Current location of the continents with outline of the tectonic plates. Label A is in lower left near South America. Label B is in lower middle near Africa. Label C is in lower right near Austraila.
Use the drop-down menus to identify the labeled plates
Answers
Label A - North America's largest tectonic plates
Label B - Africa tectonic plates
Label C - Australia's smallest tectonic plates
What are tectonic plates?A tectonic plate (also called a lithospheric plate) is a massive, irregularly shaped slab of solid rock, generally composed of both continental and oceanic lithosphere.
Tectonic plates are referred to as large motions of seven large plates.
Seven major plates: African, Antarctic, Eurasian, Indo-Australian, North American, Pacific and South American.
The dynamics of the earth are designed to balance the equator system. There are tectonic plates on seven continents of the world. The largest tectonic plate is located in southeast America.
Learn more about the tectonic plates here:
https://brainly.com/question/20740910
#SPJ1
Use the drop-down menus to identify the labeled plates.
Label A: South American Plate
Label B: African Plate
Label C: Indo-Australian Plate
---------------------------------------------------
Have a great day and God bless! :D
Related Questions
Is the following statement true or false:
Cellular respiration only occurs in animal cells.
Question 1 options:
True
False
Answers
Answer:
False
Explanation:
Humans and plans do it as well
Answer:
False
Explanation:
Cellular respiration takes place in the cells of all organisms. It occurs in autotrophs such as plants as well as heterotrophs such as animals.
The reaction of iron (III) metal with a solution of copper (II) sulfate releases iron ions into the solution through a single displacement reaction.
a. Determine the moles of iron ions produced in this reaction.
b. Name a soluble compound that could be added to precipitate all of the iron ions from the solution.
c. What mass of the soluble compound from part (c) is required to precipitate all of the iron ions you determined in part (b)? HINTs: determine a new chemical reaction with your soluble compound Fe2(SO4)3. Then, use the moles of Fe2(SO4)3 calculated in part (b)
Answers
Answer:
. Name a soluble compound that could be added to precipitate all of the iron ions from the solution.
Sodium Hydroxide.
In a 3.21g sample of the hydrate, CuSO4 • 10H2O (339.8 g/mol), how many grams of water are expected?
Answers
Therefore, 9.49 grams of water is expected in the given 3.21 g sample of CuSO4 • 10H2O.
To determine the number of water molecules in the given hydrate, CuSO4 • 10H2O, we'll need to find out the molar mass of the compound and the molar mass of water to make a comparison.
The molar mass of CuSO4 • 10H2O is calculated as:
CuSO4 → 159.6 g/mol10H2O → 180.16 g/mol (18.016 g/mol × 10)CuSO4 • 10H2O → 159.6 g/mol + 180.16 g/mol
= 339.76 g/mol (rounded to three significant figures)
Thus, we can see that the molar mass of CuSO4 • 10H2O is 339.76 g/mol.
We know that this hydrate consists of ten molecules of water, each having a molar mass of 18.016 g/mol (which is the same as the molar mass of water), and one molecule of CuSO4 with a molar mass of 159.6 g/mol.
Therefore, the number of moles of water in the sample is:
(10 × 18.016 g/mol) ÷ 339.76 g/mol = 0.527 moles
So, the mass of water is equal to its molar mass multiplied by the number of moles.
The mass of water is:
0.527 mol × 18.016 g/mol = 9.49 g
to know more about molecular mass visit:
https://brainly.com/question/15880821
#SPJ11
in this short synthetic sequence, provide the organic structures of the missing reactant and the missing product.
Answers
The first step of the reaction, we have reaction between a nucleophile and an electrophile.
We can see that in the first step of the reaction, we have reaction between a nucleophile and an electrophile. In this case, the electrophile would have to be an alkyl halide which produces a carbocation as show in the image attached. What we have here is quite similar or like most of the organic reactions, this reaction occurs in a number of detailed or smaller steps and each step of the reaction is going to help to bring us closer to the end product of the entire steps of the reaction which is wat we target as we carry out the particular reaction.The second step involves the reduction of the alkyne with the use of a Lindlar catalyst. As such the reaction is poisoned and it stops at the alkyne stage rather than going on to obtain the alkane.
Learn more about electrophile here:
https://brainly.com/question/21773561
#SPJ4

which of the following molecules can form hydrogen bonds with itself? (select all that apply.)
Answers
Kdjkdkfkfjjff
why is sodium oxide a basic oxide
Answers
Answer:
Sodium oxide is a simple strongly basic oxide. It is basic because it contains the oxide ion, O2-, which is a very strong base with a high tendency to combine with hydrogen ions. Reaction with water: Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution
Explanation:
Explanation:
Sodium oxide is a simple strongly basic oxide. It is basic because it contains the oxide ion, O2-, which is a very strong base with a high tendency to combine with hydrogen ions. Reaction with water: Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution.
Question 6 of 10
What does it mean when a reaction is spontaneous?
O
A. The reaction requires added energy.
B. The reaction goes to completion.
C. The reaction occurs rapidly.
O O
D. The reaction happens by itself.
SUBMIT
Answers
Answer:
D
Explanation:
Does not require energy ...may be slow or fast....
In a particular experiment, the reaction of 1. 0g of S with O2 produced 0. 80 g of SO3. The % yield in this experiment is how much %?
Answers
The actual yield of the product obtained in the experiment must be divided by the theoretical yield of the product that could be achieved. The reaction's percent yield is 32% as a result.
The amount of product produced in a chemical reaction or manufacturing process is referred to as yield, and it is typically expressed in mass or volume. Theoretical yield, actual yield, and percent yield are a few of the several types of yield. Theoretical yield, under the assumption that the reaction continues to completion without any losses or side reactions, is the greatest quantity of product that can be produced from a specific amount of reactants. The amount of product that is actually produced during an experiment or production process is known as the "actual yield." The actual yield to theoretical yield ratio, stated as a percentage, is known as percent yield. The efficiency and profitability of a chemical reaction or manufacturing process are significantly influenced by yield.
Learn more about yield here:
https://brainly.com/question/2506978
#SPJ4
Consider a situation in which two solid reactants are mixed together to generate an unknown gaseous product. The vapor from the gas effuses at a rate that is 1.77 times slower than the same amount of carbon dioxide (CO2) at the same temperature and pressure. What is the molar mass of this unknown gas
Answers
Two solid reactants are mixed together to generate an unknown gaseous product, the molar mass of the unknown gas is approximately 14.06 g/mol. To determine the molar mass of the unknown gas, we can use Graham's law of effusion.
Graham's law states that the rate of effusion of a gas is inversely proportional to the square root of its molar mass. Mathematically, this can be represented as:
Rate₁ / Rate₂ = √(M₂ / M₁)
In this situation, Rate₁ is the rate of effusion for CO2, and Rate₂ is the rate of effusion for the unknown gas. M₁ is the molar mass of CO2 (44.01 g/mol), and M₂ is the molar mass of the unknown gas that we want to find. The problem states that the unknown gas effuses at a rate 1.77 times slower than CO2. Therefore:
1 / 1.77 = √(44.01 / M₂)
Squaring both sides:
1 / (1.77)² = 44.01 / M₂
Now, multiply both sides by M₂ and divide by (1.77)²:
M₂ = 44.01 / (1.77)²
M₂ ≈ 44.01 / 3.13
M₂ ≈ 14.06 g/mol
The molar mass of the unknown gas is approximately 14.06 g/mol.
To know more about reactants , refer
https://brainly.com/question/26283409
#SPJ11
How are atoms and elements related?
Please explain with full and clear sentences. Thank you! :)
Answers
Answer:
closely related
Explanation:
Elements are substances containing of one type of atom, (e.g carbon element is made up of carbon atoms) . Atoms are the smallest particles into which an element can be devided.In the heating and cooling curves below, identify the letter in the diagram diagram that corresponds to each of the listed processes in the table
I’m so confused if anyone could help (and explain as if I’m a 3 yr old) that would be helpful
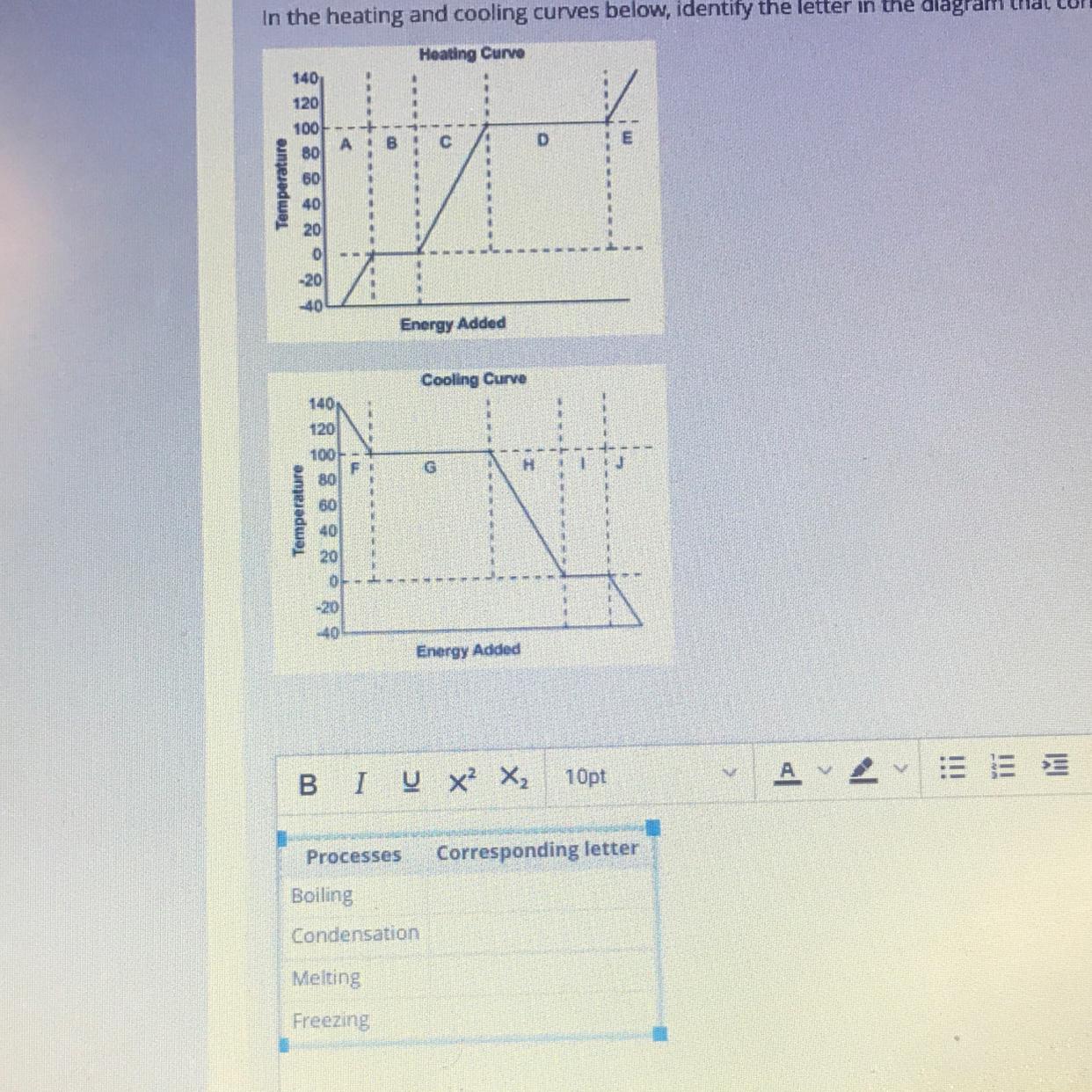
Answers
Answer:
Test for the first one is the best for
ASAP MULTIPLE CHOICE WILL MARK BRAINLIEST
Which symbol represents an ion?
4He
Ca
Mg
Na2+
Answers
What is the pH of a solution that results from mixing 25. 0 mL of 0. 200 M HA with 12. 5 mL of 0. 400 M NaOH? (Ka = 1. 0× 10-5) (C) 9. 06 (D) 11. 06 (B) 4. 94 (A) 2. 94
Answers
The pH of a solution that results from mixing 25. 0 mL of 0. 200 M HA with 12. 5 mL of 0. 400 M NaOH. is 1.70.
To answer your question, we need to use the principles of acid-base chemistry and stoichiometry. HA represents a weak acid, and NaOH represents a strong base. When these two substances are mixed together, they will undergo a neutralization reaction. The balanced chemical equation for this reaction is: HA + NaOH → \(Na^{+}\)+ H2O. Where NaA represents the salt that is formed from the acid and base.
To determine the pH of the resulting solution, we first need to calculate the moles of each reactant that are present in the mixture. We can use the formula: moles = concentration x volume (in liters)
For HA:
moles HA = 0.200 M x 0.0250 L = 0.00500 mol
For NaOH:
moles NaOH = 0.400 M x 0.0125 L = 0.00500 mol
Since the reaction between HA and NaOH is a 1:1 reaction, we can see that they react completely. Therefore, the number of moles of the excess reactant will be equal to the difference between the moles of the two reactants: moles excess = |moles HA - moles NaOH| = |0.00500 mol - 0.00500 mol| = 0 mol
Since there is no excess reactant, we can assume that all of the acid and base have reacted to form the salt \(Na^{+}\) and water. The moles of the salt Na that are formed will be equal to the moles of the acid or base that are consumed in the reaction: moles \(Na^{+}\) = moles HA (or moles NaOH) = 0.00500 mol
We can now use the volume of the mixture to calculate the concentration of the salt \(Na^{+}\): concentration \(Na^{+}\)= moles \(Na^{+}\)/ volume (in liters) = 0.00500 mol / (0.0250 L + 0.0125 L) = 0.100 M
Finally, we can use the principles of weak acid-base chemistry to determine the pH of the solution. Since \(Na^{+}\) is the conjugate base of the weak acid HA, it will hydrolyze in water to produce OH- ions:
\(Na^{+}\) + \(H_{2}0\) → HA + \(OH^{-}\)
The equilibrium constant for this reaction is given by:
Kb = [HA][\(OH^{-}\)] / [\(Na^{+}\)]
We can assume that the concentration of \(OH^{-}\) ions is equal to the concentration of NaOH that was added to the mixture (since NaOH is a strong base and completely dissociates in water). Therefore: Kb = [HA][0.400 M] / [0.100 M] = 1.60 x 10^-13
The relationship between Ka (the acid dissociation constant) and Kb for a weak acid and its conjugate base is: Ka x Kb = Kw. Where Kw is the ion product constant for water (1.00 x 10^-14 at 25°C). Therefore: Ka = Kw / Kb = 6.25 x 10^-2
Since we know the value of Ka for HA, we can use the Henderson-Hasselbalch equation to calculate the pH of the solution: pH = pKa + log([A-]/[HA]). where pKa = -log(Ka) = 1.20 (for HA), and [A-]/[HA] = [\(Na^{+}\)]/[HA] = 0.100 M / 0.200 M = 0.500. Therefore: pH = 1.20 + log(0.500) = 1.70
In conclusion, the pH of the solution that results from mixing 25.0 mL of 0.200 M HA with 12.5 mL of 0.400 M NaOH is 1.70.
How does a galvanic cell work? A. A voltage applied across two electrodes causes electrons to flow. B. A salt bridge connecting two electrodes generates electrons. O C. A redox reaction at two electrodes causes electrons to flow. D. Two electrolyte solutions react to cause electrons to flow.
Answers
Answer: C. A redox reaction at two electrodes causes electrons to flow
Explanation:

A galvanic cell work like a a redox reaction at two electrodes causes electrons to flow.
What is redox reaction?Redox reactions include a modification inside the oxidation state of the substrate. Losing of electrons or a rise in an element's oxidation state are both considered to be oxidation. Gaining electrons or lowering an object's or its atoms' oxidation state are both considered reductions.
What is galvanic cell ?An electrochemical cell wherein an electric current being produced spontaneously is called a galvanic cell as well as voltaic cell, respectively called just after scientists Luigi Galvani as well as Alessandro Volta. reduction-oxidation processes
In order to create a conduit for the passage of electrons through into the wire, each half-reaction in a galvanic cell is connected to the other by a wire, which allows the movement of electrons to be separated in the oxidation and reduction processes.
To know more about galvanic cell
https://brainly.com/question/13031093
#SPJ2
in a reaction 2.5g of sodium sulphate reacted with 4.5g of barium chloride. the products are 3.5g of barium sulphate and the rest is sodium chloride. find the mass of sodium chloride produce. state the law which justifies this reaction
Answers
The mass of sodium chloride : 3.5 g
Further explanationGiven
Reaction :
2.5g of sodium sulphate + 4.5g of barium chloride ⇒ 3.5g of barium sulphate + sodium chloride
Required
The mass of sodium chloride
Solution
Conservation of mass applies to a closed system, where the masses before and after the reaction are the same
From the reaction :
mass of reactants = 2.5 g + 4.5 g = 7.0 g
mass of products = 3.5 g + mass of sodium chloride
mass of reactants = mass of products
7.0 g = 3.5 g + mass of sodium chloride
mass of sodium chloride = 7 g - 3.5 g =3.5 g
calculate the percentage by mass of cerium in cerium carbonate
Answers
Answer:
60.88%
Explanation:
The formula for cerium carbonate is Ce₂(CO₃)₃.
Let's assume we have 1 mol of cerium carbonate. The total mass would be equal to the molar mass of Ce₂(CO₃)₃, 460.25 g/mol.
Out of those 460.25 g, the mass corresponding to cerium would be:
2 * Molar mass of Ce = 2 * 140.11 g/mol = 280.22 gNow we can calculate the percentage by mass of cerium:
% mass = 280.22 / 460.25 * 100% = 60.88%100 POINTS IF ANSWERED CORRECTLY
Think of the composition of each layer of Earth and their relative sizes. Also consider Earth's atmosphere, its oceans, its ice caps, and other materials on its surface. Think about how large or small these parts of Earth are compared to one another. Then complete the following sentence.
Overall, the Earth is made up primarily of: 1. water 2. air 3. soil 4. rock
Answers
They are extremely small infront of earth size.
We live in a very small dot like place on earth.The earth size is too big.As we are in dot like we can see objects far biggerBut according to earth It's extremely big than usA ingle replacement reaction i run in the lab between 45. 0g hydrofluoric acid,HF, and 125. 0g tin forming tin(ll) fluoride and another product
Answers
The molar mass of tin(II) chloride is: 84.9 g
Calculate the molar mass of tin(II) chloride?
The number of moles n1 is thus:
n1= m1/M1 = 2.25 mol
According to the balanced reaction, the ratio by number of moles n of tin(II) chloride produced to the number of moles n1 of HF used is 1:2
As such, the number of moles of tin(II) chloride produced is :
n = 1/2 \(n_{1}\) = 0.45 mol
The molar mass of tin(II) chloride is:
M= A\(_{r}\)(Sn)+2A\(_{r}\)(Cl) = 188.7 g/mol
The mass m produced is thus:
m = nM
= 0.45 mol × 188.7 g/mol
≈ 84.9 g
Therefore, the molar mass of tin(II) chloride is: 84.9 g.
To know more about molar mass, check out:
https://brainly.com/question/837939
#SPJ4
Freon-12 synthesized by the reaction between fluoride at carbon tetrachloride and antimony(III)Suppose 5.0 mol of antimony (III) fluoride is added to 10.0 mol of carbon tetrachloride: How many moles each compound (CCL; SbF;, CClFz, and SbCI3) are there if the reaction is |00% complete
Answers

Suppose 5.0 mol of antimony (III) fluoride is added to 10.0 mol of carbon tetrachloride, and from the 100% reaction there are the equivalents of 0 moles of SbF₃, 5.0 moles of SbCl₃, 0 moles of CCl₄, and 15.0 moles of CClF₂.
What is the significance of the balanced reaction?A balanced reaction is one where the reactants and products are present in equal amounts, and if the 100% reaction takes place, then the reactant sides have 1 mol of SbF₃ and 3 mol of CCl₄ that make the product of 1 mol of SbCl₃ and 3 mol of CClF₂. After the 100% reaction, all the reactants get converted into the product. The complete reaction is the below.
SbF₃ + 3CCl₄ → SbCl₃ + 3CClF₂
Hence, suppose 5.0 mol of antimony (III) fluoride is added to 10.0 mol of carbon tetrachloride, and from the 100% reaction there are the equivalents of 0 moles of SbF₃, 5.0 moles of SbCl₃, 0 moles of CCl₄, and 15.0 moles of CClF₂.
Learn more about the balanced reaction here.
https://brainly.com/question/14280002
#SPJ2
Aristotle did not believe that
could exist.
Answers
Answer:
aristotle din not beileve in anything. he looked at the world describe what happend
help plz really need your help if u know answer if u don't DO NOT ANSWER this is not the first time I have posted this question

Answers
T eg(1) : 97.5°C, T eg(2): 98.5°C, T eg(3): 99.2°C
∆T water(1): -2.5°C, ∆T water(2): -1.5°C, ∆T water(3): -0.8°C
∆T metal(1): 77.5°C, ∆T metal(2): 80.5°C, ∆T metal(3): 80.2°C.
ft= (m1 cp1 t1 + m2 cp2 t2 + .... + mn cpn tn) / (m1 cp1 + m2 cp2 + .... + mn cpn) (1)
where,
1000g = 1kg
ft(t eg)= final mixed temperature (°C)
m = mass of substance (kg)
cp = specific heat of substance (J/kg°C)
t = temperature of substance (°C)
A solution of sodium hydroxide was titrated against a solution of sulfuric acid. How many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers
Answer:
2 mole of Sodium hydroxide reacts with 1 mole of Sulfuric acid
Explanation:
Write down the equation in the beginning with reactants and products:
NaOH + H₂SO₄ → Na₂SO₄ + H₂0
Now try to balance it. Try with Na first:
2NaOH + H₂SO₄ → Na₂SO₄ + H₂0
Na atoms are balanced. There are 6 Oxygen atoms on the right and 5 on the left. Balance by increasing the H₂O moles:
2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂0
Check if H atoms are also balanced. They are. That means our final reaction is:
2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂0
2 Moles of NaOH reacts with 1 mole of H₂SO₄
The moles of sodium hydroxide required to neutralize 1 mole of sulfuric acid have been 2 mol.
The balanced chemical equation for the titration of sodium hydroxide against sulfuric acid has been:
\(\rm 2\;NaOH\;+\;H_2SO_4\;\rightarrow\;Na_2SO_4\;+\;2\;H_2O\)
From the balanced chemical equation, for the titration of 1 mole of sulfuric acid, 2 moles of sodium hydroxide has been required.
The moles of sodium hydroxide required for the titration of sulfuric acid has been:
\(\rm 1\;mol\;H_2SO_4=2\;mol\;NaOH\)
Thus, the neutralization of 1 mole of sulfuric acid has been required 2 mol of sodium hydroxide.
For more information about titration, refer to the link:
https://brainly.com/question/25485091
Magnesium bromide (one magnesium atom with two bromine atoms) has a
chemical formula of MgBrz. If you look at the total atomic mass of MgBr2,
does magnesium or bromine make up more of the mass?
they are exactly the same
it is impossible to tell
magnesium
bromine
Answers
Answer:bromine
Explanation:
The mass of magnesium is 24 whereas 1 bromine atom is 80 (rounded up from 79.9) and there is 2 bromine atoms which means you have to multiply 80 by 2, giving you 160.
Chlorine (Cl) has 17 electrons. How many electrons are in the n = 1, n = 2, and n = 3 levels, respectively, of a chlorine atom?
2, 8, 7
2, 6, 9
8, 2, 7
7, 8, 2
Answers
Chlorine's electrical configuration will be 2, 8, 7, because its atomic number (Z) is 17. In the L shell, there are eight electrons (second shell).
Chlorine has an atomic mass of 35.45 and an atomic number of 17, which means that each of its atoms contains 17 protons, 17 electrons, and 18 neutrons. As a result, you are already aware that for the element chlorine, the atomic number indicates how many electrons there are. In other words, a chlorine atom contains 17 electrons. This chlorine atom has 17 protons since the atomic number is equal to the number of protons in an atom. Since there are exactly as many electrons as protons in neutral atoms, we can infer that there are 17 electrons total that need to be divided among the electron shells.
Learn more about chlorine here:
https://brainly.com/question/14962130
#SPJ4
If a beach ball has a volume of 261 L at a temperature of 502 K, what will be the new
temperature of the balloon if the volume decreases to 176 L? Formula: V/T, -V2/T2
Answers
The new temperature of the balloon if the volume is decreased to the given amount is 338.5K.
Charles's law
Charles's law states that the volume occupied by a definite quantity of gas is directly proportional to its absolute temperature.
It is expressed as;
\(\frac{V_1}{T_1} = \frac{V_2}{T_2}\)
Given the data in the question;
Initial volume; \(V_1 = 261L\)Initial temperature; \(T_1 = 502K\)Final volume; \(V_2 = 176L\)Final Temperature; \(T_2 = \ ?\)
To determine the new temperature as the volume is decrease, we substitute our given values into the expression above.
\(\frac{V_1}{T_1} = \frac{V_2}{T_2}\\\\V_1T_2 = V_2T_1\\\\261L * T_2 = 176L * 502K\\\\261L * T_2 = 88352L.K\\\\T_2 = \frac{88352L.K}{261L}\\ \\T_2 = 338.5K\)
Therefore, the new temperature of the balloon if the volume is decreased to the given amount is 338.5K.
Learn more about Charles's law: https://brainly.com/question/12835309
some safety precautions people should take when using fireworks
Answers
Answer:
Do not try to re-light or handle malfunctioning fireworks. Soak both spent and unused fireworks in water for a few hours before discarding.
if you arrive at the hospital with severe dehydration or decreased blood volume, you may receive an intravenous saline solution known as normal saline (ns). ns typically contains 0.900 grams of nacl per 100.0 ml of water. what is the molar concentration of nacl in this solution?
Answers
The molar concentration of NaCl in the normal saline solution is 0.00139 M.
To calculate the molar concentration of NaCl in the normal saline solution, we need to first calculate the number of moles of NaCl present in 100.0 ml of the solution.
The molecular weight of NaCl is 58.44 g/mol (22.99 g/mol for Na and 35.45 g/mol for Cl). Therefore, the number of moles of NaCl present in 0.900 g of NaCl can be calculated as:
0.900 g NaCl / 58.44 g/mol = 0.0154 moles NaCl
Next, we need to calculate the number of moles of NaCl present in 100.0 ml of the solution. We know that the solution contains 0.900 g of NaCl in 100.0 ml of water, which is equivalent to 0.900 g/100.0 g or 0.00900 g/g of solution.
Therefore, the number of moles of NaCl in 100.0 ml of the solution is:
0.00900 g/g x 0.0154 moles/g = 0.000139 moles NaCl
Finally, we can calculate the molar concentration of NaCl in the solution as:
0.000139 moles NaCl / 0.1000 L = 0.00139 M NaCl
Therefore, the molar concentration of NaCl in the normal saline solution is 0.00139 M.
To know more about saline solution, refer here:
https://brainly.com/question/30531382#
#SPJ11
How many atoms in 98.2 g of He?
Answers
How many g of water are required to be mixed with 11.75 g of HgCl in order to make a 0.01 m solution? (Refer to the periodic table for atomic weights.)
a.) 40 g
b.) 5,000 g
c.) 2 x 10^8 g
d.) 2 x 10^6 g
The general name for a substance added to a reaction that affects the rate but is not consumed in the reaction is called a_____.
a.)constituent
b.)catalyst
c.)complex
d.)reactant
The action of a catalyst can be explained in the following manner:
a.) The catalyst makes it possible for the reaction to take place by another path that makes possible reaction at a lower energy.
b.) The catalyst takes no part in the reaction but serves as a buffer between reactants and products.
c.) The catalyst prevents the reverse reaction.
d.) The catalyst lowers the temperature of the reactants.
The energy level necessary to enable a reaction to occur is called the_____ energy.
a.) potential
b.) kinetic
c.) activation
d.) nuclear
Raising the temperature of a reacting system increases the rate of the reaction, but does NOT increase the:
a.) number of collisions
b.) average velocity of the reacting particles
c.) activation energy
d.) vibrational motions within the molecules
e.) fraction of the reacting particles which possess energies greater than the activation energy
Answers
Answer:
5,000 g
b.)catalyst
The catalyst makes it possible for the reaction to take place by another path that makes possible reaction at a lower energy.
activation
activation energy
Explanation:
Formulae for molality = number of moles of solute/mass of solvent in kilograms
Number of moles of solute= 11.75g/236 g/mol = 0.0498 moles
0.01 = 0.0498/x
x= 0.0498/0.01
x= 5 Kg or 5000 g
A catalyst refers to any substance that alters the rate of reaction but remains unaffected at the end of the reaction. The catalyst lowers the activation energy of a reaction.
For a chemical reaction to occur, the substances participating in that reaction must possess energy which is greater than the activation energy of the reaction. The activation energy is a sort of energy barrier between reactants and products which must be surmounted before a reaction can take place.
When the temperature of a reaction system is raised, the molecules move faster and the number of effective collisions in the system increases. However, raising the temperature does not raise the activation energy of the system.
What is a criterion for all products made using the process of technological design? The product must be cheaper than similar products.
The product must be available to any user who wants it.
The product must solve the problem for which it was designed.
The product must be capable of solving a variety of problems.
Answers
Answer:
C. The product must solve the problem for which it was designed.
Explanation:
Technological design is the study, development and application of technological process with the intent of designing a product to solve required problem.The process could be based on the use of a computer.
Generally, technological process and development always tend to design a problem solver product. Through this process, more new technologies are produced.
Therefore in technological process, the product must solve the problem for which it was designed.
Answer:
C) The product must solve the problem for which it was designed.