Answers
Explanation:
true true true true true true
Answer:
False
Explanation:
Because that is your friend and your friend is helping you in other things that you need some help.
Related Questions
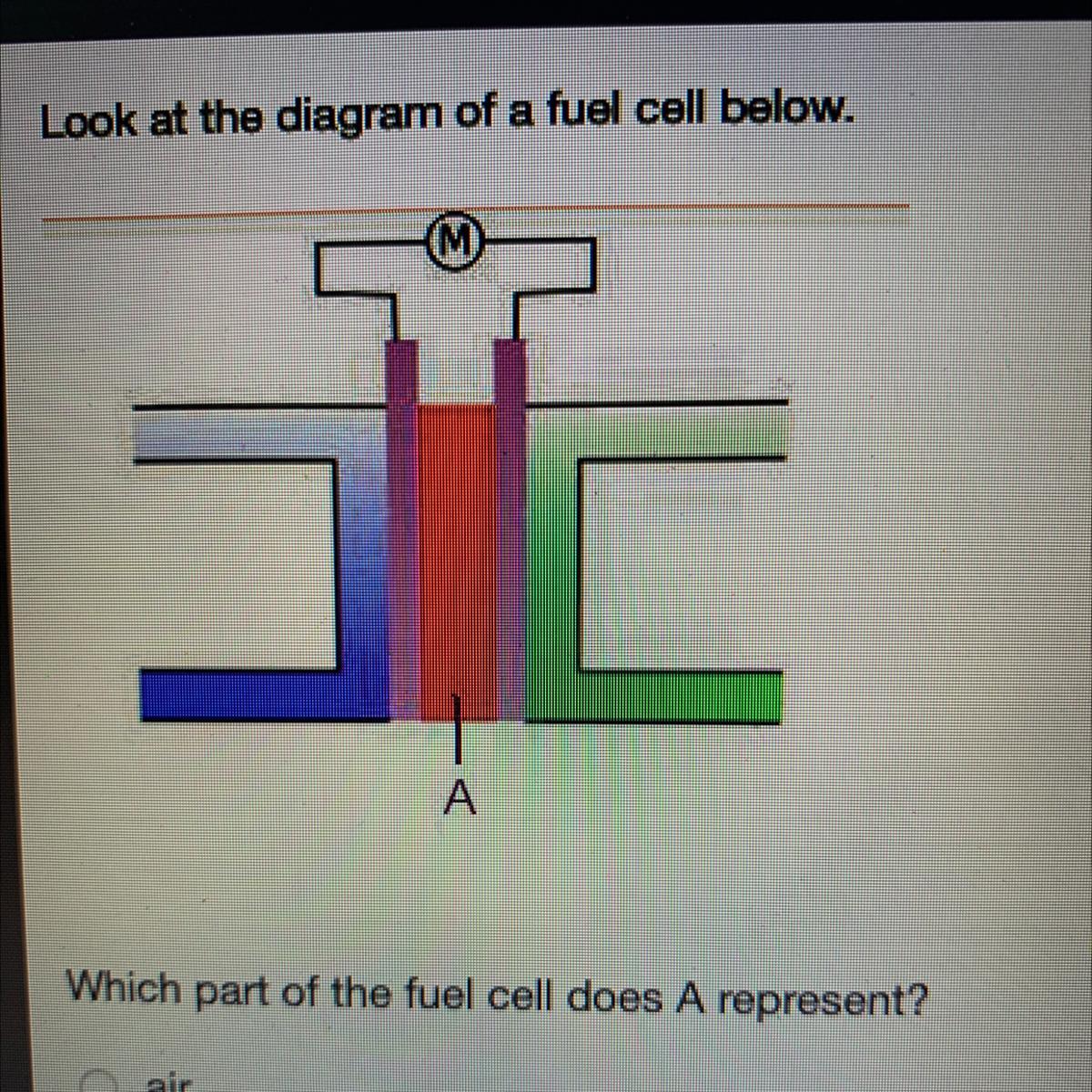
Look at the diagram of a fuel cell below.
Which part of the fuel cell does A represent?
air
anode
cathode
electrolyte
Answers
Answer:
I think its d) electrolyte
Explanation:
In the given diagram, The part of the fuel cell A represent is electrolyte.
What is Fuel Cell ?A fuel cell is the combination of two electrodes—anode (a negative electrode) and a cathode (positive electrode)— which is available around an electrolyte.
A hydrogen gas, is inserted to the anode, and air is inserted to the cathode.
Therefore, In the given diagram, The part of the fuel cell A represent is electrolyte.
Learn more about Fuel cell here ;
https://brainly.com/question/4607420
#SPJ5
The heart is an organ in the circulatory system. Muscle tissue in the heart contracts to pump blood to the body. Connective and epithelial tissues in the heart hold the muscle cells together and in place in the chest. Nervous tissue in the heart coordinates how fast and hard the muscle cells contract.
Based on the information about the heart, which of these best describes the relationship between tissues and organs?
Answers
The relationship between tissues and organs is one of interdependence. Tissues work together to form organs, and organs rely on the different types of tissues to function properly.
The relationship between tissues and organs is an important one, and it is particularly exemplified in the case of the heart. The heart is an organ, and like all organs, it is made up of various types of tissues that work together to allow it to function properly. In the case of the heart, these tissues include muscle tissue, connective tissue, epithelial tissue, and nervous tissue.
Muscle tissue is particularly important in the heart, as it contracts to pump blood to the body. Without muscle tissue, the heart would not be able to perform its vital function. Connective and epithelial tissues are also important in the heart, as they hold the muscle cells together and in place in the chest. Without these tissues, the muscle cells would not be able to work together efficiently, and the heart would not be able to function properly.
Finally, nervous tissue plays a crucial role in the heart, as it coordinates how fast and hard the muscle cells contract. This coordination is essential for the heart to function properly and maintain the circulation of blood throughout the body. In the case of the heart, the different types of tissues work together seamlessly to allow the heart to perform its vital function of pumping blood throughout the body.
For such more questions on organs
https://brainly.com/question/545314
#SPJ11
Answer: A
Explanation:
I got C wrong
1. Calculate the pH of a solution of 0.2M acetic acid and 0.35M acetate ion. The pk
of acetic acid is 4.8.
pH = pk + log ([A] : [HA])
A.
5.10
B.
5.04
c.
5.25
D.
6.10
E.
6.00
of which
Answers
Answer:
The correct answer is option C.
Explanation:
The pH of the solution with weak acid and its conjugate base is given by the Henderson-Hasselbalch equation:
\(pH=pK_a+\log[\frac{[A^-]}{[HA]}]\)
Where:
\(pK_a\)= The negative logarithm of the dissociation constant of a weak acid
\([A^-]\)= Concentration of conjugate base of a weak acid
\([HA]\)= Concentration of weak acid
We are given a solution with acetic acid and acetate ion.
\(HAc(aq)\rightleftharpoons H^+(aq)+Ac^-(aq)\)
The concentration of acetic acid in a solution= \([HAc]=0.2M\)
The concentration of acetate ion in a solution = \([Ac^-]=0.35M\)
The pK_A of the acetic acid = \(pK_a=4.8\)
The pH of the solution:
\(pH=4.8+\log[\frac{0.35 M}{0.2M}]=5.04\)
5.04 the pH of a solution of 0.2M acetic acid and 0.35M acetate ion.
Hence, the correct answer is option C.
what is use in measuring
Answers
Answer:
a ruler or measuring cups are ways of measuring
Explanation:
Answer:
Explanation:
Strictly speaking, the ruler is the instrument used to rule straight lines and the calibrated instrument used for determining length is called a measure, however common usage calls both instruments rulers and the special name straightedge is used for an unmarked rule.
what properties of a natural resource make it useful for humans as a materials or energy source?
Answers
The properties of a natural resource that make it useful for humans as a material or energy source is the ability to convert mass into energy and vice versa.
What are natural resources?The expression natural resources make reference to all types of matter and energy extracted from nature that can be used to produce goods and services.
Some examples of natural resources include for example irreversible resources such as fossil fuels (i.e., oil, or coal, gas, minerals such as metals, rocks, etc) as well as those based on the use of reversible energy such as eolic air energy, solar radiation or sunlight, soil and hydric resources or water.
Therefore, with this data, we can see that natural resources can be defined as any material and or energy obtained from nature that may be irreversible or reversibly used to produce goods and services.
Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ1
Calculate the total energy (in kJ) absorbed when 50.5 g of ice at -15.0°C is converted into liquid water at 65.0 °C.
Answers
Answer:
The total energy absorbed is 32.171 kilojoules.
Explanation:
The total energy absorbed by the ice is the sum of the sensible heat of ice and water and the latent heat of fusion of the water, that is:
\(Q = m\cdot [c_{i}\cdot (T_{2}-T_{1})+L_{f} + c_{w}\cdot (T_{3}-T_{2})]\) (1)
Where:
\(m\) - Mass of the ice, in kilograms.
\(c_{i}\) - Specific heat of ice, in kilojoules per kilogram-degree Celsius.
\(c_{w}\) - Specific heat of water, in kilojoules per kilogram-degree Celsius.
\(L_{f}\) - Latent heat of fusion, in kilojoules per degree Celsius.
\(T_{1}\) - Initial temperature of water, in degrees Celsius.
\(T_{2}\) - Fusion point of water, in degrees Celsius.
\(T_{3}\) - Final temperature of water, in degree Celsius.
\(Q\) - Total energy absorbed, in kilojoules.
If we know that \(m = 50.5\times 10^{-3}\,kg\), \(c_{i} = 2.090 \,\frac{kJ}{kg\cdot^{\circ}C}\), \(c_{w} = 4.180\,\frac{J}{kg\cdot ^{\circ}C}\), \(L_{f} = 334\,\frac{kJ}{kg}\), \(T_{1} = -15\,^{\circ}C\), \(T_{2} = 0\,^{\circ}C\) and \(T_{3} = 65\,^{\circ}C\), then the total energy absorbed is:
\(Q= (50.5\times 10^{-3}\,kg)\cdot \left[\left(2.090\,\frac{kJ}{kg\cdot ^{\circ}C} \right)\cdot (15\,^{\circ}C) + 334\,\frac{kJ}{kg}+ \left(4.180\,\frac{kJ}{kg\cdot ^{\circ}C} \right)\cdot (65\,^{\circ}C)\right]\)\(Q = 32.171\,kJ\)
The total energy absorbed is 32.171 kilojoules.
Billy is running at a rate of 8 m/s West he ran a total distance of 400 m how long does it take him to complete the distance
Answers
Answer:
50 Seconds
Explanation:
400 Divided By 8 Is 50
Answer:
50
Explanation:
Its 50 because 400/8=50
How does plastic impact the environment as a result of human activities
Answers
Answer:
As a result of human activities, plastic impacts the envionment in negative way that leads to serious environmental danger.
Explanation:
When we don't throw away plastic materials correctly, it causes littering. Well, this can also lead to piles of that, meaning that it is spreading toxins. Littering may also lead to blockages in flowing streams, lakes, river, ground water, big bodies of water, and small bodies of water. This also can contribute to global warming because plastic are made from chemicals that come from the production of fuels like oil, gas, coal and possible more.
What is the total number of peaks due to singly charged ions in the complete mass
spectrum of chlorine, Cl2
?
A Two
B Three
C Four
D Five
Answers
Five is the total number of peaks due to singly charged ions in the complete mass spectrum of chlorine, \(Cl_{2}\)
How many peaks do \(Cl_{2}\)'s molecular ions have?
The mass spectra of compounds with a single chlorine atom show two molecular ion peaks. This is because there are two isotopes of chlorine, 35Cl and 37Cl.
The molecular ion and fragment ions will both have peaks in the mass spectrum. When a mass spectrum is interpreted, a specific molecule can be located, confirmed, or its quantity can be calculated. the base summit of a mass spectrum's tallest (strongest) peak, caused by the ion with the highest relative abundance
To learn more about chlorine atom use:
brainly.com/question/30861877
#SPJ1
Five is the total number of peaks due to singly charged ions in the complete mass spectrum of chlorine, \(Cl_{2}\).
How many peaks do 's molecular ions have?
The mass spectra of compounds with a single chlorine atom show two molecular ion peaks. This is because there are two isotopes of chlorine, 35Cl and 37Cl.
The molecular ion and fragment ions will both have peaks in the mass spectrum. When a mass spectrum is interpreted, a specific molecule can be located, confirmed, or its quantity can be calculated. the base summit of a mass spectrum's tallest (strongest) peak, caused by the ion with the highest relative abundance
To learn more about chlorine atom use:
brainly.com/question/30861877
#SPJ1
Rank the relative nucleophilicity of the indicated species in ethanol. CH3OH CH3S- CH3COOH CH3O- CH3COO-
Answers
From highest to lowest, the relative nucleophilicity of ethanol is CH3O-, CH3S-, CH3COO-, CH3COOH, and CH3OH. In a substitution process, a nucleophile's capacity to oust a leaving group is referred to as nucleophilicity.
The capacity of a species to give electrons to an electrophilic centre determines its nucleophilicity. The ranking order of the given species' relative nucleophilicity in ethanol is as follows:
CH3O- (methyl oxide ion) (methyl oxide ion)
CH3S- (methyl sulphide ion) (methyl sulphide ion)
CH3COO- (methyl acetate ion) (methyl acetate ion)
CH3COOH (methyl acetic acid) (methyl acetic acid)
CH3OH (methanol) (methanol)
The most nucleophilic species among them is the methyl oxide ion (CH3O-), which can readily give electrons thanks to its negatively charged oxygen atom. Due to oxygen's strong electronegativity, the carboxylate ion (CH3COO-), which is similarly negatively charged, is less nucleophilic than CH3O- and CH3S-. Methyl acetic acid (CH3COOH) is less nucleophilic and has a less acidic character than the negatively charged species. The least nucleophilic species is neutrally charged methanol (CH3OH).
learn more about nucleophilic here:
https://brainly.com/question/29563504
#SPJ4
Which of the following properties tells the most about the stability of a metal?
A. Oxidation state
B. Electronegativity
C. Molar mass
D. Atomic number
Answers
Answer: B Electronegativity
(A-p-e-x)
B. Electronegativity tells the most about the stability of a metal.
What is Electronegativity?Electronegativity directs to the ability of an atom to attract the shared electrons in the covalent bond. The more increased the value of the electronegativity is, the more intensely that element attracts the shared electrons.
Electronegativity, symbolized as χ, stands for the tendency for an atom of a provided chemical element to attract shared electrons when creating a chemical bond. An atom's electronegativity is influenced by both its atomic number and the distance at which its valence electrons reside from the charged nucleus.
To learn more about Electronegativity refer to:
https://brainly.com/question/26436343
#SPJ2
What is the relationship between temperature and pressure
Answers
Hope this helped;)
A neutral atom of Cobalt (Co) has an atomic number of 27 and an atomic mass of 59. Therefore, Co has _________________________ neutrons
Answers
Answer:
32 neutrons
Explanation:
Step 1: Given data
Atomic number of Cobalt (Z): 27
Atomic mass of Cobalt (A): 59
Step 2: Calculate the number of neutrons
The atomic number is the number of protons, while the atomic mass is the sum of protons and neutrons. Then, we can calculate the number of neutrons using the following expression.
n⁰ = A - Z
n⁰ = 59 - 27
n⁰ = 32
Compare the number of moles of H ions to the number of moles of OH ions in the titration mixture when the HCL is exactly neutralized by the KOH
Answers
Answer:
When HCl (hydrochloric acid) and KOH (potassium hydroxide) are neutralized, they react to form water (H2O) and a salt (KCl). The balanced equation is:
HCl + KOH → KCl + H2O
In this reaction, one mole of HCl reacts with one mole of KOH to form one mole of water and one mole of KCl.
During titration of HCl with KOH, the point at which the reaction is complete is called the equivalence point. At the equivalence point, the moles of H+ ions and OH- ions are equal in the titration mixture.
Since one mole of HCl reacts with one mole of KOH, and H+ ions are present in HCl and OH- ions are present in KOH, the number of moles of H+ ions will be equal to the number of moles of OH- ions at the equivalence point.
Therefore, at the equivalence point, the number of moles of H+ ions will be equal to the number of moles of OH- ions in the titration mixture when HCl is exactly neutralized by KOH.
When the HCl is neutralized by KOH, the equivalence point is reached. During titration, the amount of HCl is determined using a basic solution of known concentration.
It is possible to calculate the amount of KOH required for complete neutralization if the initial concentration of the HCl solution is known. The balanced chemical equation for the reaction between HCl and KOH is:HCl + KOH → KCl + H2OThe stoichiometry of the reaction indicates that one mole of HCl reacts with one mole of KOH to produce one mole of H2O. Thus, the number of moles of H+ ions is equal to the number of moles of OH- ions when the equivalence point is reached.In an acid-base reaction, the number of moles of hydrogen ions (H+) produced by the acid is equal to the number of moles of hydroxide ions (OH-) produced by the base. When the HCl is exactly neutralized by the KOH, the number of moles of H+ ions is equal to the number of moles of OH- ions in the titration mixture.
This is due to the balanced chemical equation for the reaction, which shows that one mole of HCl reacts with one mole of KOH to produce one mole of water (H2O).Thus, at the equivalence point, the number of moles of H+ ions is equal to the number of moles of OH- ions. This is the point at which all of the HCl has reacted with the KOH. After the equivalence point, the excess KOH will react with the H2O to produce OH- ions, resulting in a basic solution.
for such more questions on solution
https://brainly.com/question/25326161
#SPJ8
2N2(g) +302(g) → 2N₂O3 (9)
Express your answers as integers separated by a comma.
Answers
The molar masses of the reactant and product of the equation 2N2(g) +302(g) → 2N₂O3 (9) is 152 g.
How do you calculate the molar mass of the equation?In the given in this item, we may solve directly the molar masses of the reactants and the products to see if they are matching.
Reactant:
(2N2) = (2)(28.0 g/mol) = 56 g
(3O2) = (3)(32.0 g/mol) = 96 g
total = 152 g
Product:
(2N2O3) = (2)(76 g/mol) = 152 g
The reactant and product have the same masses.
Therefore, the molar masses of the reactant and product of the equation 2N2(g) +302(g) → 2N₂O3 (9) is 152 g.
To know more about molar masses, visit:
brainly.com/question/12127540
#SPJ1
The aromatic region (7-8 ppm) of the proton NMR spectrum of compounds with para disubstituted benzene rings such as phenacetin is often referred to as an AB pattern. This pattern has two doublet signals coupled to each other. Explain the origin of this AB pattern.
Answers
The aromatic ring in phenacetin exhibits an AB pattern because it has two mutually coupled nuclei that are unrelated to other protons or whose shift differences is equal to the coupling constant.
Why are protons present on deshielded benzene?Deshielding occurs for the ring protons of benzene due to their larger chemical shift (7.3 ppm vs. 5.6 ppm for the vinylic protons in cyclohexene) and the fact that the magnetic field induced outside of the ring is the same orientation as that of the external field.
What frequency band does benzene proton NMR display?Protons are present in benzene at levels of 6–8 ppm.This value is larger than that of the protons in those other hydrocarbon like alkanes & alkenes because of the aromatic ring's deshielding effect.
To know more about protons visit:
https://brainly.com/question/1252435
#SPJ1
ENTHALPHY LAB QUESTIONS!!
PURPOSE OF EXPERIMENT: To find Heat of Solution of sodium hydroxide and to find the heat of neutralization between sodium hydroxide and hydrochloric acid.
Experiment 1 Procedure:
1. Measure 50.0 mL of water (tap) into a 100 mL graduated cylinder and pour it into a large coffee cup.
2. Determine the temperature of this water
3. Measure out 2.00 g of sodium hydroxide into a piece of paper towel *tare scale!
4. Add the sodium hydroxide to the water in the coffee cup and put a small cup over it, with the thermometer through the hole. Stir GENTLY with the thermometer and record the temperature every 30 seconds for 3 minutes or until it peaks. Record this in a properly labelled table.
5. Let this stand for 45 minutes before proceeding to Exp. 2.
WHAT WE FOUND IN EXP 1:
T (temp.) initial = 20 degrees C
T (temp) FINAL = 28.5 degrees C
moles of sodium hydroxide = 0.0518mol
the molar mass of sodium hydroxide = 39.969g/mol
C (specific heat of water) = 4.184J/g degrees C
THE NUMBER OF TRIALS FOR TEMP IN EXP 1
1st trial = 21 C
2nd trial = 24.5 C
3rd trial = 26 C
4th trial = 26 C
5th trial = 28 C
6th trial = 28.5 C
7th trial = 28.5 C (final temp)
ANALYSIS FOR EXPERIMENT ONE:
1. Determine the moles of sodium hydroxide (NaOH) from the experiment.
2. Determine Qsurroundings and Qrxn
3. Determine the enthalpy for the dissociation of sodium hydroxide (delta H sol)
4. Write the thermochemical equation for the dissociation of sodium hydroxide TWO ways and write an enthalpy diagram
5. What assumptions did you make to calculate #2? (some example assumptions to make: assume that the solution is water and that heat and density COULD be the same as water, etc)
6. Research the actual value and determine the percent error
7. In terms of bonds breaking and forming, what is RESPONSIBLE FOR ENTHALPY CHANGE?
EXPERIMENT 2 PROCEDURE:
1. Measure out 50.0 mL of 0.75 concentration M HCl into a graduated cylinder
2. Measure and record the temperature of the sodium hydroxide solution from exp. 1.
3. Add the hydrochloric acid solution to the sodium hydroxide solution, put the small cup on, and record the temperature change every 15 seconds for 1 minute. Stir GENTLY. Record this in a properly labelled table (will be given below)
4. Solutions can be discarded down the sink.
WHAT WE FOUND IN EXP. 2:
T (temp) initial = 23.5 C
T (temp) FINAL = 27 C
THE NUMBER OF TRIALS FOR TEMP IN EXP 2
1st trial = 27 C
2nd trial = 27 C
3rd trial = 27 C
4th trial = 27 C (FINAL TEMP)
ANALYSIS FOR EXPERIMENT 2:
1. Determine the moles of HCl added to this mixture
2. Write the chemical equation for this reaction
3. Determine the limiting reagent
4. Determine the Qsurr and Qrxn *CONVERT TO kJ*
5. Determine the enthalpy for the neutralization reaction.
6. Write the thermochemical equation for the dissociation of sodium hydroxide TWO WAYS and write an enthalpy diagram
7. Research the actual value and determine the percent error.
8 Explain sources of experimental error for both experiments and BE SPECIFIC! (NOT CALCULATION ERRORS, SPILLING, OR LOSING REACTANTS - DO NOT COUNT AS ERRORS! They can be EXPERIMENTAL due to heat loss/gain, room temp *specific heat capacity is for 25 C*, and atmospheric pressure is constant. And they can be MEASUREMENTS - consider the precision and the potential range of error for each measurement)
9. In terms of bonds breaking and forming, what's responsible for the enthalpy change?
CONCLUSION: write a brief statement that refers to the purpose.
Answers
In Experiment 1, we found the heat of solution of sodium hydroxide (NaOH) by dissolving 2.00 g of NaOH in 50.0 mL of water.
What was observed in the experiment?The temperature rose from 20°C to 28.5°C. The moles of NaOH were determined to be 0.0518 mol.
Using the specific heat of water (4.184 J/g°C), we calculated the enthalpy change (ΔH_sol) and compared it to the literature value, finding a percent error.
In Experiment 2, we measured the heat of neutralization between NaOH and 0.75 M HCl.
The temperature increased from 23.5°C to 27°C. We determined the moles of HCl, limiting reagent, and enthalpy change (ΔH_neut) for the neutralization reaction.
The actual value was compared to the literature value, and percent error was calculated.
Experimental errors in both experiments could arise from heat loss/gain, variations in room temperature and atmospheric pressure, and imprecise measurements.
The enthalpy changes in both experiments are due to bond breaking and forming during the dissociation of NaOH and the neutralization reaction between NaOH and HCl.
In conclusion, we determined the heat of solution for sodium hydroxide and the heat of neutralization between sodium hydroxide and hydrochloric acid, and analyzed the possible sources of experimental errors.
Read more about enthalpy here:
https://brainly.com/question/16985375
#SPJ1
calcium carbonate reacts with dilute hydrochloric acid according to the equation:
CaCO3 + 2HCl = CaCl2 + H2O + CO2
the rate of reaction decreases with time because the
Answers
Concentration of the reactants decreases over time as they are used up in the reaction. This means that there are fewer collisions between the reactant particles per unit time, leading to a decrease in the rate of reaction. Additionally, as the reaction progresses, the concentration of the product molecules increases, leading to an increase in the likelihood of the reverse reaction (i.e., CaCl2 + H2O + CO2 → CaCO3 + 2HCl) occurring. This also contributes to a decrease in the rate of the forward reaction over time.
Use the periodic table (if necessary) to determine which element the following spin diagram represents: A. BromineB. None of theseC. ChlorineD. Argon

Answers
Answer: C The chlorine element is seen in the following spin diagram.
How many electrons does chlorine have to give?Each of the chlorine and hydrogen atoms contributes one electron to the covalent link. The molecule's hydrogen valence shell is complete with two electrons, while the chlorine valence shell is fully completed with eight electrons.
How is spin calculated?The electron spin is denoted by the symbol ms, which stands for the spin quantum number, which is expressed as ms m s for electrons. An electron with a spin of +1/2 + 1 / 2 is referred to as a spin up electron, and one with a spin of 1/2 1 / 2 is referred to as a spin down electron.
To know more about element spin diagram visit:-
https://brainly.com/question/17171085
#SPJ9
What is the pH of a solution with [H⁺] = 2.5 x 10-4 M?
Answers
Answer:
\(\text{pH}=3.6\)
Explanation:
Given that,
\([H^+]=2.5\times 10^{-4}\ M\)
We need to find the pH of a solution with \([H^+]=2.5\times 10^{-4}\ M\).
We know that,
\(\text{pH}=-\text{log}[H^+]\\\\=-\text{log}[2.5\times 10^{-4}]\\\\=-(-3.6)\\\\=3.6\)
So, the pH of the solution is 3.6.
Help!!!!
A termplate of a Venn diagram representing common and differentiating characteristics of covalent and ionic bonds is
shown.
lonic
Bond
Covalent
Bond
A
C
Which of the following characteristics can be written only in space B?
O Occurs in substances that have at least one non-metal
O Occurs in substances that have a repeating lattice structure
O Occurs in substances that have discrete molecular structure
O Occurs in substances that have high melting points

Answers
Answer: it ISNT occurs in substances that have discrete molecular structure
Explanation:
I took the test
The characteristics that can be written only in space B is they occur in substances that have at least one non-metal. The correct option is A.
What is ionic and covalent bond?Ionic bonds are formed when two electrically charged elements shred their charged ions. Covalent bonds are formed when there is a mutual sharing of electrons between the atoms.
One condition that is similar in both is both bonds needed one non-metal to form the bond.
Thus, the correct option is A, which occurs in substances that have at least one non-metal.
Learn more about the ionic and covalent bonds, here:
https://brainly.com/question/12663276
#SPJ2
Mescaline a hallucinogenic amine obtained from the peyote cactus has been synthesized in two steps from 3 4 5 trimethoxybenzyl bromide The first step is nucleophile substitution by sodium cyanide. The second step is a lithium aluminum anhydride reduction. Indicate the reactions and give the structure of mescaline
Answers
Mescaline produces a wide range of psychoactive effects when ingested, including altered perception of reality, hallucinations, and euphoria. It is a powerful psychedelic drug that has been used for centuries by Native American tribes in spiritual ceremonies
Mescaline is a hallucinogenic alkaloid that is derived from the Peyote cactus. Mescaline is a complex organic molecule that can be synthesized in the laboratory from 3,4,5-trimethoxybenzyl bromide in two steps.The first step involves nucleophilic substitution using sodium cyanide, and the second step is a reduction using lithium aluminum hydride (LAH).Here's how mescaline can be synthesized from 3,4,5-trimethoxybenzyl bromide:Step 1: Nucleophilic substitution using sodium cyanideThe reaction of 3,4,5-trimethoxybenzyl bromide with sodium cyanide results in the formation of the nitrile derivative. NaCN serves as the nucleophile in this reaction, and it replaces the bromide ion.The mechanism for this reaction involves the following steps: A nucleophilic attack by the cyanide ion on the benzyl bromide. The carbon-bromine bond breaks, and the benzyl cation is formed. A second nucleophilic attack by the cyanide ion occurs on the benzyl cation, resulting in the formation of the nitrile derivative.Here's the reaction equation for this step:Step 2: Reduction using lithium aluminum hydrideThe next step is the reduction of the nitrile derivative using LAH. LAH serves as a strong reducing agent in this reaction and reduces the nitrile derivative to the amine. The mechanism for this reaction involves the following steps: A nucleophilic attack by LAH on the nitrile derivative. This results in the formation of an imine intermediate. The imine intermediate reacts with another LAH molecule, resulting in the formation of the amine.Here's the reaction equation for this step:Mescaline structure: Mescaline is a psychoactive compound that belongs to the phenethylamine class of alkaloids. The structure of mescaline is as follows: The molecule has three methoxy groups attached to the benzene ring, and it has an amine functional group. The molecule is a white crystalline powder that is soluble in water and alcohol.
for such more questions on psychoactive
https://brainly.com/question/30551262
#SPJ8
A hot air balloon's gondola is suspended below a cloth envelope containing
2.775 x 10⁰ liters of hot air. How many milliliters of hot air is this?
Answers
The milliliters of hot air that a balloon's gondola is suspended below is 2775millilitres.
How to convert litres to milllilitres?Litres and millilitres are both unit of measures of volume.
According to this question, a hot air balloon's gondola is suspended below a cloth envelope containing 2.775 x 10⁰ liters of hot air.
The conversion factors of litres to milllilitres;
1000mL = 1L
2.775 × 10⁰ × 1000 = 2775millilitres
Therefore, the milliliters of hot air that a balloon's gondola is suspended below is 2775millilitres.
Learn more about volume at: https://brainly.com/question/1578538
#SPJ1
What is the amount of energy required to raise the temperature of 150 grams of aluminum by 10°C?
Group of answer choices
A. 13.45 J
B. 0.897 J
C. 1345.5 J
D. 4.18 J
Answers
Answer:
C.) 1345.5 J
Explanation:
To find the energy, you need to use the following equation:
Q = mcΔT
In this equation,
-----> Q = energy (J)
-----> m = mass (g)
-----> c = specific heat (J/g°C)
-----> ΔT = change in temperature (°C)
The specific heat of aluminum is 0.89 J/g°C. You can plug the given values into the equation and solve.
Q = mcΔT
Q = (150 g)(0.89 J/g°C)(10 °C)
Q = 1335
*It is up to you whether you wish to trust this answer. My answer may be slightly different due to using a different specific heat.
Which of the following represents C3H8?

Answers
Among the given options the compound with the formula C₃H₈ is option C. It is an organic compound under named as propane.
What is propane?
Propane is an organic compound classified as a hydrocarbon with saturated carbon - hydrogen bonds. It's formula is C₃H₈. It contains 3 carbons and 8 hydrogens.
The two end carbons contains 3 hydrogens each and the middle carbon contains 2 hydrogens. They are all bonded through sigma bonding. The compound in A is C₅H₁₂ and in B, it is C₆H₁₄.
The formula of the compound in option D is C₅H₁₀. Hence, the skeleton showing the compound with the formula of C₃H₈ is option C.
To find more on propane, refer here:
https://brainly.com/question/24159489
#SPJ2
Question 2 of 10
What is the percent yield of a reaction?
The amount of product obtained x 100
amount possible
B. The amount of product actually obtained in a reaction
C. The amount of product that is possible from a reaction
D. The difference between measured and calculated amounts
A.
Answers
Answer:
c
Explanation:
Solar and wind energy are both intermittent resources that cannot be relied upon for a constant stream of energy production. Explain why developing better ways to store energy is an important part of making these energy sources more practical to use.
Answers
By removing the need to build additional transmission lines and equipment, energy storage may reduce costs for utilities and their customers.
By removing the need to build additional transmission lines and equipment, energy storage may reduce costs for utilities and their customers. Energy storage's inherent ability to offer backup power in the event of grid failure is a feature that both residential consumers and commercial owners find highly desirable.
To know more about energy, here:
https://brainly.com/question/1932868
#SPJ1
How is the mass in grams of the element converted to amount in atoms
Answers
What is radiochemical dating?
Answers
Answer: The technique of comparing the abundance ratio of a radioactive isotope to a reference isotope to determine the age of a material
Explanation:
A material that consists of only one type of element or compound.
Answers
Answer:
Pure substance
Explanation:
A material that consists of only one type of element or compound is regarded as a pure substance.
A pure substance has the following properties;
All the parts are the same throughout i.e. they are homogenousThey have a definite composition. They cannot easily be separated or broken down into simpler substances by physical means. Separation by physical methods is not easyThey have unique sets of properties.Compounds and elements are pure substances too.