Can you use melting point to determine the identity of an unknown solid? A piece of gray metal appears to be melting. The metal is observed to begin to melt when the temperature of its container is approximately 29°C. The metal is most likely.
Answers
Answer:
E - Gallium
Explanation:
This is for edge
Related Questions
which of the following would be expected to form hydrogen bonds with water? choose all that apply. acetamide cyclopentane butanoic acid ethyl methyl ketone
Answers
Acetamide and butanoic acid would be expected to form hydrogen bonds with water. Hydrogen bonding occurs when a hydrogen atom is covalently bonded to a highly electronegative atom (such as oxygen or nitrogen) and is also attracted to another electronegative atom through a dipole-dipole interaction.
Both acetamide and butanoic acid contain highly polar functional groups (amide and carboxylic acid, respectively) with hydrogen atoms that can form hydrogen bonds with water. On the other hand, cyclopentane and ethyl methyl ketone do not contain highly polar functional groups that can form hydrogen bonds with water. Cyclopentane is a nonpolar molecule with only C-H bonds, and ethyl methyl ketone contains a carbonyl group, which is polar but not strongly polar enough to form hydrogen bonds with water. Acetamide and butanoic acid would be expected to form hydrogen bonds with water due to the presence of polar functional groups with hydrogen bonding capability, while cyclopentane and ethyl methyl ketone would not.
Learn more about acetamide here:
https://brainly.com/question/8434658
#SPJ11
name an element in the fourth period (row) of the periodic table with: a. five valence electrons b. four 4p electrons c. three 3d electrons d. a complete outer shell
Answers
The metals of fourth period are Arsenic, Germanium, Scandium and Xenon.
(a)
Fourth Period
Five valence electrons
Five valence electrons means: This is V A group element.
V A group elements are: N, P, As, Sb, Bi
N = 7: 1s² 2s² 2p³
P = valence configuration = 3s²3p³
As valence configuration = 4s²4p³
So, the metal is As (Arsenic).
(b)
Fourth Period
Two 4-p electrons
Four valence electrons means: This is IV A group element.
IV A group elements are: C, Si, Ge, Sn, Pb
C = 6: 1s² 2s² 2p²
Si = valence configuration = 3s²3p²
Ge valence configuration = 4s²4p²
So, the metal is Ge (Germanium).
(c)
Fourth Period + one 3d electron
It is d-block element.
Sc = 21: 1s² 2s² 2p⁶3s²3p⁶4s²3d¹
3d¹ (one 3d one electron)
4s² (indicates fourth period)
Hence, the metal is Sc (scandium).
(d)
Fifth period + complete outer-shell
Complete outer-shell elements = He, Ne, Ar, Kr, Xe, Rn
He = 2: 1s²
Ne = 10: 1s² 2s² 2p⁶
Ar outer-shell = 3s²3p⁶
Xe outer-shell = 5s²5p⁶
Hence the metal is Xenon (Xe).
Learn more about elements from the link given below:
https://brainly.com/question/9410546
#SPJ4
approximate the work for the following reaction at 300 k. ch4 (g) + 2o2 (g) → co2 (g) + 2h2o (l)
Answers
The work done during the reaction is equal to 4.988 KJ.
What is work?According to the work-energy principle, the work done by a force on a body can be measured in terms of change in kinetic energy.
In mathematical form, the work by a system is given by the equation:
Work = - P.δV = Δn.RT
Given, the chemical equation of the combustion of methane is :
\(CH_4 (g) + 2O_2 (g) \longrightarrow CO_2 (g) + 2H_2O (l)\)
Filling the given values into equation (1), we have;
Δng =(gaseous moles of products -gaseous moles of reactants)
Δng = 1 - (2 + 1)
Δng = 1-3 = -2
Work done, W = - Δng. RT
W = -( -2) ×8.314 × 300
W = 4988 J
W = 4.988 KJ
Learn more about work, here:
brainly.com/question/10063455
#SPJ1
The strongest forces of attraction occur between molecules of
Answers
Answer:
Dipole-dipole
- are the strongest intermolecular force of attraction.
Explanation:
Yun lang <3
what is the water write example
Answers
Answer:
Water, a substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid states. It is one of the most plentiful and essential of compounds. A tasteless and odourless liquid at room temperature, it has the important ability to dissolve many other substances.
Explanation:
Answer/Explanation:
Water is a colorless and tasteless liquid that makes up an organism or body of any sort.
The meaning of the (holy)water is the spirit that flows in each person that will keep their hearts satisfied. Jesus used this term when talking to a Samaritan at a well. Water is also the source of the living water that springs up into eternal life. When talking about this water, Jesus was referring to himself. For His water(blood) had not yet been poured out and it could not yet cleanse (those who believed in him would be gushing out with the living water).
HELP MEEE PLEASEEEEEEEEEEEEEE
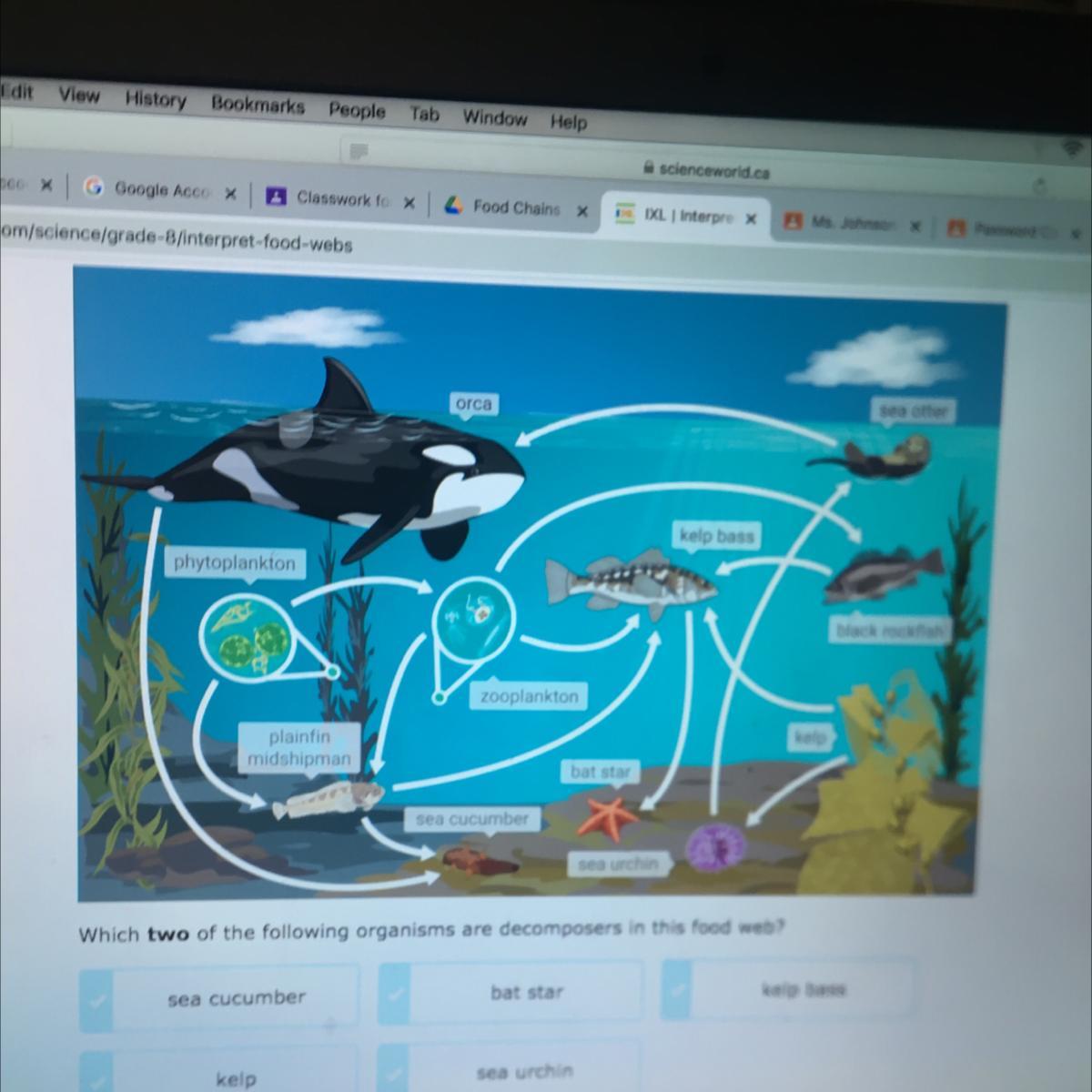
Answers
I think its the sea cucumber and sea urchin
but it could be the star too
What conversion factor will help solve the problem?
2HgO → 2Hg + O2
How many moles of mercury (II) oxide are needed to make 125 grams of O2
125 g O2 x ___1 mol__O2 x ____________ = mols HgO
32.00 g O2
a
2 mol Hg
1 mol O2
b
216.6 g HgO
1 mol HgO
c
1 mol O2
2 mol HgO
d
2 mol HgO
1 mol O2
Answers
Answer:
i fond nothing sooory
pic so PC
Potassium metal reacts with chlorine gas to form solid potassium chloride. Answer the following:
Write a balanced chemical equation (include states of matter)
Classify the type of reaction as combination, decomposition, single replacement, double replacement, or combustion
If you initially started with 78 g of potassium and 71 grams of chlorine then determine the mass of potassium chloride produced.
Answers
The 149.2 grams of potassium chloride would be produced if 78 grams of potassium and 71 grams of chlorine completely reacted.
The balanced chemical equation for the reaction between potassium metal (K) and chlorine gas (Cl₂) to form solid potassium chloride (KCl) is:
2K(s) + Cl₂(g) → 2KCl(s)
This equation indicates that two atoms of potassium react with one molecule of chlorine gas to yield two molecules of potassium chloride.
The type of reaction is a combination reaction, also known as a synthesis reaction. In this type of reaction, two or more substances combine to form a single product.
To determine the mass of potassium chloride produced, we need to calculate the limiting reactant. The molar mass of potassium is approximately 39.1 g/mol, and the molar mass of chlorine is approximately 35.5 g/mol.
First, we convert the given masses of potassium (78 g) and chlorine (71 g) into moles by dividing them by their respective molar masses:
Moles of potassium = 78 g / 39.1 g/mol = 2 mol
Moles of chlorine = 71 g / 35.5 g/mol ≈ 2 mol
Since the reactants have a 1:1 stoichiometric ratio, it can be seen that both potassium and chlorine are present in the same amount. Therefore, the limiting reactant is either potassium or chlorine.
Assuming potassium is the limiting reactant, we can calculate the mass of potassium chloride produced. Since 2 moles of potassium react to form 2 moles of potassium chloride, we can use the molar mass of potassium chloride (74.6 g/mol) to calculate the mass:
Mass of potassium chloride = 2 mol × 74.6 g/mol = 149.2 g
For more such questions on potassium chloride
https://brainly.com/question/24879357
#SPJ11
Question 7 What is the molarity for the following solution: 5. 50 L of 13. 3-MH₂CO (the formaldehyde used to "fix" tissue samples)? (A) 0. 022 mol/L (B) 13. 3 mol/L 2. 2 mol/L D) 0. 0133 mol/L 3 Points
Answers
The molarity of a solution is calculated by dividing the number of moles of solute by the volume of the solution in litres.
Therefore, the molarity of the H₂CO solution is 13.30 mol/L.
In this case, we have 5.50 L of a 13.3 M H₂CO solution. To find the molarity, we need to calculate the number of moles of H₂CO and divide it by the volume of the solution.
The formula weight of H₂CO is 30.03 g/mol. To convert from molarity to moles, we multiply the molarity by the volume in liters:
13.3 mol/L × 5.50 L = 73.15 mol
So we have 73.15 moles of H₂CO in 5.50 L of solution.
Finally, to find the molarity, we divide the number of moles by the volume of the solution:
73.15 mol ÷ 5.50 L = 13.30 mol/L
To know more about solute refer to this:
https://brainly.com/question/8851236
#SPJ11
In one to two sentences, describe an experiment that would show that intramolecular forces (attractions between atoms within molecules) are stronger than intermolecular forces (attractions between molecules)
Answers
In order to demonstrate that intramolecular forces are stronger than intermolecular forces, a block of ice will be heated in a sealed container until it turns into steam.
Why do intramolecular forces outweigh intermolecular forces?
Because the forces holding together compounds are stronger than the forces holding together molecules, intramolecular forces are stronger than intermolecular forces.
Intermolecular forces exist between molecules, but intramolecular forces exist between atoms within a molecule. This is the primary distinction between intermolecular and intramolecular forces.
Look for the molecule with the most polarity, the most electronegative atoms, or the most hydrogen bonding groups if the molecules have identical molar weights and similar intermolecular forces. That one will have the overall stronger IMFs.
Learn more about intramolecular forces at:
https://brainly.com/question/26096719
#SPJ1
A 10.0 mL portion of 0.010 M HCl is added to 100.0 mL of water. What is the pH of the resulting solution? (B) between 2.90 and 3.01 (D) between 1.90 and 2.01 (A) between 3.02 and 3.10 (C) between 2.02 and 2.10
Answers
The pH of the resulting solution is 2.98. Option B is Correct.
The pH of a solution is a measure of its acidity or basicity, on a scale of 0 to 14. The pH of a solution is calculated by taking the negative logarithm of the hydrogen ion (H+) concentration.
The pH of the resulting solution, we need to know the concentration of H+ ions in the initial solution and in the final solution. We can use the following equation to calculate the pH:
pH = -log[H+]
The initial concentration of H+ ions is 0.010 M, and the final volume of the solution is 100.0 mL. To find the concentration of H+ ions in the final solution, we can use the formula:
[H+] = [solute] * V
here [solute] is the concentration of the HCl and V is the volume of the solution.
The concentration of HCl in the initial solution is not given, but we can assume it is also 0.010 M. Therefore, the concentration of H+ ions in the final solution is:
[H+] = 0.010 M * 100.0 * 2.98 mL
= 2.98 mM
The pH of a solution can be calculated using the following equation:
pH = -log[H+]
pH = -log(2.98 mM)
pH = 2.98
Therefore, the pH of the resulting solution is 2.98. Option B is Correct.
Learn more about solution visit: brainly.com/question/25326161
#SPJ4
Biotic components of ecosystem include
Answers
Answer:
Animals, plants, fungi, bacteria, protists, etc.
Explanation:
Biotic means they are alive.
Nitric acid+ calcium oxide gives what
Answers
Answer:
HNO3 + Ca(OH)2 ====> H2O + Ca(NO3)2
it gives water and Calcium nitrate
Hope to helpfull.
Answer:
HNO3 + Ca(OH)2 ====> H2O + Ca(NO3)2
predict what will happen to the silicone compound when mixed with dilute hydrochloric acid.
Answers
Answer:
to produce silicon tetrachloride and water
Explanation:
A container with a volume of 893L contains how may moles of air at STP?
Answers
Answer:
i think 54
Explanation:
In the container with a volume of 893 liters, there are 39.9 moles of air at STP conditions.
We can find the number of moles with the Ideal gas law equation:
\( PV = nRT \)
Where:
P: is the pressure = 1 atm (STP)
V: is the volume = 893 L
n: is the number of moles =?
R: is the gas constant = 0.082 L*atm/(K*mol)
T: is the temperature = 273 K
Hence, the number of moles is:
\( n = \frac{PV}{RT} = \frac{1 atm*893 L}{0.082 L*atm/(K*mol)*273 K} = 39.9 moles \)
Therefore, there are 39.9 moles in the container.
You can find more about Ideal gas law here: https://brainly.com/question/4147359?referrer=searchResults
I hope it helps you!

In an ozone molecule, the three atoms must be connected, so there must at least be a single bond between them. Place
dots in pairs around the oxygen atoms until each oxygen atom has eight valence electrons, starting with the atoms on the
outside and doing the central atom last if there are enough. Do not exceed the total number of valence electrons
identified in part A. Remember that the dashes between the oxygen atoms, which represent single bonds, each indicate
the presence of two valence electrons.
lol
Answers
The structure of an ozone molecule is a resonance structure of three oxygen atoms covalently bonded together.
What is the structure of the ozone molecule?An ozone molecule is a form of oxygen molecule that is composed of three atoms of oxygen covalently bonded to each other.
The formula of the ozone molecule is given as O₃.
An ozone molecule is an important form of oxygen in that it is found in the layer of the atmosphere and acts as a protective layer against harmful ultraviolet radiation from the sun.
The lewis dot structure of ozone shows that an ozone molecule has two resonance structures.
Learn more about ozone at: https://brainly.com/question/14465914
#SPJ1

2. What is the concentration in mol dm-3 of a solution which contains 3 moles of a given solute in 0.25dm³ (250cm³) of solution.
Answers
The concentration in mol dm-³ of a solution which contains 3 moles of a given solute in 0.25dm³ (250cm³) of solution is 12 mol/dm³.
How to calculate molarity?The concentration of a solution can be calculated by dividing the number of moles of the solution by its volume as per the following formula;
Concentration = moles ÷ volume
According to this question, a solution contains 3 moles of a given solute in 0.25dm³ (250cm³) of solution. The concentration of the solution can be calculated as follows:
Concentration = 3moles ÷ 0.25dm³
Concentration = 12 mol/dm³
Therefore, the concentration in mol dm-³ of a solution which contains 3 moles of a given solute in 0.25dm³ (250cm³) of solution is 12 mol/dm³.
Learn more about concentration at: https://brainly.com/question/202460
#SPJ1
DO the postulates of daltons atomic theory explain the law of consrevetion of mass and thelaw of constant composition
Answers
Answer:
see answer
Explanation:
DO the postulates of Daltons atomic theory explain the law of conservation of mass and the law of constant composition.
Dalton's Atomic Theory Postulates1. Everything is composed of atoms, which are the indivisible building blocks of matter and cannot be destroyed or divided any further.
2. All atoms of a single pure element are the same in size and mass
3. Atoms of different elements vary from each other in size and mass.
4. Compounds are produced through different simple whole-number combinations of atoms to produce molecules.
5. Anywhere a compound exists, it will have the same atoms in the same ratio.
6. A chemical reaction between compounds is a re-arrangement of atoms
The law of conservation of mass is shown in 2, 4. and 5.
The law of constant co,position is shown in 2, 4, 5, and 6
a compound is found to contain 53.70% iron and 46.30% sulfur. Find The Empirical Formula Of A Compound Containing 53.70% Iron And 46.30% Sulfur
Answers
Fe2S3 is The Empirical Formula Of A Compound Containing 53.70% Iron And 46.30% Sulfur.
Fe = 0.961 mol and S = 1.44 mol, Now divide them to get lowest empirical formula. When the subscripts in a chemical formula represent the simplest ratio of the kinds of atoms in the compound, the formula is called an empirical formula.
Most ionic compounds are described with empirical formulas. A molecular formula describes the actual numbers of atoms of each element in a molecule. A compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us recognize many of the compounds that are ionic:
When a metal is combined with one or more nonmetals, the compound is usually ionic. This guideline works well for predicting ionic compound formation for most of the compounds typically encountered in an introductory chemistry course. However, it is not always true (for example, aluminum chloride, AlCl3, is not ionic).
To know more about empirical formula here
https://brainly.com/question/1439914
#SPJ4
all zero greenhouse gas emission fuel sources are also renewable.
a. true b. false
Answers
"All zero greenhouse gas emission fuel sources are also renewable". The statement is false.
While many renewable energy sources such as solar, wind, and hydropower produce zero greenhouse gas emissions, not all zero-emission fuels are renewable.
For example, nuclear power is a zero-emission source of electricity, but it is not considered a renewable energy source because it relies on the mining and processing of non-renewable uranium.
Renewable energy sources are defined as those that can be replenished naturally and sustainably within a human timescale. These include solar, wind, hydropower, geothermal, and biomass. Zero-emission fuels refer to any fuel source that emits no greenhouse gases during use, such as hydrogen fuel cells.
While renewable energy sources often overlap with zero-emission fuels, not all zero-emission fuels are renewable. Therefore, it is important to differentiate between the two terms when discussing the sustainability and environmental impact of different energy sources.
Visit here to learn more about greenhouse gas:
brainly.com/question/12684997
#SPJ11
What is the legacy of J. J. Thomson's experiment that led to the identification of the electron?
Please help
Answers
The legacy of J. J. Thomson's experiment that led to the identification of the electron was that he was involved in the experiment using cathode material which provided the particles and its emission means that particle was a fundamental part of all atom.
What is an Atom?This is referred to as a particle of matter that uniquely defines a chemical element and it consists of a central nucleus that is surrounded by one or more negatively charged electrons.
Thomson concluded that the particle was a fundamental part of all atom because of the electron which was emitted in the experiment.
Read more about Atom here https://brainly.com/question/6258301
#SPJ1
Question 23
Marks: 1
The rate at which atoms of radioactive sources (radionuclides) disintegrate are measured in
Choose one answer.
a. rems
b. rods
c. curies
d. roentgens
Answers
The rate at which atoms of radioactive sources, or radionuclides, disintegrate is measured in curies. A curie is a unit of measure for the amount of radioactive material present. It represents the amount of radioactive material in which 37 billion atoms disintegrate per second.
The disintegration of radionuclides produces ionizing radiation, which can be measured in rems or roentgens.
A rem is a unit of measurement for the amount of ionizing radiation absorbed by living tissue, while a roentgen is a unit of measurement for the amount of ionizing radiation in the air.
In summary, the rate at which atoms of radioactive sources disintegrate is measured in curies, while the amount of ionizing radiation produced by the disintegration can be measured in rems or roentgens. It is important to understand these units of measurement in order to properly monitor and regulate exposure to ionizing radiation, as it can have harmful effects on living organisms.
The rate at which atoms of radioactive sources (radionuclides) disintegrate is measured in curies (c).
To explain further, radioactive sources contain unstable atoms, called radionuclides. These radionuclides undergo disintegration or decay, during which they emit radiation. To quantify this process, we use various units.
Curies (Ci) is a unit of measurement specifically used to express the activity of a radioactive substance, or how quickly atoms in the radioactive source are disintegrating. One curie represents 37 billion disintegrations per second.
It's important to note the other units you mentioned:
- Rems (roentgen equivalent in man) is a unit used to measure the biological impact of ionizing radiation on human tissue.
- Roentgens (R) is a unit used to measure the exposure to ionizing radiation, specifically the amount of radiation that produces a certain amount of ionization in air.
- Rods is not a unit related to radioactivity, but might be confused with control rods, which are used in nuclear reactors to control the rate of nuclear reactions.
In summary, the appropriate unit for measuring the rate at which atoms of radioactive sources disintegrate is curies.
Learn more about radioactive at : brainly.com/question/1770619
#SPJ11
10. As the temperature of a fixed volume of a gas increases, the pressure will _______
answer is increase
Answers
How much of a sample remains after five half-lives have occurred?
1/5 of the original sample
1/25 of the original sample
1/32 of the original gample
1/64 of the original sample
Answers
The first half-life, we have 1 • 1/2 = 1/2 left.
After two: 1/2 • 1/2 = 1/4
Three: 1/4 • 1/2 = 1/8
Four: 1/8 • 1/2 = 1/16
And five: 1/16 • 1/2 = 1/32.
Answer:
1/32
Explanation:
Why would we want to correct for water vapor?
Answers
Answer:
The vapor pressure due to water in a sample can be corrected for in order to get the true value for the pressure of the gas.
Explanation:
when magnesium reacts with chlorine, the chlorine atom gain electrons. what happens to chlorine in this reaction?
a. it is oxidized
b. it is synthesized
c. it is decomposed
d. it is reduced
Answers
Answer:
D
Explanation:
Reduction refers to gain in electrons. Hence it is D
Oxidation occurs when the element loses electrons. Hence A is wrong
what is the most dangerous element to a star?
hydrogen
lithium
helium
iron
Answers
Which element is this?

Answers
It’s It is tin
The chemical formula for water is h2o. According to this formula what is the composition of a water molecule
Answers
Answer:
two hydrogen atoms and one oxygen atom
Explanation:
2. During which of the following stato changes would the particles in the matter become more spread out?
changing from liquid to gas?
changing from gas to liquid?
changing from gas to solid?
Answers
Answer
changing from liquid to gas
Explanation
changing from liquid to gas