Chini is sliding blocks of different materials down a steel ramp. All of the blocks have similar sizes and masses. The table shows the coefficient of friction
for several materials when sliding on steel. The larger the coefficient of friction, the greater the force of friction. In which order would the blocks reach the
bottom of the ramp?
Material
Coefficient
of friction
aluminum
chromium
glass
0. 25
0. 21
0. 12
steel
0. 23
titanium
vanadium alloy
0. 31
Drag the materials into the correct order from the material that will slide down the ramp the fastest to the material that will slide down the ramp the
slowest
chromium
glass
aluminum
titanium vanadium alloy
steel
Answers
The blocks will reach the bottom of the ramp in the following order: Glass, Chromium, Aluminum, Steel, and Titanium vanadium alloy.
How to explain the informationThe coefficient of friction is a measure of the resistance between two surfaces in contact, in this case, between the materials of the blocks and the steel ramp. It indicates how "sticky" or "slippery" the surfaces are relative to each other.
When an object slides down a ramp, the force of gravity pulls it downwards, and there is also a force of friction acting in the opposite direction. The force of friction depends on the weight of the object and the coefficient of friction between the surfaces. A higher coefficient of friction means a stronger force of friction, which resists the motion of the object.
Learn more about friction on,
https://brainly.com/question/24338873
#SPJ1
Related Questions
The concentration of chemical exposure depends on: the persistence of the chemical. the LD50 of the chemical. the solubility of the chemical.
Answers
The concentration of chemical exposure is influenced by various factors, including the persistence of the chemical, the LD50 (lethal dose 50%) of the chemical, and the solubility of the chemical.
The persistence of a chemical refers to its ability to remain in the environment or a biological system without breaking down or being eliminated. Chemicals that are persistent tend to accumulate over time, leading to higher concentrations and potential long-term exposure.
For example, persistent organic pollutants (POPs) like certain pesticides and industrial chemicals can bioaccumulate in organisms and concentrate in the food chain, resulting in higher exposure levels. The LD50 of a chemical represents the dose or concentration at which 50% of the exposed population is expected to die. While the LD50 primarily reflects the toxicity of a substance, it indirectly affects concentration.
Highly toxic chemicals with a low LD50 require lower concentrations to cause harm, potentially leading to higher exposure risks. The solubility of a chemical refers to its ability to dissolve in a particular solvent, such as water or fat. Solubility influences the distribution and availability of a chemical in different environmental or biological compartments.
For example, highly soluble chemicals may be more readily absorbed and distributed throughout the body, potentially resulting in higher systemic exposure.
know more about chemical exposure here:
https://brainly.com/question/29549631
#SPJ11
I have a question for us gals, is it hard for all of you when you try to get your crushes attention and he/she just keeps ignoring you to the point where you could just give up?
Answers
Answer:
Yes it is hard. For some of us. I already gave up, but now hes coming back. Id say sometimes its useless to get his attention if he never even pays attention to you anyways. It sucks because its so hard to get then to notice you for once.
Explanation:
lead is a well known heavy metal with a density of 11.53 g/cm^3. what would be the volume of lead if you are given a 0.255 pound sample? (dimensional analysis)
Answers
Since lead has a density of 11.53 g/cm^3, the volume of a 0.255 pound sample of lead is equal to 10.03 cm³.
What is density?In Science, the density of a chemical substance such as lead can be calculated by using this formula:
Density = M/V
Where:
M represents the mass of a chemical substance or physical object.V represents the volume of a physical substance or object.Next, we would convert the value of the mass in pound to grams by multiplying it by 453.6 as follows;
Mass = 0.255 × 453.6
Mass = 115.668 grams
Making volume the subject of formula, we have:
Volume = Mass/Density
Volume = 115.668/11.53
Volume = 10.03 cm³.
Read more on density here: brainly.com/question/14035509
#SPJ1
give the symbol and the nuclei number of an isotope that is used as a nuclear fuel
Answers
Answer: The nuclear symbol of an isotope indicates the number of protons and neutrons in an atom of the element. It does not indicate the number of electrons. The number of neutrons is not stated. Instead, you have to figure it out based on the number of protons or atomic number.
Explanation:
brain me
does anybody knows the field in science for the levels of organization
Answers
Answer:
Taxonomy
Explanation:
Taxonomy is the science of naming, describing and classifying organisms
i hoped this helped you!
The homogeneity of the chloride level in a water sample from a lake was tested by analyzing portions drawn from the top and from near the bottom of the lake, with the following results
Top (ppm Cl)
Bottom (ppm Cl)
26.30
26.22
26.43
26.32
26.28
26.20
26.19
26.11
26.49
26.42
Apply the t-test at the 95% confidence level to determine if the chloride level from the top of the lake is different from that at the bottom.
Now use the paired t-test and determine whether there is a significant difference between the top and bottom values at the 95% confidence level.
Why is a different conclusion drawn from using the paired t- test than from just pooling the data and using the normal t- test for differences in means?
Answers
The paired t-test yields a different conclusion than the normal t-test because it accounts for the paired nature of the data, comparing the measurements taken at the top and bottom of the lake separately.
In this scenario, the paired t-test is appropriate because it analyzes the data as pairs, considering the chloride levels measured at the top and bottom of the lake for each sample. By comparing the differences within each pair, the paired t-test determines whether there is a significant difference between the chloride levels at the top and bottom of the lake.
Using the paired t-test, the differences between the paired observations are calculated, and the null hypothesis assumes that the mean difference is zero (no significant difference between the top and bottom chloride levels). The test then determines whether the observed differences are statistically significant at a chosen confidence level, in this case, 95%.
The normal t-test for differences in means, on the other hand, would treat the top and bottom chloride levels as separate and unrelated groups, disregarding their paired nature. By pooling the data and conducting a standard t-test, the analysis assumes that the two sets of measurements are independent, which may not be appropriate in this case. This can lead to a different conclusion compared to the paired t-test.
The different conclusion drawn from using the paired t-test compared to pooling the data and using the normal t-test is due to the consideration of the paired nature of the measurements. The paired t-test takes into account the potential correlation or connection between the measurements taken at the same location (top and bottom of the lake) and assesses the differences within each pair.
Pooling the data and using the normal t-test treats the measurements as independent, disregarding the pairing. This can result in a loss of valuable information and may lead to an inaccurate conclusion. The paired t-test is more appropriate when dealing with dependent or related measurements, ensuring a more accurate assessment of the differences between the top and bottom chloride levels.
Learn more about paired t-test
brainly.com/question/32245864
#SPJ11
Calculate the number of hydrogen atoms in180 grams of glucose
Answers
Answer:
Explanation:
Calculating the number of hydrogen atoms in 180 grams of glucose involves using the molecular formula of glucose (C6H12O6) and the Avogadro constant (6.022 x 10^23).
First, calculate the molar mass of glucose by adding the atomic masses of its elements:
C6H12O6 = (6 x 12.01) + (12 x 1.01) + (6 x 16.00) = 180.18 g/mol
Next, use the molar mass to find the number of moles of glucose in 180 grams:
180 g / 180.18 g/mol = 0.999 moles
Finally, multiply the number of moles by Avogadro's constant to find the number of hydrogen atoms:
0.999 mol x 6.022 x 10^23 atoms/mol = 6.02 x 10^23 hydrogen atoms
Which of the following parameters would be different for a reaction carried out in the presence of a catalyst, compared with the same reaction carried out in the absence of a catalyst? ΔG∘, ΔH‡, Ea, ΔS‡, ΔH∘, Keq, ΔG‡, ΔS∘, k
Check all that apply.
a. ΔH‡
b. Keq
c. ΔH∘
d. Ea
e. k
f. ΔG∘
g. ΔS‡
h. ΔG‡
i. ΔS∘
Answers
The parameters that would be different for a reaction carried out in the presence of a catalyst compared to the same reaction carried out in the absence of a catalyst are: ΔH‡, Ea, and k.
When a catalyst is present in a chemical reaction, it provides an alternative pathway with lower activation energy (Ea) for the reaction to occur. This means that the catalyst lowers the energy barrier for the reaction, making it easier for the reactants to reach the transition state and form the products. Consequently, the activation energy (Ea) is reduced in the presence of a catalyst.
The enthalpy change of the transition state (ΔH‡) is also affected by the presence of a catalyst. Since the catalyst provides an alternative pathway, the transition state formed in the presence of the catalyst may have a different enthalpy compared to the transition state in the absence of a catalyst.
Additionally, the rate constant (k) of the reaction is influenced by the catalyst. The catalyst increases the rate of the reaction by providing an alternative reaction pathway with a lower activation energy. As a result, the rate constant (k) is generally higher when a catalyst is present.
Therefore, the parameters that differ for a reaction carried out in the presence of a catalyst compared to the absence of a catalyst are ΔH‡, Ea, and k.
Learn more about parameters
brainly.com/question/28249912
#SPJ11
In a 0.57 M solution of propanoic acid, HOC6H5, 0.0684% of the acid has dissociated. a. Find the concentrations of all aqueous species in the solution at equilibrium. b. Find the pH of the solution. c. What concentration of HBr would produce a solution with the same pH as a 0.57 M solution of propanoic acid, HOC6H5? Justify your answer.
Answers
a) [HOC₆H₅] ≈ 0.57 M ; b) pH of the solution is approximately 3.41.c) concentration of [HBr] = (3.9012 × 10⁻⁴)²/Ka ≈ 2
What is propanoic acid?Propanoic acid is a carboxylic acid with chemical formula as C₃H₆O₂ and is also known as propionic acid.
a.) HOC₆H₅(aq) + H₂O(l) ⇌ H₃O⁺(aq) + OC₆H₅⁻(aq)
[H₃O⁺] = [OC₆H₅⁻] = 0.0684/100 × 0.57 M = 3.9012 × 10⁻⁴ M
Initial concentration of HOC₆H₅ is 0.57 M, and since only small fraction of it has dissociated, we can assume that its concentration at equilibrium is approximately equal to initial concentration. Therefore:
[HOC₆H₅] ≈ 0.57 M
b.) pH = - ㏒ [H₃O⁺]
pH = - ㏒ (3.9012 × 10⁻⁴) ≈ 3.41
Therefore, pH of the solution is approximately 3.41.
c.) pH = pKa + ㏒([OC₆H₅⁻]/[HOC₆H₅])
The pKa of propanoic acid is 4.87, so:
3.41 = 4.87 + ㏒([OC₆H₅⁻]/[HOC₆H₅])
㏒([OC₆H₅⁻]/[HOC₆H₅]) = -1.46
[OC₆H₅⁻]/[HOC₆H₅] = 3.47 × 10⁻²
[OC₆H₅⁻] = (3.47 × 10⁻²) × 0.57 M ≈ 1.97 × 10⁻² M
HBr(aq) + H₂O(l) ⇌ H₃O⁺(aq) + Br⁻(aq)
Ka = [H₃O⁺][Br⁻]/[HBr]
Ka = (3.9012 × 10⁻⁴)²/1.97 × 10⁻² ≈ 7.69 × 10⁻⁹
Ka = [H₃O⁺][Br⁻]/[HBr] = [H₃O⁺]²/[HBr]
[H₃O⁺] = √(Ka[HBr])
3.9012 × 10⁻⁴ = √(Ka[HBr])
[HBr] = (3.9012 × 10⁻⁴)²/Ka ≈ 2
To know more about propanoic acid, refer
https://brainly.com/question/15241093
#SPJ1
The complete combustion of propane, C3H8 in the presence of an excess amount of
oxygen gas, results in the formation of carbon dioxide gas and water. If 220g of propane fully reacted, how many grams of water were produced ?
Answers
The balanced equation is given by
2 C3H8 + 9 O2 → 4 CO2 + 2 CO + 8 H2O + HeatMoles of propAne
Given mass/Molar mass220/445mol2 mol of propane produces 8 mol water
1 mol produces 4mol water
moles of water
4(5)20molMass of water
20(18)360gMore crimes occur when the temperature increases outdoors. This statement is a(n) _____.
a. problem
b. hypothesis
c. scientific law
d. opinion
Answers
The correct answer is b. The statement "More crimes occur when the temperature increases outdoors" is a hypothesis.
It is a tentative explanation for a phenomenon that is based on observations and preliminary data. This hypothesis suggests that there may be a correlation between temperature and crime rates, but it does not establish a causal relationship.
To determine whether this hypothesis is valid, it would be necessary to conduct research using rigorous scientific methods, such as collecting data from multiple locations and over an extended period of time, controlling for confounding factors, and using statistical analysis to determine the strength and direction of the relationship between temperature and crime rates.
It is important to note that hypotheses are subject to revision or rejection based on further research and evidence. While this hypothesis may have some initial support, it should not be considered a scientific law or an opinion without further investigation and confirmation.
For more such questions on hypothesis
https://brainly.com/question/31330789
#SPJ11
Which actions would increase the rate at which salt dissolves in water? Stir the water. Crush the salt. Use less water. Heat the water. Cool the salt.
Answers
Answer:
Crushing or grinding increases the surface area of the salt that is exposed to the molecules of water. Stirring increases the speed at which the particles of salt come in contact with the water molecules.
You need to prepare an acetate buffer of pH 5. 17
from a 0. 660 M
acetic acid solution and a 2. 63 M KOH
solution. If you have 930 mL
of the acetic acid solution, how many milliliters of the KOH
solution do you need to add to make a buffer of pH 5. 17
? The pa
of acetic acid is 4. 76. Be sure to use appropriate significant figures
Answers
The volume that is needed is 173 mL of KOH solution is needed to prepare this buffer.
The reaction between acetic acid (CH₃COOH) and KOH can be written as follows.
CH₃COOH + KOH -------------> CH₃COOK + H₂O
CH3COOH is a weak acid and CH₃COOK is its strong salt, therefore together they make a buffer system.
Let's say we add "x" moles of base KOH . Let's draw ICE table to find out moles at equilibrium
Initial moles of CH₃COOH are 0.654 mol/L * 625 mL * 1 L / 1000 mL = 0.40875 mol
CH3COOH KOH CH3COOK H2O
I 0.40875 x 0 -
C -x -x +x -
E 0.40875 - x 0 x
At equilibrium, we have 0.40875 - x moles of acid and x moles of its conjugate base.
Let's use Henderson Hasselbalch equation to solve for x.
pH = pKa + log ( base/ acid)
the required pH is 5.87 and pKa is given as 4.76
5.87 = 4.76 + log ( x / 0.40875 - x )
5.87 - 4.76 = log ( x / 0.40875 - x )
1.11 = log ( x / 0.40875 - x )
10¹°¹¹ = ( x / 0.40875 - x )
12.88 = x / 0.40875 - x
12.88 ( 0.40875 - x ) = x
5.266 - 12.88 x = x
5.266 = 13.88 x
x = 5.266 / 13.88
x = 0.379
From ICE table, we know that x is moles of KOH
Molarity of KOH is given as 2.19M
Molarity = moles of KOH / liters
2.19 = 0.379 / Liters
Liters of KOH = 0.379 / 2.19
Liters of KOH = 0.173 L
173 mL of KOH solution is needed to prepare this buffer.
To learn more about molarity check the link below-
https://brainly.com/question/30404105
#SPJ4
When sodium sulfate and water react they form sodium hydroxide and hydrogen sulfate. Write the balanced equation and classify it.
Answers
The balanced chemical equation of the reaction between sodium sulfate and water to form sodium hydroxide and hydrogen sulfate is as follows:
Na₂SO₄ + 2H₂O → 2Na(OH) + H₂SO₄
What is a chemical reaction?A chemical reaction is a process, typically involving the breaking or making of interatomic bonds, in which one or more substances are changed into others.
A chemical equation is a symbolic representation of a chemical reaction where reactants are represented on the left, and products on the right.
According to this question, sodium sulfate and water react to form sodium hydroxide and hydrogen sulfate. The balanced chemical equation is as follows:
Na₂SO₄ + 2H₂O → 2Na(OH) + H₂SO₄
The equation is said to be balanced because the number of atoms of each element are the same on both sides of the equation.
Learn more about chemical equation at: https://brainly.com/question/29039149
#SPJ1
What’s an example of phase change you’ve seen in real life?
Answers
Answer:
Melting and boiling will be seen in this example. Condensation might be seen as well. In the Phases of Water Gizmo™, you can heat up or cool down a container of water.
Explanation:
4. What kept the concept of continental drift from originally being
accepted?
Answers
Answer:
The fact that there was no driving force or motive mechanism behind the theory of continental drift.
Explanation:
The concept of continental drift was first proposed by Abraham Ortelius in 1596, and further improved on by Alfred Wegener in 1912. The theory proposes that the earth's continents have moved across the ocean bed. The continents also move with geologic time relative to each other. The concept was not accepted by scientists because there was no driving force or motive mechanism to explain the theory.
A scientist named Alfred Holmes, came up with the mantle convection theory which explains that the slow motion of the earth was caused by convection currents. This explanation was still not satisfactory to scientists. Today, the theory of plate tectonics has gained more acceptance than the theory of continental drift.
What are the 3 chemical properties of / for Neon ?
Answers
3 chemical properties of for Neon is colorless, odorless and tasteless
Neon is a rare atmospheric gas and as such is non toxic and chemically inert and neon poses no threat to the environment and can have no impact at all because its chemically unreactive and form no compound and no known ecological damage caused by this element and the properties of neon include the colorless and tasteless and odorless inert gas and it changes to reddish orange color in vacuums tube and it is chemically inactive
Know more about chemical properties
https://brainly.com/question/489869
#SPJ1
The electron configuration of an element is 1s22s22p6. Describe what most likely happens when an atom of this element comes near an atom having seven valence electrons.
Answers
Answer: Nothing will happen
Explanation:
Elements want to fill their valence shell with electrons (8).
Referring to both of the table below we will know that the element with that electron configuration is Neon (Ne). It is on the far right side of the periodic table so it contains all 8 electrons in its valence shell. The elements on the right side of the periodic table are called noble gases and are not reactive because they have the maximum number of electrons.

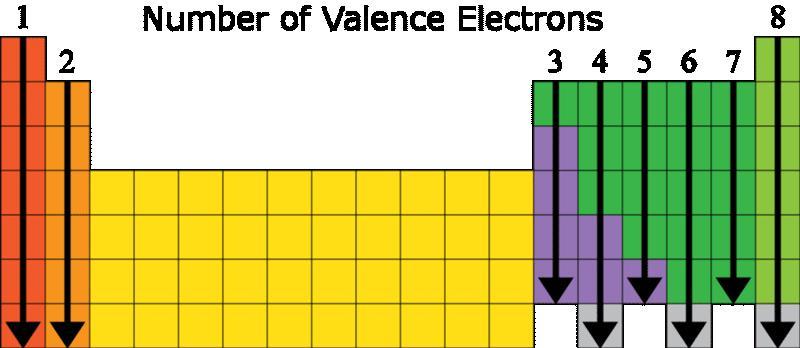
A single electron from the outermost shell of this element will move into the outermost shell of the atom with seven electrons.Nothing will happen in these state.
What is an electronic configuration ?Electron configurations define the arrangement of electrons around an atom's nucleus. Lithium, for instance, contains two electrons in the 1s subshell and one electron in the 2s subshell, according to its electron configuration, 1s22s1.
We may determine that Neon is the element with that electron configuration by looking at both of the tables below (Ne). It has all 8 electrons in its valence shell since it is on the extreme right side of the periodic table.
Noble gases are the elements on the right side of the periodic table that contain the most electrons and are thus non-reactive.
Thus, Electronic configuration an element is 1s22s22p6, it is stable.
To learn more about an electronic configuration, follow the link;
https://brainly.com/question/29757010
#SPJ5
How many moles are in 3. 612x1024 atoms of Carbon?
YOU MUST SHOW YOUR WORK IN ORDER TO RECEIVE CREDIT
Answers
There are approximately 6 moles in given set of atoms.
To find the number of moles in 3.612x10^24 atoms of Carbon, you will need to use Avogadro's number, which is 6.022x10^23 atoms/mol.
1. Determine the number of atoms given: 3.612x10^24 atoms of Carbon
2. Use Avogadro's number to convert atoms to moles:
(3.612x10^24 atoms) * (1 mol / 6.022x10^23 atoms)
3. Perform the calculation:
(3.612x10^24) / (6.022x10^23) = 6 moles (approximately)
So, there are approximately 6 moles in 3.612x10^24 atoms of Carbon.
To know more about Avogadro's number:
https://brainly.com/question/1513182
#SPJ11
PLS I NEED HELP give me examples of an atom
Answers
Answer:
Neon (Ne)
Hydrogen (H)
Argon (Ar)
Iron (Fe)
Calcium (Ca)
Deuterium, an isotope of hydrogen that has one proton and one neutron.
Plutonium (Pu)
F-, a fluorine anion.
Explanation:
I got u
Chlorine is safely used to disinfect waste liquids, some medical instruments, and various surfaces. It is readily available in liquid household bleach but bleach must be diluted because high concentrations are toxic and corrosive. To obtain an effective concentration of chlorine when using household bleach, a 1:100 dilution is made. This means that ______ mL of bleach is added to ______ mL of water.
Answers
To obtain an effective concentration of household bleach, where a 1:100 dilution is made. This means that 1mL of bleach is added to 100mL of water.
According to the question, the dilution factor is 1:100 in the order quantity of bleach to that of water respectively.
The question posed, more specifically, demands for the quantity of bleach to be added to what quantity of water to achieve the 1:100 dilution.Ultimately, 1mL of bleach is added to 100mL of water to achieve the 1:100 dilution.
Read more:
https://brainly.com/question/20885742
a pure liquid has a constant boiling point, but a liquid with a constant boiling point is not neccesarily pure. explain.
Answers
A pure liquid has a constant boiling point because it is composed of only one type of molecule while a liquid with a constant boiling point may or may not be pure depending on the type and amount of molecules it contains.
A pure liquid refers to a liquid that contains only one type of molecule, while a liquid with a constant boiling point means that the temperature at which the liquid boils remains the same, even if the pressure changes.
Pure liquids have fixed boiling points that are equal to the liquid's vapor pressure. The vapor pressure is determined by the liquid's molecular structure and the temperature at which it is exposed. It is the pressure exerted by the gas molecules that are in equilibrium with the liquid surface.
As such, these molecules all have the same amount of energy, which means the liquid requires the same amount of energy to reach its boiling point. On the other hand, a liquid with a constant boiling point is not necessarily pure because it may be composed of a mixture of molecules with different boiling points. The molecules with the lowest boiling point will start to evaporate first, leading to a decrease in the overall boiling point of the mixture. The decrease in boiling point will be slower if the molecules in the mixture have a similar boiling point.
In conclusion, a pure liquid has a constant boiling point because all of its molecules have the same amount of energy, while a liquid with a constant boiling point may or may not be pure depending on the type and amount of molecules it contains.
For more such questions on Liquid.
https://brainly.com/question/19578142#
#SPJ11
x=2 and y=3
find the value of
3x + y
3x² - 4
solve this please ty
Answers
Answer:
3x+y = 9
3x² - 4 = 8
Explanation:
3x+y
3(2)+3
9
3x2-4
3(2)^2-4
3
i need help on this please

Answers
how many electrons will be unpaired in the complex ion [rh(cn)6]3− ?
Answers
The complex ion [Rh(CN)6]3− has a rhodium atom in the center, surrounded by six cyanide ligands. Each cyanide ligand is a strong field ligand, which causes the splitting of the d-orbitals of the rhodium atom.
The electron configuration of rhodium in its ionic state is [Kr]4d^6. When it forms the complex [Rh(CN)6]3−, all the six cyanide ligands occupy the lower energy t2g orbitals, leaving the upper energy eg orbitals unoccupied. Therefore, there are three unpaired electrons in the eg orbitals, which gives the complex a magnetic moment of 3.87 Bohr magnetons. Hence, the complex [Rh(CN)6]3− has three unpaired electrons.
In the complex ion [Rh(CN)6]3−, Rhodium (Rh) is the central metal ion. The oxidation state of Rhodium in this complex is +3, making its electronic configuration 4d^6. According to the crystal field theory, the d-orbitals split into two sets: t2g (lower energy) and eg (higher energy). As [Rh(CN)6]3− has a low-spin octahedral configuration, the electrons will fill the t2g orbitals first. Three electrons will occupy the t2g orbitals, and the other three will pair up, leaving no unpaired electrons. Therefore, the complex ion [Rh(CN)6]3− has zero unpaired electrons.
To know about electrons visit:
https://brainly.com/question/12001116
#SPJ11
which of the following conditions would favor the carboxylate ion instead of the carboxylic acid? a. low ph b. high ph c. both low ph and high ph d. neither low ph or high ph
Answers
The carboxylate ion would be favored over the carboxylic acid under the condition of high pH. The correct option is b.
Carboxylic acids are organic compounds that contain a carboxyl functional group. Carboxylic acids are acidic and can be converted to carboxylate ions or salts when they react with a base, releasing a proton. The carboxylic acid group is a critical structural feature in amino acids and fatty acids. A carboxylate ion is an anion with the formula RCOO−, where R is a hydrogen atom, a carbonyl group, or an alkyl group. Carboxylate ions are formed by removing a proton (H+) from a carboxylic acid, which converts the carboxylic acid into a salt. Carboxylate ions are negatively charged and have a relatively high degree of solubility in water.
The correct option is b.
To know more about carboxylic acids; https://brainly.com/question/26855500
#SPJ11
How do the four properties of liquids relate to the polarity of a molecule?Include the following terms in your answer: bonds, polar, non-polar, adhesion, cohesion,surface tension, capillary rise. This summary should be several sentences long. Needs to be a short summary including those words. Please HELP!
Answers
Answer
In summary
A number of properties of liquids, such as cohesion and adhesion, are influenced by the intermolecular forces within the liquid itself. Cohesion are various intermolecular forces that hold solids and liquids together while adhesion is the ability of a liquids to stick to an unlike substance. The rise or fall of a liquid in a capillary tube is governed by the balance of cohesive and adhesive forces. Lastly, surface tension is a fundamental property of the surface of liquid, it is responsible for the curvature of the surfaces of liquids dues to the polarity that exists in the liquid molecules.
Explanation
Liquids flow because the intermolecular forces between molecules are weak enough to allow the molecules to move around relative to one another. The forces are attractive when a negative charge interacts with a nearby positive charge and repulsive when the neighboring charges are the same, either both positive or both negative. Molecules are held together by polar covalent bonds – which means that the electrons are not evenly distributed between the bonded atoms. This uneven distribution in the covalent bonds of the molecules results in a partial charge.
The liquid molecules don’t interact particularly strongly with each other because the intermolecular forces are weak. The primary intermolecular forces – are London dispersion forces, which for small molecules are the weakest types of intermolecular forces. These weak forces lead to low cohesion. The molecules don’t interact strongly with each other, so they can slide right past one another.
Adhesion is the tendency of a compound to interact with another compound. (Remember that, in contrast, cohesion is the tendency of a compound to interact with itself.) Adhesion helps explain how liquids interact with their containers and with other liquids. One example of an interaction with high adhesion is that between water and glass. Both water and glass are held together by polar bonds. Therefore, the two materials can also form favorable polar interactions with each other, leading to high adhesion.
Carbon tetrachloride (CCIA) is: covalent or Ionic?
Answers
Answer:
covalent
Explanation:
Which would a chemist be most likely to study?
Answers
Answer: Chemistry, the science that deals with the properties, composition, and structure of substances (defined as elements and compounds), the transformations they undergo, and the energy that is released or absorbed during these processes.
Explanation:
Mention and discuss briefly the adverse effects of chemistry.
Answers
Depending on the chemical, these longer-term health effects might include:
organ damage. weakening of the immune system. development of allergies or asthma. reproductive problems and birth defects. effects on the mental, intellectual or physical development of children. cancer.