Cells that have a high level of reactive oxygen species would need which of the following pathway(s) to be active in order to reduce the high level of oxidative stress?
A. Oxidative Phase of PPP
B. Non-Oxidative Phase of PPP
C. Glycolysis
D. Gluconeogenesis
More NADPH --> oxidative phase
non-oxidative phase--> fructose 6- bisphosphate and 3 glyceraldehyde
gluconeogensis: convert it to fructose 6- bisphosphate and 3 glyceraldehyde to make even more glucose
Since the cell needs high levels of NADPH. The pathways that would provide the most NADPH would be the oxidative phase of PPP, the non-oxidative phase of PPP producing fructose 6-phosphate and glyceraldehyde 3-phosphate, and gluconeogenesis using fructose 6-phosphate and glyceraldehyde 3-phosphate to produce more glucose 6-phosphate that can be used to generate more NADPH.
Answers
The oxidative segment PPP is the pathway(s) to be active in order to reduce the high level of oxidative stress.
This segment is made up of two irreversible steps: Step 1: Glucose-6-phosphate is oxidized to shape lactone. NADPH is produced as a byproduct of this response as NADP +begin superscript, plus, cease superscript is decreased as glucose-6-phosphate is oxidized.
More NADPH --> oxidative phasenon-oxidative segment--> fructose 6- bisphosphate and three glyceraldehydegluconeogensis: convert it to fructose 6- bisphosphate and three glyceraldehyde to make even extra glucose
Since the mobileular wishes excessive ranges of NADPH. The pathways that might offer the maximum NADPH will be the oxidative segment of PPP, the non-oxidative segment of PPP generating fructose 6-phosphate and glyceraldehyde 3-phosphate, and gluconeogenesis the usage of fructose 6-phosphate and glyceraldehyde 3-phosphate to provide extra glucose 6-phosphate that may be used to generate extra NADPH.
Read more about glycolysis;
https://brainly.com/question/28874793
#SPJ4
Related Questions
How many mL of C8H18 are needed to react with 0.0500 mol O2? 2 C8H18 (l) + 25 O2 (g) - 16 CO2(g) + 18 H20 (g) molar masses: C8H18 = 114.22g/mole H2O = 18.02 g/mole CO2 = 44.01 g/mole O2 = 32.00 g/mole and make sure the answer has significant figures
Answers
Answer:
0.45688mL
Explanation:
1) First you should look on the coefficients.
\(2C_8H_{18(l)}\ + 25O_2_{(g)}=\ 16CO_{2(g)}+ 18H_2O_{(g)}\)
2) Do the ratio between C8H18 and O2:
\(C_8H_{18}\ \ \ \ \ \ \ \ \ O_2\\2 : \ \ \ \ \ \ \ \ \ \ \ \ \ 25\\X: \ \ \ \ \ \ \ \ \ \ \ 0.0500moles\\2X0.0500/25= 0.004\\X=0.004 \moles\ of\ C_8H_{18}\)
3) grams= moles X molecular mass
\(0.004*114.22= 0.45688g\)
[1g=1mL] => 0.45688mL
dentify the compound that does NOT have hydrogen bonding. Identify the compound that does NOT have hydrogen bonding. HF (CH3)2N(CH2) 4 CH3 H2O CH3(CH2) 4 OH CH3(CH2) 2 NH2
Answers
Answer:
(CH3)2N(CH2)
Explanation:
A hydrogen bond occurs in a molecule when hydrogen is covalently bonded to a highly electronegative atom.
That means that if we are looking for a compound in which there is no hydrogen bonding, we are actually looking for the compound in which hydrogen is not directly linked to an electronegative atom.
If we look at the options carefully, the compound that has such characteristic is only (CH3)2N(CH2) which is a tertiary amine. The nitrogen is directly linked to three alkyl groups and no hydrogen atom hence the molecule does not exhibit hydrogen bonding.
An object momentum is determined by the objects:
A. Weight and velocity
B. Mass and Acceleration
C. Mass and velocity
D. Weight and acceleration
Answers
Into a 125 mL Erlenmeyer flask was added some KI and H2SO4 as described in the procedure section of this experiment. Then 3.0 mL of bleach solution was added to the flask and it was immediately titrated with 0.1261 M sodium thiosulfate solution. It required 41.26 mL of this sodium thiosulfate solution to reach the end point of the titration. What is the percentage of NaOCl in the bleach
Answers
Answer:
answer is a
Explanation:
i took quiiiiiiz
Photoelectric effect will occur only if frequency of light striking an electron in a metal is above a certain threshold frequenci
Answers
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. The frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
The statement is correct. The photoelectric effect refers to the phenomenon where electrons are ejected from the surface of a material when it is exposed to light. However, for the photoelectric effect to occur, the frequency of the incident light must be above a certain threshold frequency.
The threshold frequency is the minimum frequency of light required to dislodge electrons from the material. Below this threshold frequency, regardless of the intensity or duration of the light, no electrons will be emitted.
This behavior can be explained by the particle-like nature of light, where light is composed of discrete packets of energy called photons. The energy of a photon is directly proportional to its frequency. Only photons with energy greater than or equal to the binding energy of the electrons in the material can dislodge them.
Therefore, the frequency of light striking an electron in a metal must be above the threshold frequency in order for the photoelectric effect to occur.
For more question on photoelectric
https://brainly.com/question/1458544
#SPJ8
what lactol (cyclic hemiacetal) is formed from intramolecular cyclization of the following hydroxy aldehyde?
Answers
The hydroxy aldehyde will undergo intramolecular cyclization to produce a lactol (cyclic hemiacetal), which has the formula C3H6O2.
In this reaction, an aldehyde molecule combines with a hydroxyl group from a different molecule of itself, which is an example of an aldol condensation reaction. The aldehyde molecule can then create a cyclic hemiacetal by combining a carbonyl group and a hydroxyl group to create a hemiacetal. A lactol is the name for this cyclic hemiacetal.
An,-unsaturated carbonyl compound (usually an aldehyde or a ketone) is created by the chemical reaction known as the Aldol condensation reaction from two aldehyde or ketone molecules. In order to produce the unsaturated product, the reaction calls for the nucleophilic addition of the enolate of one molecule to the carbonyl group of the other molecule.
For more information on cyclic hemiacetal click on
https://brainly.com/question/28030326
#SPJ4
Define biotechnology. } List two advantages in the use of biotechnology
Answers
Advantages of biotechnology:
Improvement of plants and animal breeds to give a high yield of their products.
Pests and pathogen control in agriculture which will reduce the loss of yield in food crops.
Synthesis of biocatalyst which can be used for enhancing the reactions which can be carried out in vitro or laboratory conditions.
Sewage treatment or water recycling can be done with the help of transgenic microbes which have better efficiency and speed.
Biotechnology is the use of living organisms or other biological systems in the manufacture of drugs or other products or for environmental management, as in waste recycling: includes the use of bioreactors in manufacturing, microorganisms to degrade oil slicks or organic waste, genetically engineered bacteria to produce human hormones, and monoclonal antibodies to identify antigens.
Biotech offers the possibility of improving human health, the environment, and agriculture while creating more sustainable modes of production.
Help needed within the next 10 minutes

Answers
16.2 g of Water are formed

The pedigree represents how a certain sex-linked recessive trait is inherited.
Which is the most likely genotype of the grandfather?
XdY
XDXd
XDY
XdXd
Answers
Answer:c
Explanation:
i think that what is was for me
Arrange the following compounds in order of increasing solubility in
water and explain your sequence.
C7H15OH C6H13OH C6H6 C2H5OH
Answers
Answer:
C6H6<C7H15OH<C6H13OH<C2H5OH
Explanation:
Organic substances are ordinarily nonpolar. This means that they do not dissolve in water. However, certain homologous series of organic compounds actually dissolve in water because they possess certain functional groups that effectively interact with water via hydrogen bonding.
A typical example of this is alcohol family. All members of this homologous series contain the -OH functional group. This group can effectively interact with water via hydrogen bonding, leading to the dissolution of low molecular weight alcohols in water.
Low molecular weight alcohols are miscible with water in all proportions. This implies that they are highly soluble in water. However, as the size of the alkyl moiety in the alcohol increases, the solubility of the alcohol in water decreases due to less effective interaction of the -OH group with water via hydrogen bonding. This explains the fact that C2H5OH is the most soluble alcohol in the list.
C6H6 is insoluble in water since it is purely a hydrocarbon with no -OH group capable of interaction with water via hydrogen bonding.
Question 24
15.0 g of Cu are combined with 46.0 g of HNO3 (molar mass = 63.02), according to the
reaction
3 Cu + 8 HNO3 - 3 Cu(NO3)2 + 2 NO + 4 H20
Which reactant is limiting, and how many grams of H20 are produced?
O HNO3, 0.737 g
O HNO3, 6.13 g
O Cu, 5.67 g
O Cu, 3.79 g
Answers
Explanation:
ee9siotsutozirzizitzizifz
The following Lewis diagram represents the valence electron configuration of a main-group element.
This element is in group
.
According to the octet rule, this element would be expected to form an ion with a charge of
.
If is in period 5, the ion formed has the same electron configuration as the noble gas
.
The symbol for the ion is
.
Answers
This element is in group 1.
According to the octet rule, this element would be expected to form an ion with a charge of +1.
If X is in period 5, the ion formed has the same electron configuration as the noble gas Krypton
The symbol for the ion is Rb⁺
What is electronic configuration?Electronic configuration refers to the arrangement of electrons in the orbitals of an atom or molecule, indicating the energy level of the electrons, the number of electrons in each energy level, and the number of electrons in each orbital.
Considering the given element:
It has one valence electron, hence it is in group 1. Group 1 elements form ions with a charge of +1.
Losing one electron will give the ion the same electron configuration as Kyrton since it is the noble gas in Period 4.
The element is rubidium and the ion is Rb⁺.
Learn more about electronic configuration at: https://brainly.com/question/26084288
#SPJ1

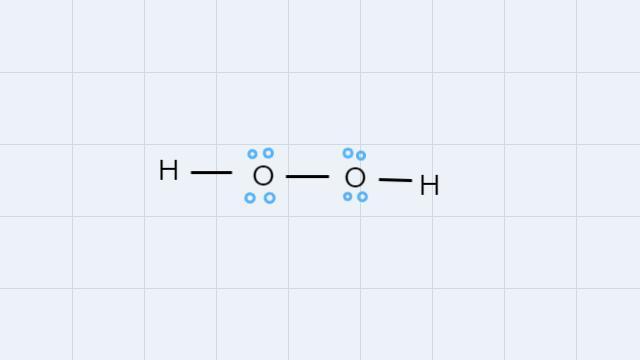
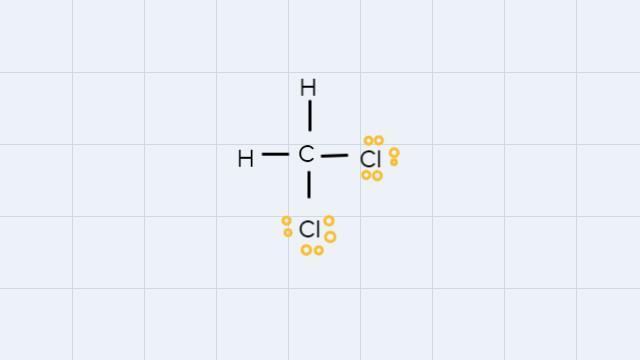
How to draw the lewis structures for the following? PCl3H2O2CH2Cl2H2CO2
Answers
Answer and explanation:
To draw the Lewis structure we have to make sure that each element in the molecule has 8 electrons (Octet rule) in the valence shell, or 2 electrons in the hydrogen atom.
• PCl3:
In the PCl3 molecule, each chlorine atom has an Octet with 8 electrons, and the phosphorus atom also has an Octet but it is expanded (that means, that P atomd can have more that 8 electrons). From period 3 of the Periodic Table, elements can expand their octet and have more than 8 electrons.
• H2O2:
In the H2O2 molecule, oxygen atoms have an Octet with 8 electrons and hydrogen atoms have an Octet with 2 electrons.
• CH2Cl2:
In the CH2Cl2 molecule, all atoms have octets with 8 electrons, except for hydrogen, which has an octet with 2 electrons.
• H2CO2:
In this case, the Octet rule is fulfilled and also for this, an oxygen atom must form a double bond with the chlorine atom.


7. What is the volume of the
composite
solid?
4 in.
3 in.
3 in.

Answers
Answer:
The volume of Component 1 is 36 cubic inches.
Explanation:
To calculate the volume of a composite solid, we need to determine the individual volumes of the different components and then add them together.
In this case, the composite solid consists of multiple components with the following dimensions:
Component 1:
Length: 4 inches
Width: 3 inches
Height: 3 inches
To find the volume of Component 1, we multiply the length, width, and height together:
Volume of Component 1 = Length x Width x Height = 4 in x 3 in x 3 in = 36 cubic inches
Therefore, the volume of Component 1 is 36 cubic inches.
Please provide the dimensions of the remaining components of the composite solid, and I will calculate the total volume by summing up the individual volumes.
__________ 1. What valuable contribution will my study make to the field?
Answers
Answer:
I'm not entirely sure what your study is about, but I can tell you that any research or study that contributes new knowledge or insights to a particular field can be valuable. It's important to identify gaps in the existing literature and to approach your research with a clear and focused question or objective. Ultimately, the value of your study will depend on the quality of your research and the significance of your findings.
what is the element for gas
Answers
Answer:
If by gas you mean oxygen it is O.
A change in the number of neutrons in an atom will change an isotope. What will happen when the number of protons changes in an atom?
Answers
A student weighs out a 2.17 g sample of KOH, transfers it to a 300. mL volumetric flask, adds enough water to dissolve it and then adds water to the 300. mL tick mark.
What is the molarity of potassium hydroxide in the resulting solution?
Answers
The molarity of potassium hydroxide in the resulting solution is 0.129 M.
How to calculate molarity?Molarity of a substance refers to the concentration of a substance in solution, expressed as the number of moles of solute per litre of solution.
According to this question, a student weighs out a 2.17g sample of KOH, transfers it to a 300. mL volumetric flask, adds enough water to dissolve it and then adds water to the 300. mL tick mark.
No of moles of KOH = 2.17g ÷ 56.11g/mol = 0.039 moles
Molarity = 0.039 moles ÷ 0.3L = 0.129 M
Learn more about molarity at: https://brainly.com/question/31545539
#SPJ1
Calculate the number of grams of Ne contained in 18.4 L of Ne gas.
Express your answer to three significant figures and include the appropriate units

Answers
In 18.4 liters of neon gas at STP, you have16.42g of the gas.
A balloon with a 22.4 liter volume at STP contains how many helium atoms?Reason: A MOLAR quantity of gas is defined as a volume of 22.4 L of gas at standard conditions of pressure and temperature (1 atm, 273.15 K). That means, each helium atom has a mass of 6.022 1023.
To determine the value of moles, use the ideal gas law.
The ideal gas law is PV=nRT
T is the temperature in Kelvin which 273 K for standard conditions.
Rearrange the equation to solve for n
n = PV/RT
n = ( 1 atm) ( 18.4L)/[(0.0821 L atm/mol K)(273)]
n=0.821 moles of Neon gas
mass of neon= 0.821*20g
mass of neon= 16.42g
To know more about moles visit:-
https://brainly.com/question/20486415
#SPJ1
What does a compound do in a chemical reaction?
Answers
Answer:Compounds are chemical substances that contain more than one element. They're created during a chemical reaction where atoms are rearranged into new compound molecules. For example, if carbon atoms react with oxygen atoms they form carbon dioxide molecules.
Explanation:
BRAINILY ANSWER NOW OLEASE I NEED HELP
explain why the Sun appears to move across the sky throughout the day.
Answers
Answer:
From Earth, the Sun looks like it moves across the sky in the daytime and appears to disappear at night. This is because the Earth is spinning towards the east. The Earth spins about its axis, an imaginary line that runs through the middle of the Earth between the North and South poles.
Explanation:
Answer:
The Earth rotates on its axis always, so it looks like the sun is moving but it is the Earth that's moving.
Explanation:
Hope this helps!
Please help me ASAP ANYONE THANK YOU VERY MUCH(:!!??

Answers
The recipe conversion factor (RCF) is 2.5 (option A).
How to calculate conversion factor?Recipes often need to be adjusted to meet the needs of different situations. The most common reason to adjust recipes is to change the number of individual portions that the recipe produces.
The most common way to adjust recipes is to use the conversion factor method.
The conversion factor can be obtained by dividing the required yield by the old yield i.e.
Conversion factor = required/new yield/recipe/old yield
Conversion factor = 25/10
Conversion factor = 2.5
Learn more about conversion factor at: https://brainly.com/question/3280652
#SPJ1
wurtz reaction can be used to prepare higher hydrocarbons. how many product may be formed by reaction of ethyl iodide and methyl iodide with sodium? a) 1 b) 2 c)3 d)4
Answers
Answer:
b) 2
Explanation:
The Wurtz reaction is a reaction in which the hydrocarbon chain in lengthened by reaction two alkyl halides with sodium. The reaction is carried out in dry ether.
When methyl iodide reacts with ethyl iodide, two products are formed; the hydrocarbon product and sodium iodide.
Na/Ether
CH3CH2I + CH3I ----------> CH3CH2CH3 + 2NaI
To identify a halide, you can react a solution with chlorine water in the presence of mineral oil.
If the unknown halide is a
Choose...weaker/stronger
reducing agent than chlorine, the halide will be oxidized to
Choose...its elemental form/ its ionic form/ a solid
which would change the color of the
Choose...mineral oil/ aqueous
layer.
Answers
Answer:
- Stronger reducing agent than Chlorine
- Oxidized to it's elemental form
- Change the colour of the aqueous layer.
Explanation:
Halides are electronegative elements in group seven of the Periodic table which have gained electrons to complete their electronic configuration.
They include F-, CL-, Br- and I-.
As you descend the group electro negativity decreases as the number of outermost shells increases. Hence F- is the most electronegative while I- is the least electronegative.
In terms of oxidising and reducing abilities amongst the halogens, since an oxidizing agent readily accepts electrons and is thereby reduced, oxidizing power decreases down the group.
For example, Fluorine being the strongest oxidising agent in the group readily accepts electrons from other members of the group and is reduced to the fluoride ion
F + e = F -
Therefore in terms of oxidizing abilities,
F > Cl > Br > I
Conversely, , as the oxidising power decreases down the group, the reducing powers increases
Therefore, in terms of reducing powers,
I > Br > Cl > F
In the test for halide ions using aqueous chlorine, since chlorine is a stronger oxidizing agent/weaker reducing agent than Bromine or iodine, it readily accepts their electrons forming the chloride ion.
Cl2 + 2 Br- = 2 Cl- + Br2
The bromide ion (assuming the unknown halide is bromide) being a stronger reducing agent/weaker oxidizing agent than Chlorine would readily lose it's electrons and get oxidized to it's elemental form changing the colour of the aqueous layer to brown.
That is : Br2- = Br2 + 2e
The fill in the blanks could be filled with stronger, elemental form and mineral oil.
Identification of a halide:In the case when the halide is not known so it should be stronger. The halide should be oxidized with respect to the elemental form and it should change the color of mineral oil. Due to this, halide should be oxidized for elemental halogen i.e. more soluble for mineral oil.
Learn more about the water here: https://brainly.com/question/21281574
A tank has 14 gallons of water in it when water begins draining from the tank. (recall that water weighs 8. 345 pounds per gallon. ).
Answers
If 12 pounds of water have left the tank . There are 12.56 gallons of water remaining in the tank.
How to find the number of gallons of water?First, we need to convert the weight of water that has left the tank from pounds to gallons. Since 1 gallon of water weighs 8.345 pounds, we can find the number of gallons that have left the tank as follows:
12 pounds / 8.345 pounds per gallon = 1.44 gallons
So, 1.44 gallons of water have left the tank.
To find how many gallons of water are remaining in the tank, we can subtract the gallons that have left from the initial amount of water in the tank:
14 gallons - 1.44 gallons = 12.56 gallons
Therefore, there are 12.56 gallons of water remaining in the tank.
Learn more about gallons of water here:https://brainly.com/question/26007201
#SPJ1
The complete question is:
A tank has 14 gallons of water in it when all of a sudden the water begins draining from the tank. Recall that water weights 8.345 pounds per gallon.
If 12 pounds of water have left the tank how many gallons of water are remaining
Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction entropy of the following chemical reaction:
S8 + 24F2------ 8S + F6
Round your answer to zero decimal places.J/K
Answers
Answer:
\(\Delta _rS\°=-2965.27J/K\)
Explanation:
Hello,
In this case, for the considered reaction, the standard reaction entropy is computed considering the standard entropy of each product and reactant and their stoichiometric coefficients as shown below:
\(\Delta _rS\°=8*S\°_{SF_6}-S\°_{S_8}-24*S\°_{F_2}\)
Thus, considering the ALEKS Data tab, we obtain:
\(\Delta _rS\°=8*(291.52J/K)-(430.23J/K)-24*(202.8J/K)\\\\\Delta _rS\°=-2965.27J/K\)
Regards.
is UF6 a covalent compound or an ionic compound?
Answers
Hey there,
Given - Is UF6 a covalent compound or an ionic compound?
Answer- Covalent compound
Reason - Even though, still a subject of debate, theoretical results indicate that the U–F bond is partially covalent and could even possess some multiple bond characters, resulting from F → U π interaction cause.
~ Benjemin360

Potential in a different kind of cell.
A typical mammalian cell at 37
∘
C, with only potassium channels open, will have the following equilibrium:
K+ (intracellular) ⇌ K+ (extracellular),
with an intracellular concentration of 150 mM K+, and 4.0 mM K+ in the extracellular fluid.
What is the potential, in volts, across this cell membrane? Note: in this case, n = the charge on the ion, and Eo for a concentration cell = 0.00 V. explain please
Answers
The potential across this cell membrane with only potassium channels open is -0.082 V, which means that the inside of the cell is negatively charged relative to the outside.
The potential across a cell membrane can be calculated using the Nernst equation:
E = (RT/zF) ln([ion]out/[ion]in)
E = potential in volts, R= gas constant (8.314 J/mol*K), T= temperature in Kelvin, z = charge on the ion, F= Faraday constant (96,485 C/mol), and [ion]out and [ion]in are the concentrations of the ion outside and inside the cell, respectively.
K+ (intracellular) ⇌ K+ (extracellular)
The charge on potassium ions is +1, so z = 1.
The temperature is 37°C or 310 K.
The concentrations of potassium ions are [K+]in = 150 mM and [K+]out = 4.0 mM.
Substituting these values into the Nernst equation,
E = (RT/zF) ln([K+]out/[K+]in)
E = (8.314 J/mol.K × 310 K)/(1 × 96,485 C/mol) ln(4.0 mM/150 mM)
E = -0.082 V
Learn more about the nerst equation here.
https://brainly.com/question/31703564
#SPJ1
Determine the empirical formula. a 3.880g sample contains 0.691g of magnesium , 1.84 g of sulfur , and 1.365 g of oxygen .
Answers
Answer:
Mg S2 O3
Explanation:
.691 g of Mg is .284 mole
1.84 g of S is .5739 mole
1.365 g of O is .8531 mole you can see the ratio is ~ 1 :2 :3
Mg S2 O3
list several characteristics of ligroin
Answers
- Petroleum fraction consisting mostly of \(C_{7}\) and \(C_{8}\) hydrocarbons
- The fraction is also called heavy naphtha
- Boiling in the range 90‒140 °C
- Ligroin is used as a laboratory solvent
- Ligroin it also esed as a motor fuel or as a solvent for fats and oils in dry cleaning
[] All of these are different characteristics of ligroin
[] If you need more let me know
Have a nice day!
I hope this is what you are looking for, but if not - comment! I will edit and update my answer accordingly. (ノ^∇^)
- Heather