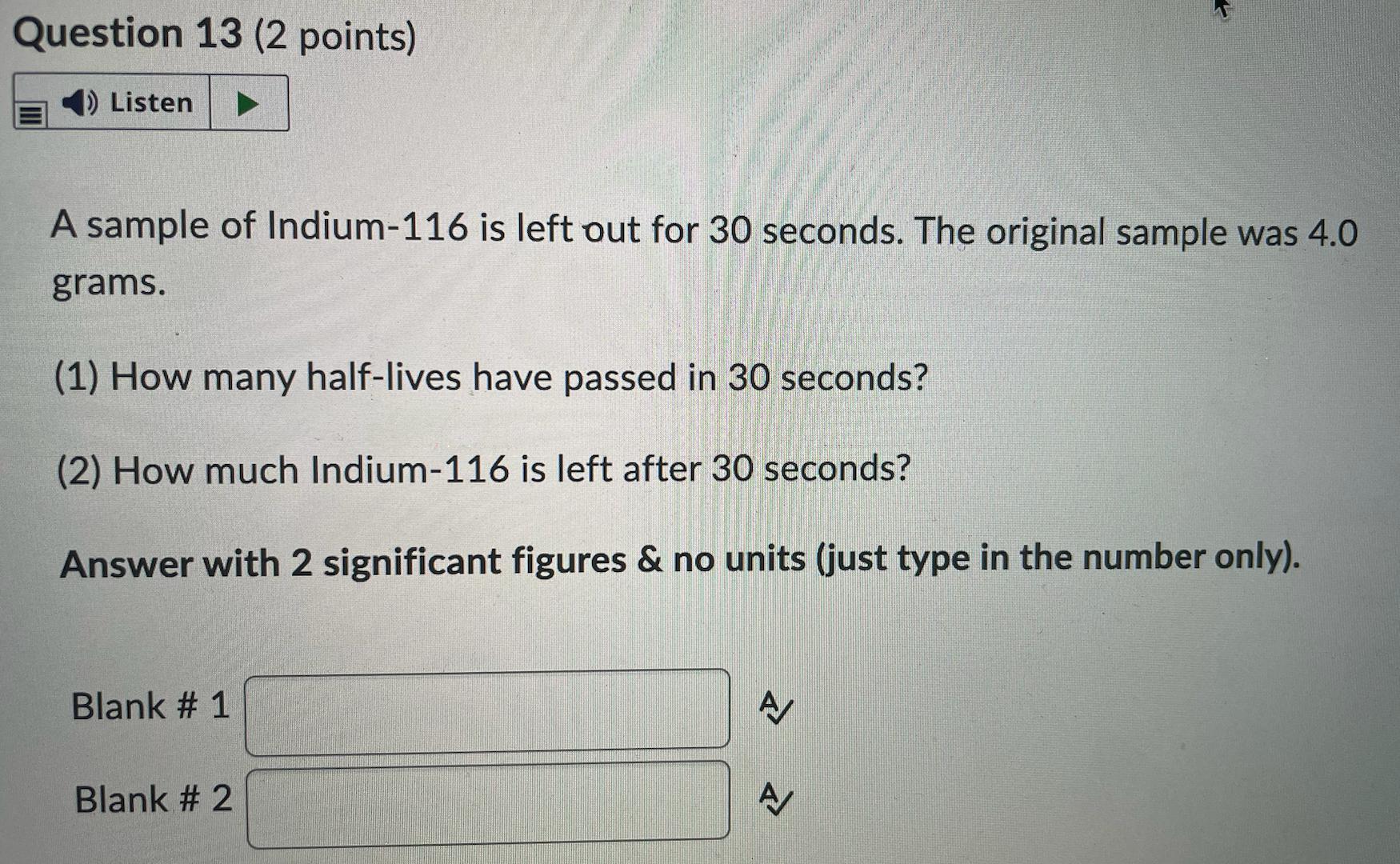
Carbon-14 is commonly used to determine how old fossils and artifacts are. You have a 0.5 milligram sample of Carbon-14 and it is put into storage for 100 years.(1) How many half-lives have passed in 100 years?(2) How much Carbon-14 is left after 100 years?Answer with 3 significant figures & no units (just type in the number only).Question 14 options:Blank # 1:Blank # 2:

Answers
Answer:
Explanations:
The formula for calculating the exponential decay of an element is expressed as:
\(N(t_{\frac{1}{2}})=N_0e^{-\lambda t\frac{1}{2}}\)No is the original sample
N(t) is the final sample after time "t"
λ is the decay constant
t is the time
Given the following parameters
No = 4.0grams
t = 30 seconds
Determine the decay constant
\(undefined\)Related Questions
How many atoms of hydrogen are in 3 moles of ammonia, NH3?
Answers
Answer:
\(5.4198\) × \(10^{24}\) atoms of hydrogen
Explanation:
Firstly, we need to know how many atoms of \(NH_{3}\) are there in 3 moles of \(NH_{3}\).
We know that there is \(6.022\) × \(10^{23}\) atoms in a mole of any substance.
\(6.022\) × \(10^{23}\) is Avogadro's Constant
\(6.022\) × \(10^{23}\) × 3 = \(1.8066\) × \(10^{24}\)
Therefore in 3 moles of ammonia, there are \(1.8066\) × \(10^{24}\) atoms.
Next, you should determine how many molecules of an element is present in ammonia.
There is 1 Nitrogen molecule and 3 Hydrogen molecules for every mole of ammonia.
Finally, since we know 3 moles of ammonia contains \(1.8066\) × \(10^{24}\) atoms, we just have to multiply accordingly to get the individual amount of atoms of an element a compound has.
For Nitrogen :
1 × \(1.8066\) × \(10^{24}\) = \(1.8066\) × \(10^{24}\) atoms of nitrogen
For Hydrogen :
3 × \(1.8066\) × \(10^{24}\) = \(5.4198\) × \(10^{24}\) atoms of hydrogen
Therefore, there is \(5.4198\) × \(10^{24}\) hydrogen atoms in 3 moles of ammonia.
Hope this answer helped!How many grams of CO are produced when 41.0 g of C reacts?
Answers
Answer:
95.7 g CO to the nearest tenth.
Explanation:
2C + O2 ---> 2CO
Using relative atomic masses:
24 g C produces 2*12 + 2*16 g CO.
So 41 g produces ( (2*12 + 2*16) * 41 ) / 24
= 95.7 g CO,
Which elements cannot have more than an octet of electrons? Select all that apply
C
S
O
N
Br
Answers
Answer:
{ Carbon, Oxygen, Nitrogen }
Explanation:
Elements can only have more than an octet of electrons if they demonstrate an expanded octet. This is if they belong to groups in or beyond the third group. Why? Well these elements have d - orbitals that they can rely on to expand the number of electrons that could otherwise be limited. * Here we are focusing on main group elements, P - block elements more specifically. *
Carbon belongs to the 2 group, and thus doesn't have an empty d - orbital. Thus, it can't have more than an octet of electrons. Sulfur belongs to group 3, hence has an empty d - orbital, and can have more than an octet of electrons. Oxygen belongs to the 2 group, and thus doesn't have an empty d - orbital, so it can't have more than an octet of electrons. Same goes for Nitrogen. Bromine belongs to group 4, thus has empty d - orbitals, and can expand further than Sulfur can - it can have more than an octet of electrons.
Solution = { Carbon, Oxygen, Nitrogen }
the picture below shows an asteroid and a comet. Which of these statements is most likely correct about both asteroids and comets?
A. They are created from space debris.
B. They have the same number of satellites.
C. They do not consist of ice and dust.
D. They do not experience gravitational force.
Answers
Answer: They are created from space debris.
The following Lewis diagram represents the valence electron configuration of a main-group element.
This element is in group
.
According to the octet rule, this element would be expected to form an ion with a charge of
.
If is in period 5, the ion formed has the same electron configuration as the noble gas
.
The symbol for the ion is
.
Answers
This element is in group 1.
According to the octet rule, this element would be expected to form an ion with a charge of +1.
If X is in period 5, the ion formed has the same electron configuration as the noble gas Krypton
The symbol for the ion is Rb⁺
What is electronic configuration?Electronic configuration refers to the arrangement of electrons in the orbitals of an atom or molecule, indicating the energy level of the electrons, the number of electrons in each energy level, and the number of electrons in each orbital.
Considering the given element:
It has one valence electron, hence it is in group 1. Group 1 elements form ions with a charge of +1.
Losing one electron will give the ion the same electron configuration as Kyrton since it is the noble gas in Period 4.
The element is rubidium and the ion is Rb⁺.
Learn more about electronic configuration at: https://brainly.com/question/26084288
#SPJ1

Determine the mass of H2O produced if 1.75g of AL(CH)3 reacts with 2.00g of H2SO4 if H2SO4 is a limitating reagent
Answers
Answer: We must first compute the quantity of H2O that can result from the reaction of AL(CH)3 and H2SO4 in order to determine the mass of H2O produced.
Explanation:
Let's begin by formulating the reaction's balanced chemical equation:
Al2(SO4)3 + 6 H2O + 2 CH4 = 2 AL(CH)3 + 3 H2SO4
According to the equation, 2 moles of AL(CH)3 and 3 moles of H2SO4 combine to form 6 moles of H2O.
1.75 g of AL(CH)3 is equal to: 1.75 g / 78.0 g/mol = 0.0224 mol of AL(CH)3 as 1 mole of AL(CH)3 has a molar mass of roughly 78.0 g/mol.
2.00 g of H2SO4 is equal to: 2.00 g / 98.1 g/mol = 0.0204 mol of H2SO4 as 1 mole of H2SO4 has a molar mass of roughly 98.1 g/mol.
In order to calculate the amount of H2O produced, we must now identify the limiting reagent.
We contrast the moles of AL(CH)3 and H2SO4 in use. AL(CH)3 and H2SO4 have a stoichiometric ratio of 2 to 3. As a result, 3 moles of H2SO4 are required for every 2 moles of AL(CH).
The equation states that 2 moles of AL(CH)3 will yield 6 moles of water. Therefore, we can calculate the moles of H2O created for 0.0224 mol of AL(CH)3 as follows: 0.0224 mol AL(CH)3 (6 mol H2O / 2 mol AL(CH)3) = 0.0672 mol H2O
Finally, we may use the molar mass of H2O to convert its moles to grams. H2O has a molar mass of about 18.0 g/mol:
18.0 g/mol x 0.0672 mol H2O = 1.21 g H2O
As a result, the mass of H2O generated is roughly 1.21 grams if H2SO4 is the limiting reagent.
HELP!!!
I DON'T UNDERSTAND!!!

Answers
Answer:
I dont either lol
Explanation:
what happens when muscle cells are triggered?
Answers
Answer:
When myocytes (aka muscle cells) are triggered at a certain point for awhile it can cause strain and pain throughout the muscle.
How many moles of carbon dioxide are present if the sample contains
11.8 x 10^23 atoms of oxygen? Assume all the oxygen atoms are part of
carbon dioxide molecules.
Answers
Answer: 0.983 moles of carbon dioxide are present
Explanation:
To determine the number of moles of carbon dioxide (CO₂) present when there are 11.8 x 10²³ atoms of oxygen, we need to consider the molecular formula of carbon dioxide.
The molecular formula of carbon dioxide (CO₂) indicates that there is one carbon atom and two oxygen atoms in each molecule of carbon dioxide.
Given that there are 11.8 x 10²³ atoms of oxygen, we can divide this number by 2 to determine the number of carbon dioxide molecules present, since each molecule of carbon dioxide contains two oxygen atoms.
Number of carbon dioxide molecules = \(\frac{11.8 X 10^2^3 atoms of oxygen }{2}\)
Next, we can use Avogadro's number, which states that there are approximately 6.022 x 10²³molecules per mole, to convert the number of carbon dioxide molecules to moles.
Number of moles of carbon dioxide = (11.8 x 10²³ atoms of oxygen / 2) / (6.022 x 10^23 molecules per mole)
Evaluating this expression, we get:
Number of moles of carbon dioxide = 0.983 moles
For a reaction Y → X with a very high equilibrium constant, K, which of the following is true? You can refer to the following two equations in formulating your answer.$$$$Choose one:A. The standard free-energy change is large and positive.B. The amount of product and substrate are equal at equilibrium.C. The forward reaction rate greatly exceeds the reverse reaction rate at equilibrium.D. The amount of product will be higher than the amount of substrate at equilibrium.
Answers
Answer:The amount of product will be higher than the amount of substrate at equilibrium
Explanation:
Recall that the equilibrium constant K depends on the amounts of reactants and products present in the system at equilibrium.
Considering the equation; K = [X]/[Y], as the concentration of X increases above that of Y, the equilibrium constant K becomes very high, hence the answer above.
How does light travel through a solid, liquid, gas, and a vacuum?
Answers
Light travels in a straight line as a wave. It does not require any medium or material to travel like sound waves which means it can travel in vacuum.The speed of the light from the special theory of relativity is a constant value of 38x10^8m/second
The statement “the scientific process is open ended” means:
Answers
Answer:
think it helps you
Explanation:The statement “the scientific process is open ended” means: Would an element with 7 valence electrons be more or less reactive than an element with 3 valence electrons? Element 1 is a hard dark-red solid
Helium is a....
a. metalloid
b. nonmetal
c. metal
Answers
Answer:
NonmetalExplanation:
Describe the molecular structure around the indicated atom or atoms:
(a) the sulfur atom in sulfuric acid, (b) the chlorine atom in chloric acid, (c) the oxygen atom in hydrogen peroxide, HOOH
(d) the nitrogen atom in nitric acid, (e) the oxygen atom in the OH group in nitric acid, (f) the central oxygen atom in the ozone molecule, (g) each of the carbon atoms in propyne, (h) the carbon atom in Freon, (i) each of the carbon atoms in allene,
Answers
(a) tetrahedral (b) tetrahedral (c) bent or V-shaped (d) trigonal planar (e) bent or V-shaped (f) bent or V-shaped (g) linear (h) tetrahedral (i) trigonal planar
(a) The molecular structure is tetrahedral around the sulfur atom, with the four oxygen atoms forming the vertices of the tetrahedron.
(b) The molecular structure is tetrahedral around the chlorine atom, with the three oxygen atoms and hydrogen atom.
(c) The molecular structure is bent or V-shaped around the oxygen atom, with the two hydrogen atoms at the bottom of the V.
(d) The molecular structure is trigonal planar around the nitrogen atom, with the three oxygen atoms and hydrogen atom.
(e) The molecular structure is bent or V-shaped around the oxygen atom, with the hydrogen atom and nitrogen atom.
(f) The molecular structure is bent or V-shaped around the central oxygen atom, with the two other oxygen atoms at the bottom of the V.
(g) The molecular structure around each carbon atom is linear, with the two other carbon atoms forming a straight line with the carbon atom at the center.
(h) The molecular structure around the carbon atom is tetrahedral, with the two other carbon atoms and two fluorine atoms forming the vertices of the tetrahedron.
(i) The molecular structure around each carbon atom is trigonal planar, with the two other carbon atoms forming a straight line.
To know more about the molecular structure, here
brainly.com/question/503958
#SPJ4
--The complete question is, Describe the molecular structure around the indicated atom or atoms:
(a) the sulfur atom in sulfuric acid,
(b) the chlorine atom in chloric acid,
(c) the oxygen atom in hydrogen peroxide, HOOH
(d) the nitrogen atom in nitric acid,
(e) the oxygen atom in the OH group in nitric acid,
(f) the central oxygen atom in the ozone molecule,
(g) each of the carbon atoms in propyne,
(h) the carbon atom in Freon,
(i) each of the carbon atoms in allene--
How many moles are in 25.58 grams of H20? SHOW WORK
Answers
Answer:
1.42 moles
Explanation:
25.58 g of H₂O .
Molecular weight of water = 2 x 1 + 16 = 18
1 mole of water = 18 g of water
18 g of water = 1 mole
25.58 g of water = 25.58 / 18 mole
= 1.42 moles .
Calculate the number of kilograms of sodium fluoride needed per year for a city of 100,000 people if the daily consumption of water per person is 150.0 gallons.
Answers
Fluoridation is the process of adding fluorine to drinking water to avoid tooth decay.
The question is incomplete; the complete question is;
Fluoridation is the process of adding fluorine compounds to drinking water to help fight tooth decay. A concentration of 1 ppm of fluorine is sufficient for the purpose. (1 ppm means one part per million, or 1 g of fluorine per 1 million g of water.) The compound normally chosen for fluoridation is sodium fluoride, which is also added to some toothpastes. Calculate the quantity of sodium fluoride in kilograms needed per year for a city of 50,000 people if the daily consumption of water per person is 150 gallons.
(Sodium fluoride is 45.0 percent fluorine by mass. 1 gallon = 3.79 L; 1 year = 365 days; 1 ton = 2000 lb; I lb = 453.6 g; density of water = 1.0 g/mL.)
The quantity of sodium fluoride in kilograms needed per year for a city of 50,000 people if the daily consumption of water per person is 150 gallons is \(9.32 * 10^9 Kg\).
From the question, we know that;
Daily consumption of water per person = 150 gallons.
Population of the city = 100,000 people
Total daily city consumption =\(100,000 * 150 gallons = 15 * 10^6 gallons\)
Annual water consumption = \(15 * 10^6 gallons * 365 = 5.475 * 10^9 gallons\)
1 gallon = 3.785 L = 3.785 kg
\(5.475 * 10^9 gallons = 5.475 * 10^9 gallons * 3.785 kg/ 1 gallon = 2.1 * 10^10 Kg\)
Since sodium fluoride accounts for 45% by mass of water
Mass of sodium fluoride= \(0.45 * 2.1 * 10^10 Kg= 9.32 * 10^9 Kg\)
Learn more; https://brainly.com/question/14805580
In run 1, you mix 7.9 mL of the 43 g/L MO solution (MO molar mass is 327.33 g/mol), 3.13 mL of the 0.040 M SnCl2 in 2.0 M HCl solution, 5.49 mL of 2.0 M HCl solution, and 3.43 mL of 2.0M NaCl solution. What is the [H3O+]? Remember that there is a contribution of H3O+ from two solutions.
Answers
Answer:
Concentration of H3O⁺ [H3O⁺] = 0.864 M
Explanation:
Given that:
The mass concentration of MO = 43 g/L
The volume of MO = 7.9 mL = 7.9 × 10⁻³ L
Recall that
The mass number of MO = Mass concentration of MO × Volume of MO
The mass number of MO = (43 g/L) * (7.9 × 10⁻³ L)
The mass number of MO = 0.3397 g
number of moles of MO = (mass number of MO) / (molar mass of MO)
number of moles of MO = (0.3397 g) / (327.33 g/mol)
moles of MO = 0.00104 mol
The total volume = 7.9 mL + 3.13 mL + 5.49 mL + 3.43 mL
The total volume = 19.95 mL = 19.95 × 10⁻³ L
Concentration of MO [MO} =(number of moles of MO) / (total volume)
[MO] = 0.00104 mol / 19.95 × 10⁻³ L
[MO] = 5.2130 × 10⁻⁸ M
the number of moles of H3O⁺ = molarity of HCl in the solution × the volume of HCl in solution
the number of moles of H3O⁺ = [(2.0 M) * (3.13 mL)] + [(2.0 M) * (5.49 mL)]
the number of moles of H3O⁺ = 17.24 mmol
Concentration of H3O⁺ [H3O⁺] = (the number of moles of H3O⁺) / (total volume)
Concentration of H3O⁺ [H3O⁺] = (17.24 mmol) / (19.95 mL)
Concentration of H3O⁺ [H3O⁺] = 0.864 M
The Concentration of H3O⁺ [H3O⁺] is 0.864 M
Calculation of the H3O⁺ concentration:But before that following calculations should be needed.
The mass number of MO = Mass concentration of MO × Volume of MO
= (43 g/L) * (7.9 × 10⁻³ L)
= 0.3397 g
Now
number of moles of MO = (mass number of MO) / (molar mass of MO)
= (0.3397 g) / (327.33 g/mol)
= 0.00104 mol
Now
The total volume = 7.9 mL + 3.13 mL + 5.49 mL + 3.43 mL
= 19.95 mL
= 19.95 × 10⁻³ L
Now
Concentration of MO [MO} =(number of moles of MO) / (total volume)
= 0.00104 mol / 19.95 × 10⁻³ L
5.2130 × 10⁻⁸ M
Now
the number of moles of H3O⁺ = molarity of HCl in the solution × the volume of HCl in solution
= [(2.0 M) * (3.13 mL)] + [(2.0 M) * (5.49 mL)]
= 17.24 mmol
Now
Concentration of H3O⁺ [H3O⁺] = (the number of moles of H3O⁺) / (total volume)
= (17.24 mmol) / (19.95 mL)
= 0.864 M
Hence, The Concentration of H3O⁺ [H3O⁺] is 0.864 M
learn more about concentration here: https://brainly.com/question/17844928
• How does the name of the salt tell us that:
a) there is just one other element combined with the metal?
b) there is oxygen present in the salt?
Answers
The name of the salt tells us that:
a) there is just one other element combined with the metal by looking at the suffix of the salt's name.
b) the presence of oxygen in a salt can be indicated by the name of the salt.
a) The name of a salt can tell us that there is just one other element combined with the metal by looking at the suffix of the salt's name. If the salt name ends in "-ide," it indicates that the salt is composed of a metal and a single non-metal element.
For example, sodium chloride (NaCl) and potassium bromide (KBr) are salts where the metal (sodium and potassium) is combined with a single non-metal element (chlorine and bromine, respectively). The "-ide" suffix suggests the presence of only one other element in the salt.
b) The presence of oxygen in a salt can be indicated by the name of the salt. If the salt name includes the element oxygen, it suggests that oxygen is present in the salt compound.
For example, sodium carbonate (Na₂CO₃) and calcium sulfate (CaSO₄) contain the element oxygen in their chemical formulas. The presence of oxygen in the salt is implied by the name and the combination of elements in the compound.
Therefore, the name of salt tells us that there is just one other element combined with the metal and there is oxygen present in the salt
Learn more about salt here:
https://brainly.com/question/31814919
#SPJ 1
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
How many moles of KBr are present in 500 ml of a 0.8 M KBr solution?
1. 1.6
2. .4
3. .625
4. 625
5. .0016
Answers
Answer:
2) 0.4 mol
Explanation:
Step 1: Given data
Volume of the solution (V): 500 mLMolar concentration of the solution (M): 0.8 M = 0.8 mol/LStep 2: Convert "V" to L
We will use the conversion factor 1 L = 1000 mL.
500 mL × 1 L/1000 mL = 0.500 L
Step 3: Calculate the moles of KBr (solute)
The molarity is the quotient between the moles of solute (n) and the liters of solution.
M = n/V
n = M × V
n = 0.8 mol/L × 0.500 L = 0.4 mol
Which relationship or statement best describes ΔS° for the following reaction?
KCl(s) → K+(aq) + Cl−(aq)
Explain why.
A. ΔS° ≈ 0
B. ΔS° = ΔH°/T
C. ΔS° > 0
D. ΔS° < 0
E. More information is needed to make a reasonable prediction.
Answers
The ΔS° value for the reaction KCl(s) → K+(aq) + Cl−(aq) is ΔS° > 0, as the products have a higher degree of disorder than the reactant due to an increase in the number of particles in solution. Hence the correct option is (C) ΔS° > 0.
The ΔS° value for a reaction represents the change in the entropy of the system, which is a measure of the disorder or randomness of the system. The reaction KCl(s) → K+(aq) + Cl−(aq) involves a solid compound breaking down into two separate aqueous ions, which means that the products have a higher degree of disorder than the reactant. This increase in the number of particles in solution results in an increase in entropy, which means that ΔS° > 0. Option (A) is incorrect because the reaction involves a change in state, which results in an increase in entropy. Option (B) is incorrect because it represents the relationship between enthalpy and entropy, not the ΔS° value for this particular reaction. Option (D) is incorrect because the reaction results in an increase in entropy, not a decrease. Option (E) is incorrect because the given information is sufficient to predict the sign of ΔS°.
To know more about reaction please refer: https://brainly.com/question/28984750
#SPJ1
PLEASE HELPPP AOSAPAOPSP
What are some of the difficulties in identifying particular drugs? Why is it important for forensic scientists to be able to identify particular drugs?
Answers
some medicines contain legal ingredients like starch and sugar, and forensic scientists must be able to distinguish between the two. Because courts rely on the data forensic scientists find in their research, it is crucial for them to be able to recognise specific substances.
How do you identify forensic drugs?The ideal technique for this evaluation is Gas Chromatography/Mass Spectrometry (GC/MS), which is frequently used in forensic laboratories. The method offers a quick, semi-automated analysis of the material and often produces enough data to pinpoint the problematic substances. To ascertain whether an unlawful substance is present in the supplied material is the aim of forensic drug chemistry. Law enforcement can seek criminal charges based on the study' findings, and the court can decide on the right sentence.
To know more about forensic drugs visit:
https://brainly.com/question/1347938
#SPJ1
Match the solution with the correct concentration.

Answers
The solution with correct concentration is -- 4 m: One kilogram of water contains 4 moles of H₂SO₄ (molality )
4M : One Liter of water contains four moles of H₂SO₄ (molarity)4 Molal : When 4 moles of the solute are dissolved in 1 kilogram of solvent—usually water—this is referred to as a solution concentration. 4 Molar: A solution concentration in which 4 moles of the solute are dissolved in 1 Liter of solvent, typically water, is referred to as this.In chemistry, what does concentration mean?In chemistry, the abundance of a component divided by the total volume of a mixture is what is meant by the term "concentration." Quantitative descriptions can be broken down into four categories: concentrations of mass, molarity, number, and volume are all measured in these units.
The term "concentration" can be used to describe any combination of chemicals, but solutes and solvents in solutions are the most common examples. There are two types of molar concentration, also known as quantity: osmotic concentration and the standard concentration.
What is molality ?The "total moles of a solute contained in a kilogram of a solvent" is how molality is measured. Molal concentration is another name for molality. It is a measurement of how much solute is in a solution.
Incomplete question :
Match the solution with the correct concentration.
4 m:
4 M:
4 Molal:
4 Molar
1. 4 moles of H₂SO₄ are placed into 1 Liter of water
2. 4 moles of H₂SO₄ are placed into 1 kg of water
3. a solution concentration where 4 moles of solute are dissolved in 1 kg of solvent
4. a solution concentration where 4 moles of solute are dissolved in 1 Liter of solvent
Learn more about Concentration :
brainly.com/question/26255204
#SPJ1
A laser emits light that has a frequency of 6.69 x 1014 s-1. If the laser emits a "pulse" (a burst of light) that contains 7.0 x 1017 photons, what is the total energy of that pulse in Joules? Do not use scientific notation in your answer. Give the answer ( in Joules) to 2 significant figures.
Answers
Answer:
0.31 Joule
Explanation:
ΔE(per photon) = h·f = (6.63 x 10⁻³⁴J·s)(6.69 x 10¹⁴s⁻¹) = 4.44 x 10⁻¹⁹ J/photon X 7 x 10¹⁷photons = 0.31 Joules
Question 2 of 10
What is the percent yield of a reaction?
The amount of product obtained x 100
amount possible
B. The amount of product actually obtained in a reaction
C. The amount of product that is possible from a reaction
D. The difference between measured and calculated amounts
A.
Answers
Answer:
c
Explanation:
Can someone please help me with this question. I got half of the question and I am stuck on the rest.

Answers
The mean of the data set is approximately 4.0626, and the 90% confidence interval is [4.060925, 4.064275].
What is the mean and 90% confidence interval of the given data?The sample mean (x) is calculated as follows:
x = (4.0620 + 4.0550 + 4.0650 + 4.0740 + 4.0550 + 4.0660) / 6
x ≈ 4.0626 (rounded to four decimal places)
The 90% confidence interval is calculated as follows;
Standard deviation (s):
(4.0620 - 4.0626)² = 0.00000036
(4.0550 - 4.0626)² = 0.00000576
(4.0650 - 4.0626)² = 0.00000006
(4.0740 - 4.0626)² = 0.00001328
(4.0550 - 4.0626)² = 0.00000576
(4.0660 - 4.0626)² = 0.00000012
average of the squared differences:
(0.00000036 + 0.00000576 + 0.00000006 + 0.00001328 + 0.00000576 + 0.00000012) / 6 ≈ 0.00000624
s = √(0.00000624)
s ≈ 0.002496
the standard error of the mean (SEM):
SEM = 0.002496 / √6
SEM ≈ 0.001018
For a 90% confidence interval, the z value is approximately 1.645.
ME = 1.645 * 0.001018 ≈ 0.001675
CI = x ± ME
CI = 4.0626 ± 0.001675
CI ≈ [4.060925, 4.064275]
Learn more about mean and confidence intervals at: https://brainly.com/question/20309162
#SPJ1
An absorption measurement with a 1 cm path length yields a reading of 0.002 absorbance units with a noise of 0.0005 absorbance units and a mean noise reading of 0 for 5 scans averaged together. Assuming the noise for a single scan does not change if the path length is increased to 5 cm and the number of scans is increase to 65, what is the signal to noise of the new measurement. Remember signal averaging
Answers
Answer:
Assuming the noise remains constant, the total noise for the longer path length, 65 scans should be 0.0005 x 65 = 0.0325 absorbance units. The new reading should be 0.01 absorbance units. The signal to noise ratio (SNR) of the new measurement will be 0.01/0.0325 = 3.08.
Signal averaging decreases the magnitude of the residual noise and increases the SNR. The total noise decreases by the square root of the number of scans, in this case √65 = 8, so for 65 scans, the noise level is 8 times lower than for a single scan.
Which action would most likely create a light waye?
A. producing a fire fram fuel
B. dropping a pebble in a pond
O
C. pouring water into a pile of dirt
D. hitting a drum with a drumstick
Answers
Answer:
A. Producing a fire gram fuel
Explanation:
None of the other actions create light. Fire gives off light and heat.
60 points!! Look at picture please don’t troll
Answers
Which example is an biotic factor of an aquarium environment?
Answers
Answer: fish
Explanation: they are living organisms; they live in water