Consider an aqueous solution of ammonium hypochlorite. This is a(n) ____ salt. The resulting solution is ____.
a. insoluble; acidic
b. soluble; acidic
c. insoluble; basic
d. soluble; basic
e. soluble; neutral
Answers
An aqueous solution of ammonium hypochlorite is a soluble salt. Ammonium hypochlorite is an ionic compound formed by the reaction between ammonium and hypochlorite ions.
When this salt dissolves in water, it dissociates into ammonium and hypochlorite ions, both of which are highly soluble in water. Therefore, the resulting solution is also soluble and can conduct electricity.
Ammonium hypochlorite is a weak acid salt. This means that the resulting solution will be slightly basic. The ammonium ion can undergo hydrolysis in water, producing ammonium hydroxide and hydrogen ions. The hydrogen ions combine with the hypochlorite ions to form hypochlorous acid, which is a weak acid. This reaction results in a slight increase in the pH of the solution, making it basic.
Therefore, an aqueous solution of ammonium hypochlorite is a soluble salt, and the resulting solution is slightly basic.
To know more about hypochlorite please visit...
brainly.com/question/27548463
#SPJ11
Related Questions
In the equation. C + O2 --------> CO2; CO2 is the
A.coefficient
B.precipitate
C.reactant
D.product
Answers
Answer: Product
Explanation:
A Chemical reaction shows how substances, written in symbols known as reactants, are converted to different substances called products. An arrow from the left hand side (reactants) points to right hand side ( products )shows the direction of flow of reaction.
In the equation. C + O2 --------> CO2
reactant------> product
CO2 is the product
Compare and contrast the political system
(institutions, branches of government, electoral rules) of France
and Russia. How do they compare? What are the key distinguishing
features? What are the stre
Answers
Russia is a federation with a semi-presidential political system. The President is the head of state while the Prime Minister is the head of government. The Federal Assembly is a bicameral legislature that is made up of the State Duma (lower house) and the Federation Council (upper house).
The political system in Russia and the United States are different. In the US, it is a presidential system where the President is both the head of state and government, while in Russia, the President is the head of state while the Prime Minister is the head of government.
In the US, the Congress is made up of the Senate (upper house) and the House of Representatives (lower house) while in Russia, the Federal Assembly is made up of the State Duma (lower house) and the Federation Council (upper house).
The key distinguishing features between the political systems in Russia and the US include the role of the President, the structure of the legislature, and the nature of the judiciary. In Russia, the President has a lot of power and is able to appoint the Prime Minister and other members of the executive branch.
The judiciary is also less independent compared to that of the US. On the other hand, the US has a more balanced system of power between the three branches of government, with the judiciary being independent of the executive and legislative branches.
The strengths of the political system in Russia include a strong centralized government that is able to make quick decisions and a strong military. However, the lack of political pluralism and the weak judiciary system are key weaknesses of the system.
The US political system has a strong commitment to individual rights and democratic principles. However, the system is often characterized by gridlock and polarization between political parties, leading to slow decision-making and a lack of progress on important issues.
To know more about Federal Assembly here
https://brainly.com/question/28863069
#SPJ11
Sandban
Grade 8 Science Winter Interim
Question
Q Zoom
Signed in as NOTRE
Q Review Finish Test
Question
>
Normal
ABC
Question 3
Twoiron balls are dropped from a height of 100 meters One Dal Frame A) is not moving when it is dropped and the second ball is moving horizontally at a constant velocity of 10 m/s when it is dropped (Frame B). The balls follow the
trajectories indicated by the arrows and fall for 4,52 s before hitting the ground. (Ignore air resistance)
Frame A
Frame B
1kg
-10%
10kg
Answers
~~20 points~~ What is the mass number of an element which has 43 protons, 46 neutrons, and 43 electrons?
a. 132
b. 3
c. 86
d. 83706
e. 89
f. -46
g. there is not enough information given to correctly answer the question
Answers
Answer:
e. 89
Explanation:
The mass number is the number of protons and neutrons in an atom. It doesn't include electrons.
43 + 46 = 89
Which mass and atomic number is correct for the following isotope: carbon-14
explain your answer

Answers
Answer:
Explanation:
Isotope notation is of the form
A
Z
X
where
Z
is the atomic number (number of protons),
A
is the mass number (sum of protons and neutrons), and X is the element.
In Carbon- 14 , 14 is the mass number (sum of protons and neutrons). This is our A .
Carbon has the atomic symbol C , and it has 6 protons. This is the atomic number, or A . Thus, the isotope notation for Carbon- 14 is 14 6 C
Hope this helps!
Explanation:
Which of the following is a natural recourse?
Labor
Skills
Talent
Soil
Answers
a 3.82-g sample of magnesium nitride is reacted with 7.73 g of water: mg3n2 3h2o 2nh3 3mgo the yield of mgo is 3.60 g. what is the percent yield in the reaction?
Answers
The percent yield of MgO in the reaction is 78.6%
What is stoichiometry?To calculate the percent yield, we first need to calculate the theoretical yield of MgO. We can do this by using stoichiometry to determine the amount of MgO that should be produced from the given amount of Mg3N2.
From the balanced chemical equation:
1 mole of Mg3N2 produces 3 moles of MgO
The molar mass of Mg3N2 is:
24.31 g/mol (Mg) x 3 + 14.01 g/mol (N) x 2 = 100.95 g/mol
So, the number of moles of Mg3N2 used in the reaction is:
3.82 g / 100.95 g/mol = 0.038 mol
According to the balanced chemical equation, 3 moles of MgO are produced for every 1 mole of Mg3N2 that reacts. Therefore, the theoretical yield of MgO can be calculated as follows:
Theoretical yield of MgO = 3 × 0.038 mol × 40.31 g/mol (MgO)
= 4.58 g
The percent yield can now be calculated using the following formula:
Percent yield = (Actual yield / Theoretical yield) × 100%
Substituting the given values, we get:
Percent yield = (3.60 g / 4.58 g) × 100%
= 78.6%
Therefore, the percent yield of MgO in the reaction is 78.6%.
Learn more about stoichiometry
brainly.com/question/30215297
#SPJ11
(PLS HELP)
Describe three of your favorite foods. How do they change phases of matter when they are heated?
Answers
Answer:
ice.
Explanation:
it becomes a liquid
Which property of matter is conserved in chemical reactions and shown by balanced equations? mass volume density shape
Answers
Answer:
A. mass
Explanation:
On edgenuity 2020
The property of matter that is conserved in chemical reactions is mass. The correct option is A.
What is law of conservation of mass?The law of conservation of mass implies that mass is never be created nor destroyed in a chemical reaction.
The law of conservation of mass, also known as the principle of mass conservation, implies that for any system closed to all transfers of matter and energy.
The mass of the system must remain constant over time, because the system's mass cannot change, and thus quantity cannot be added or removed.
The law of conservation of mass was critical to the advancement of chemistry.
It assisted scientists in understanding that substances did not disappear as a result of a reaction, as they may appear to do, but rather transformed into another substance of equal mass.
Thus, the correct option is A.
For more details regarding law of conservation, visit:
https://brainly.com/question/24131537
#SPJ6
What is solar energy?
Answers
Answer:
Solar energy is energy from the sun converting it to thermal or electric energy. It is renewable resource meaning it will never die out. It is also called radiant energy.
Explanation:
The reactants of a reaction are 2AgClO3 and Na2CO3. According to the law of
conservation of matter, which of the following could be a product?
O A. NaOH
O B. Ag2CO3
O C. CaCO3
O D. AgNO3
Answers
Answer:
The answer is "Option B"
Explanation:
Law:
In the law of the conservation of matter, states the volume of matter throughout the process remains constant. In any particular case, it is closed to the movement of matter. It is mostly on conservation of energy means, that the total mass of the products should consider the sum of the mass, which is the reactant of the event of chemical processes.
Reaction:
\(2AgClO_3+ Na_2CO_3= Ag_2CO_3+Na_2ClO_3\)
In the above equation when we react to \(2AgClO_3\) from the \(Na_2CO_3\), it will give \(Ag_2CO_3 \ \ \text{and} \ \ Na_2ClO_3\). that's why option b is correct.
Answer:
Ag2CO3
Explanation:
Calculate the change in pH when 0.16mol OH^- is added to 1.00 L of each of the following buffers. (a) 1.18 M solution of sodium dihydrogen phosphate containing 1.18 M sodium hydrogen phosphate (b) 0.590 M solution of sodium dihydrogen phosphate containing 0.590 M sodium hydrogen phosphate
Answers
(a) The change in pH when 0.16 mol OH^- is added to 1.00 L of a 1.18 M sodium dihydrogen phosphate buffer is approximately -0.43.
(b) The change in pH when 0.16 mol OH^- is added to 1.00 L of a 0.590 M sodium dihydrogen phosphate buffer is approximately -0.08.
Explanation to the above short answers are written below,
To calculate the change in pH when a base is added to a buffer solution, we need to consider the acid-base equilibrium of the buffer components and the Henderson-Hasselbalch equation.
The Henderson-Hasselbalch equation is given by:
pH = pKa + log([A-]/[HA])
(a) For the sodium dihydrogen phosphate buffer, the acid is the sodium dihydrogen phosphate (NaH2PO4) and its conjugate base is the sodium hydrogen phosphate (Na2HPO4).
The pKa for this acid-base pair is around 7.20. Since the initial concentrations of the buffer components are the same (1.18 M), the logarithmic term becomes 0, and the pH remains close to the pKa value.
Therefore, the change in pH when 0.16 mol OH^- is added is approximately -0.43.
(b) Similarly, for the 0.590 M sodium dihydrogen phosphate buffer, the initial concentrations of the acid and its conjugate base are the same (0.590 M). As a result, the change in pH when 0.16 mol OH^- is added is smaller, around -0.08.
These calculations assume that the volume of the buffer solution remains constant and that the dissociation constants (pKa values) remain the same over the course of the reaction.
To know more about "Henderson-Hasselbalch equation" refer here:
https://brainly.com/question/31732200#
#SPJ11
PLEASE HELP ME!!! It would be great
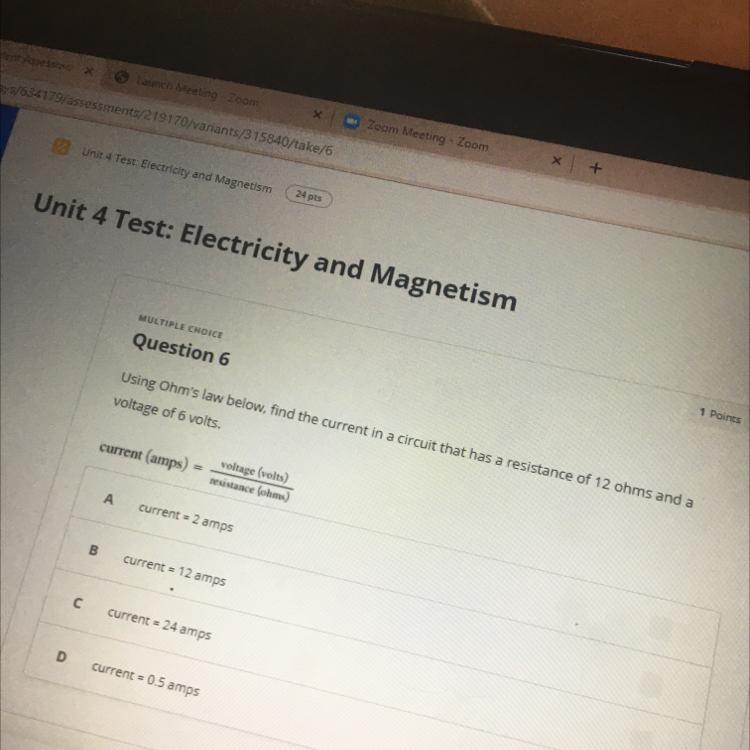
Answers
What is the difference between a plain and a plateau? A. Only a plain is flat, a plateau is steep mountainside. B. Both are flat, but a plateau is bordered by cliffs. C. A plain is rolling hills with cliffs, and a plateau is large flat area.
Answers
Answer:
B
Explanation:
Both plain and plateau have flat surfaces. However, a plain is located in a low-lying area while a plateau is located on an elevated area. In essence, a plateau can be viewed as an elevated plain or a plain that is bordered by cliffs.
The correct option is B.
This phytoplankton has cell walls of calcium carbonate (CaCO3) and are responsible for the sediments that ultimately formed the White Cliffs of Dover, UK.
a) diatoms
b) bacteriaplankton
c) dinoflagellates
d) copepods
e) coccolithophorids
Answers
The phytoplankton responsible for the sediments that formed the White Cliffs of Dover, UK are coccolithophorids.
The phytoplankton responsible for the sediments that formed the White Cliffs of Dover, UK are coccolithophorids. These tiny organisms have cell walls made of calcium carbonate (CaCO3) plates called coccoliths. When these organisms die, their coccoliths sink to the ocean floor and accumulate over time, forming sedimentary rocks like those seen in the White Cliffs. Coccolithophorids are found in oceans all around the world and play an important role in the global carbon cycle, as they can both absorb and release carbon dioxide. To provide a detailed explanation of the specific type of phytoplankton responsible for the formation of the White Cliffs.
To know more about phytoplankton visit: https://brainly.com/question/10279696
#SPJ11
Find the kinetic energy of a boy of mass 6.5kg running at a velocity of 6.0m/s
Answers
Explanation:
Given,
Mass(m)=6.5kg
Velocity(v)=6 m/s
K.E.=1/2×mv^2
=1/2×6.5×6^2
=1/2×6.5×36
=117 J
Which of the following is NOT an atom that hydrogen bonds?
Select one:
a. Oxygen
b. Nitrogen
c. Fluorine
d. Sodium
Answers
Answer:
The answer is D sodium
Explanation:
6. 3 mL of 0.115 M KOH was needed to arrive at the equivalence point when 15.0 mL of HNO3 was titrated. What is the molarity of HNO3
Answers
To determine the molarity of HNO3, we can use the concept of stoichiometry and the volume and concentration information provided. The balanced chemical equation for the reaction between KOH and HNO3 is: KOH + HNO3 -> KNO3 + H2O
From the given information, we know that 3 mL of 0.115 M KOH is required to reach the equivalence point when titrating 15.0 mL of HNO3.
Using the concept of stoichiometry, we can set up a ratio between the volumes and concentrations of the two solutions:
(Molarity of KOH) x (Volume of KOH) = (Molarity of HNO3) x (Volume of HNO3)
(0.115 M) x (3 mL) = (Molarity of HNO3) x (15.0 mL)
Solving for the molarity of HNO3, we get:
Molarity of HNO3 = (0.115 M) x (3 mL) / (15.0 mL)
Molarity of HNO3 = 0.023 M
Therefore, the molarity of HNO3 is 0.023 M.
Learn more about molarity here;
brainly.com/question/31545539
#SPJ11
How does water get up to the atmosphere, and how does it get back down to Earth’s surface?
Answers
Answer: water goes into atmosphere through evaporation and get back down on earth surface by precipitation.
Explanation: Evaporation is the process where the water gets evaporated to the atmosphere where the liquid state of the water is changed into the vapor state . Then the vapor condenses into the clouds till the clouds get filled and the water falls as precipitation to the ground or earth. This whole process is called the water cycle.
At pH 3, how many charged groups are present in the pentapeptide Ala-Asp-His-Ser-Lys?
a) 1
b) 2
c) 3
d) 4
e) 5
Answers
There are 4 charged groups present in the pentapeptide at pH 3. Therefore, the correct answer is (d) 4.
At pH 3, the carboxyl group of Ala, Asp, and Lys will be protonated, making them positively charged. The amino group of the N-terminal Ala will also be protonated, making it positively charged. The imidazole group of His, however, will be fully protonated, making it neutral. Therefore, there are a total of four charged groups present in the pentapeptide Ala-Asp-His-Ser-Lys at pH 3. The answer is d) 4.
At pH 3, the charged groups present in the pentapeptide Ala-Asp-His-Ser-Lys are:
1. Asp (aspartic acid) with a carboxyl group (COOH), which is negatively charged at pH 3.
2. Lys (lysine) with an amino group (NH3+), which is positively charged at pH 3.
3. The N-terminal amino group (NH3+) of Ala, which is positively charged at pH 3.
4. The C-terminal carboxyl group (COOH) of Lys, which is negatively charged at pH 3.
Visit here to learn more about carboxyl group brainly.com/question/31319088
#SPJ11
calculate the effective nuclear charge of s and cl using the simple formula zeff = z–s. do not use slater's rules.
Answers
The effective nuclear charge of sulfur is 14, and the effective nuclear charge of chlorine is 15.
Effective nuclear charge (Zeff) is a measure of the positive charge felt by the valence electrons. The effective nuclear charge (Zeff) of s and Cl is calculated using the simple formula zeff = z – s, where z is the atomic number and s is the screening constant. Screening constant (s) is the number of electrons between the nucleus and the valence electrons that shield the valence electrons from the full nuclear charge of the nucleus.
For sulfur (S), the atomic number is 16, and there are two electrons in the innermost shell and four electrons in the second shell. So, the number of valence electrons in sulfur is 6. The screening constant of S is 2. Effective nuclear charge of sulfur = z – s= 16 - 2= 14
For chlorine (Cl), the atomic number is 17, and there are two electrons in the innermost shell and eight electrons in the second shell and seven valence electrons. Therefore, the screening constant of Cl is 2. Effective nuclear charge of chlorine = z – s= 17 - 2= 15
Learn more about effective nuclear charge: https://brainly.com/question/30459988
#SPJ11
For some transformation having kinetics that obey the Avrami equation, the parameter n is known to have a value of 1.5. If the reaction is 25% complete after 125 s, how long (total time) will it take the transformation to go to 90% completion
Answers
The Avrami equation is a mathematical model used to describe the kinetics of certain types of transformations, such as phase transformations in materials. The equation takes the form of a power law, where the extent of transformation is related to the time of the reaction and a parameter called "n". For the given transformation, it is known that n has a value of 1.5.
To determine the total time required for the transformation to reach 90% completion, we can use the Avrami equation and the information that the reaction is 25% complete after 125 seconds. From the equation, we know that:
X = 1 - exp(-(kt)^n)
where X is the extent of transformation, k is the rate constant, t is time, and n is the Avrami parameter. Solving for k, we get:
k = (ln(1/(1-X)))^(1/n) / t
Substituting X = 0.9 (90% completion) and n = 1.5, we can solve for k. Then, we can use k and the initial extent of transformation (X=0.25) to solve for the total time required for 90% completion:
t = ((ln(1/(1-0.9)))^(1/1.5) - (ln(1/(1-0.25)))^(1/1.5)) / k
The resulting value of t will give us the total time required for the transformation to go from 25% to 90% completion.
TO KNOW MORE ABOUT The Avrami equation CLICK THIS LINK -
brainly.com/question/31494223
#SPJ11
i pick D am i right if so or not why

Answers
Answer:
it would be b
Explanation:
because when the roller coaster is going downhill it is moving faster so that means there is potential energy becoming kinetic energy because kinetic energy is made from movement
Answer: 1
Explanation: Because it is not moving
Match the separation techniques with the correct descriptions.
1. Heating a solid until it passes directly from the solid phase into the gaseous phase.
2. Separating a liquid from an insoluble solid sediment by carefully pouring the liquid from the solid without disturbing the solid.
3. The process of vapor returning to the solid phase without a liquid phase in between.
4. Heating a mixture to vaporize a volatile liquid component to make the remaining component dry.
5. Separating a solid from a liquid using a porous material, such as paper, charcoal, or sand, as a filter.
6. Using a solvent to selectively dissolve one or more components from a solid mixture.
A. Sublimation
B. Solids that Sublime
C. Extraction
D. Decantation
E. Evaporation
F. Filtration
Answers
Answer:
Explanation:
1 = A
Sublimation is the process where by a sample is heated to pass through solid phase to gaseous phase without the intermittent liquid phase. Example of substance that sublime is camphor.
2 = D
Decantantion
5 = F
Filtration is the process a liquid from solid using a porous material. This technique requires a set up and a good porous material eg filter paper.
6 = B
This technique is to separate a mixture of solids by converting them from solid phase to gaseous phase since they sublime.
3 = deposition.
The answer isn't in the option but deposition is the process of substance in gaseous phase to change into solid state without passing through liquid phase. Deposition is the opposite of sublimation.
4 = E
the cleaning action of soaps and detergents is attributable to:
their ability to evaporate quickly. their ability to form micelles. their short hydrocarbon tail. their acidic character.
Answers
The cleaning action of soaps and detergents is attributable to their ability to form micelles. Micelles are small clusters of molecules that are formed when the hydrophobic (water-repelling) tail of a soap or detergent molecule faces inward, while the hydrophilic (water-attracting) head faces outward.
This arrangement allows the soap or detergent to surround and suspend dirt, oil, and other particles in water, making them easier to remove from surfaces. Soaps and detergents do not evaporate quickly, nor do they have short hydrocarbon tails or acidic character that contribute to their cleaning action.
Therefore, their ability to form micelles is the primary reason for their effectiveness in cleaning.
To know more about micelles visit:
https://brainly.com/question/31587558
#SPJ11
Gibbs free energy differences between axial-substituted and equatorial-substituted chair conformations of cyclohexane were given in Table 4.(a) Calculate the ratio of equatorial to axial tert-butylcyclohexane at 25°C.(b) Explain why the conformational equilibria for methyl, ethyl, and isopropyl substituents are comparable but the conformational equilibrium for tert-butylcyclohexane lies considerably farther toward the equatorial conformation.
Answers
(a) The ratio of equatorial to axial tert-butylcyclohexane at 25°C can be calculated by using the Gibbs free energy difference between the two conformations, which is given in Table 4.
The ratio can be calculated using the following equation:
ratio = e^(-ΔG/RT),
where ΔG is the Gibbs free energy difference,
R is the gas constant, and
T is the temperature. At 25°C (298 K),
the ratio of equatorial to axial tert-butylcyclohexane is:
e^(-3.8/8.314*298)= 1.70
(b) The conformational equilibria for methyl, ethyl, and isopropyl substituents are comparable, but the conformational equilibrium for tert-butylcyclohexane lies considerably farther toward the equatorial conformation because of the steric hindrance caused by the bulky tert-butyl group.
In the axial conformation, the tert-butyl group experiences more steric strain than in the equatorial conformation because it is closer to the other axial substituents.
This steric strain leads to a higher energy state for the axial conformation, making it less favorable than the equatorial conformation. As a result, the equilibrium between the two conformations is shifted toward the equatorial conformation for tert-butylcyclohexane.
To learn more about Gibbs free energy, visit;
https://brainly.com/question/15409485
#SPJ11
please help me need it fast

Answers
Answer:
Do you need 3 ways or just one?
1. Temperature.
2. Pressure.
3. Polarity.
Explanation:
Eh hope these help, Idr understand the question but those are 3 ways to increase the solubility of a solid in water.
write the chemical formula for the ligand in the coordination compound tetracarbonylplatinum(iv) chloride.
Answers
The ligand in the coordination compound tetracarbonylplatinum(IV) chloride is carbonyl. A ligand is a molecule or ion that binds to a central metal atom or ion to form a coordination complex.
Tetracarbonylplatinum(IV) chloride is a coordination compound that consists of a platinum(IV) ion coordinated with four carbonyl ligands and one chloride ion. The chemical formula of the carbonyl ligand is CO, which represents a carbon atom bonded to an oxygen atom through a double bond.
In this compound, each platinum atom is surrounded by four carbonyl ligands, which means there are four CO ligands attached to the central platinum(IV) ion. The coordination number of the platinum(IV) ion is four, indicating that it forms four bonds with the carbonyl ligands.
Additionally, there is one chloride ion present as a counterion to balance the charge of the complex. Therefore, the chemical formula for the ligand in tetracarbonylplatinum(IV) chloride is CO.
Learn more about ligand here:
https://brainly.com/question/31836087
#SPJ11
which alkaline earth metal is the components of Epsom salt
Answers
Answer:
Epsom salt is magnesium sulfate.
Alkaline earth metal, which is also sold in other products like milk of magnesia and Epsom salt magnesium sulfate and magnesium hydroxide.
What is alkaline earth metal ?The elements that make up group 2 of the current periodic table are the alkaline earth metals. Beryllium, magnesium, calcium, strontium, barium, and radium are among the elements in this group.
The physical and chemical characteristics of the elements in this group are quite similar. However, they are not often encountered in their elemental forms in nature.
Beryllium and radium are the first two elements in the second group of the periodic table, which is made up of the alkaline earth metals. They are all glossy, silvery-white metals with good reactivity, albeit not as good reactivity as the alkali metals.
Thus, alkaline earth metal is the components of Epsom salt are magnesium sulfate and magnesium hydroxide.
To learn more about alkaline earth metal, follow the link;
https://brainly.com/question/12835232
#SPJ6
An empty flask has a mass of 47. 392 g and 47. 816 g when filled with acetone vapor (C3H60) at 100. C and 745 mmHg. If the volume of the flask is 247. 31, what is the molar mass of the acetone?
Answers
A flask is 47.392 g when empty & 47.816 g when filled with acetone vapor at 100 °C and 745 mm Hg.
A flask is 47.392 g when empty & 47.816 g when filled with acetone vapor at 100 °C and 745 mm Hg.
What is the molar mass of the acetone if the flask has a 247.3 mL volume?
What is the molar mass of the acetone if the flask has a 247.3 mL volume?
(R = 0. To answer this question, we can find the acetone's molar mass.
So, we must determine the mass of acetone, which is equal to the master of the flask when it is filled with acetone, which is 47.816 g less the mask of the flask. When it is empty, it is 47.392 grams. This is equivalent to 2.4 to 4 g.
The ideal gas equation Next, solve the gas laws for a mole of acetone, which is equal to force times space equals ideal gas temp. Given here is a pressure of 7 45 H G as this is a vaporizing asset.
One atmosphere, size 760 mm H G, was extant while writing this. One liter is equal to 1000 ml is when capacity of 247.3 M. L is split by these. calculated as follows:.08-0 21 later air per mole Calvin Also, the temperature ranged from 73.15 to plus 100. A mole of acetone is 7.91 times that much in this case. After that, find the molar mass at -3 mol. It was indeed overly much. It's was obtained by dividing by the process of acetone exchange. 4-4 g of pressure then is derived for a mole of acetone.
It was too sticky Divided using the process of acetone replacement, we obtained this. 4-4 g Muller divided the mold, that is 7.91 time and rises to -3 mol. Acetone's molar mass in Sequels is therefore 53.6 grams per mole. 0821L.atm/mol.K)
To know more about molar mass click here
brainly.com/question/12127540
#SPJ4