2 = 3x
3N2 = 3(0.0205)
= 0.0615
= 0.062 = 6.2 × 10^-2
Which option shows the correct conversion of units from 3 L to mL?
3 L ∙ 100 mL1 L
3 L ∙ 1,000 mL1 L
3 L ∙ 1 mL100 L
3 L ∙ 1 mL1,000 L
Answers
Answer:
345
Explanation:
When the ball is at rest, what forces are acting on it?
Answers
Why is it not necessary to test the reactivity of the elemental metals with solutions of the same metal ion?
Answers
It is not necessary to test the reactivity of the elemental metals with solutions of the same metal ion because it won't react with itself.
What is Reactivity?
This is referred to as the relative capacity or how readily a substance undergoes a chemical reaction.
Reactivity of elements are dependent on various types of parameters such as the number of valence electrons, shells etc and can be tested with other substances. However, it can't be tested with the solutions of the same metal ion because no reaction will occur thereby making it the most correct reason in this scenario.
Read more about Reactivity here https://brainly.com/question/28815106
#SPJ1
Write the unbalanced chemical
equation for the following reaction:
butane (C4H10) + oxygen gas yields
carbon dioxide + water

Answers
Answer:
C₄H₁₀ + O₂ --> CO₂ + H₂O
Explanation:
The other reactant is stated below the reaction, which is oxygen.
Oxygen is a diatomic element meaning there are usually 2 bonded oxygens. O is incorrect since oxygen is never found as a single atom.
The products are stated below the question. The products are:
- Carbon dioxide
- Water
The chemical formula for carbon dioxide is CO₂
The chemical formula for water is H₂O
Both of the above formulas are very common and are widely known.
