Describe what happens to the overburden once all the coal has been taken out of a surface coal mine.
Answers
The overburden is the material that lies on top of the coal deposit in a surface coal mine. The removal of this material is a crucial aspect of the mining process. Once all of the coal has been extracted, the overburden is replaced in the mine to restore the surface of the land to its original contour. This is known as reclamation.
In a surface coal mine, the overburden is the rock, soil, and other material that lies above the coal deposit. To access the coal, miners remove the overburden and set it aside. The overburden may contain valuable minerals or materials that can be used in other industries.
Once all of the coal has been extracted, the overburden is replaced in the mine. This is done to restore the land to its original contour and to minimize the environmental impact of mining. The process of reclamation involves several steps, including grading the land, seeding and planting vegetation, and installing erosion control measures.
In conclusion, the overburden in a surface coal mine is the material that lies on top of the coal deposit. Once the coal has been extracted, the overburden is replaced in the mine to restore the surface of the land to its original contour. This process is known as reclamation, and it is an important aspect of modern mining practices.
To know more about reclamation, visit:
https://brainly.com/question/31635846
#SPJ11
Related Questions
A student started with a 0.032 g sample of copper which he took through the series of reactions described in this experiment. At the end of the experiment he obtained 0.038 g of a black product. What was his percent yield? What is the most likely source of the error in his experiment? (Hint: consider question 2 above Post-lab Questions: 1. Copper (II) hydroxide is converted into copper (II) oxide by heating the test tube containing Cu(OH) 2 in a hot water bath. Is it necessary to use distilled water in this water bath? Why or why not? 2. Copper metal doesn't "rust" in the presence of oxygen at room temperature. However, it will react with O2 at elevated temperatures. Write a balanced chemical equation describing the formation of copper (II) oxide when copper metal is heated in air. 3. When zinc is dissolved in sulfuric acid a gas is produced. What is the chemical identity of this gas? How is it produced? 4. A student started with a 0.032 g sample of copper which he took through the series of reactions described in this experiment. At the end of the experiment he obtained 0.038 g of a black product. What was his percent yield? What is the most likely source of the error in his experiment? (Hint: consider question 2 above
Answers
Percent yield: 118.75%.
Most likely source of error: Experimental loss or contamination during the reactions.
The percent yield is calculated using the formula: (Actual yield / Theoretical yield) x 100%. In this case, the actual yield is 0.038 g and the theoretical yield can be calculated based on the stoichiometry of the reactions involved. Since the series of reactions is not provided, it is not possible to determine the theoretical yield accurately.
However, assuming the reactions were carried out properly and stoichiometrically, the theoretical yield should be lower than the actual yield, resulting in a percent yield greater than 100%. Therefore, the percent yield is calculated as (0.038 g / Theoretical yield) x 100%.
The most likely source of error in the student's experiment is experimental loss or contamination during the reactions. It is possible that some of the copper or the product was lost during the transfer or handling processes, leading to a lower actual yield than expected. Contamination from impurities or reactants that were not properly removed or separated during the reactions could also contribute to the discrepancy between the actual and theoretical yields.
It is important to handle and transfer the substances carefully, use proper techniques to minimize loss, and ensure the purity of reagents and equipment to obtain more accurate results. Additionally, errors in measuring or recording the masses of the copper sample and the product could also contribute to the difference in yields.
To learn more about Percent yield, here
https://brainly.com/question/17042787
#SPJ4
PLS HELP ASAP!!!The pedigree diagram shows how a dominant trait is inherited in a family. The red circle and squares show family members with the trait.
Based on the pedigree diagram, who must be heterogeneous for the trait?
A. The child without the trait
B. Both parents
C.The child with the trait
D. Only one parent

Answers
Answer:To determine who must be heterozygous for the trait based on the given pedigree diagram, we need to look for patterns of inheritance and consider the possible genotypes of the individuals.
If the trait is dominant, individuals who exhibit the trait can either be homozygous dominant (TT) or heterozygous (Tt). Homozygous dominant individuals would have both parents with the trait, while heterozygous individuals would have one parent with the trait.
From the pedigree diagram, we can observe that the child without the trait does not have any parent with the trait. This means that the child without the trait must have two recessive alleles (tt) and is therefore homozygous recessive, not heterozygous.
Both parents in the pedigree diagram have the trait, indicating that they are both either homozygous dominant (TT) or heterozygous (Tt). Therefore, it is possible for one or both parents to be heterozygous for the trait.
Based on this information, the correct answer is:
D. Only one parent
Explanation:
Answer:
B. Both Parents
Explanation:
If the trait is dominant, a person who is homozygous dominant or heterozygous for the trait will express the trait. In the pedigree given, we see that both affected individuals (the red circle and squares) have at least one affected parent. This suggests that the trait is likely inherited from both parents, which means both parents must be heterozygous (i.e., carry one dominant and one recessive allele for the trait).
The child without the trait is homozygous recessive, meaning they inherited two copies of the recessive allele from both parents who are both heterozygous for the trait. Therefore, the correct answer to the question is B, "Both parents."
Balance the chemical equation. Based on the equation, how many grams of bromine are produced by the complete reaction of 11 grams of potassium bromide? Use the periodic table to get the weights of the elements. Carry out your calcuation in the same way as you did in parts A, B, and C. That is, begin by converting grams of KBr to moles of KBr, then use the mole ratio of KBr to Br2.
Drag the labels to the correct locations to complete the analysis. Each label can be used more than once
Answers
The balanced chemical equation for this question is \(Cl_{2} + 2KBr \rightarrow 2KCl + Br_{2}\).
A balanced chemical equation occurs when the number of the atoms involved in the reactants side is equal to the number of atoms in the products side. Chemical equations are balanced using coefficients. A chemical symbol or formula is preceded by a coefficient, which is a number. It reveals how many of the substance's atoms or molecules took part in the process. Two molecules of water would be expressed as 2 H2O and two molecules of hydrogen as 2 H2, respectively.
The amount of bromine is calculated as follows:
\(11.0 g KBr* (1mol KBr/119.002g KBr )* (1mol Br2/2mol KBr) *( 159.808g Br_{2}/1mol Br_{2} )= 7.39 g Br_{2}.\)
So, 7.39 g of bromine is calculated which is the answer.
Ans- 7.39g
Learn more about balanced chemical equation:
brainly.com/question/28294176
#SPJ4
The reaction of C2H4 +Cl2 is what type of reaction.
Answers
Answer:
C2H4(g) + Cl2(g)→C2H4Cl2(g)
This is an example of synthesis reaction, when compounds are joined together.
All covalent compounds donot exist as giant aggregates. Why?
Answers
Which components of a galaxy move in circular patterns or revolve around a star?
A:Gas
B: Dust
C:Other orbiting objects
D: Sun
Answers
Answer:
B
Explanation: hoped this helped!!!
Answer:
it is c no cap
Explanation: C
Select the correct answer. What is the molecular formula of a compound with the empirical formula SO and molecular weight 96. 13? A. SO B. S2O2 C. SO2 D. S3O3.
Answers
The molecular formula of the SO compound is \(\rm S_2O_2\). Thus, option B is correct.
The empirical formula is the whole number formula for the compound. The molecular formula is the descriptive formula for each number of atoms of elements in the compound.
Computation for molecular formula of SOThe molecular mass is the sum of each atom of an element in the formula unit. The molecular mass of the given compound is 96.13 g/mol.
The mass of Empirical formula of compound (\(\rm M_E\)) is:
\(\rm M_E=Mass\;of \;S\;+\;Mass\;of\;O\\M_E=32.065\;g/mol\;+\;16\;g/mol\\M_E=48.065\;g/mol\)
The mass of the empirical formula unit is 48.165 g/mol.
The number of empirical formula units in 96.13 g/mol are:
\(\rm 48.165\;g=1\;unit\\\\96.13\;g=\dfrac{1}{48.165}\;\times\;96.13\;units\\\\ 96.13\;g=2\;units\)
The number of empirical mass units is 2. Thus, the formula of SO will be \(\rm (SO)_2=S_2O_2\). Hence, option B is correct.
Learn more about empirical formula, here:
https://brainly.com/question/11588623
Many factors influence the amount of a solute which will dissolve in a solvent.
True
False
Answers
Answer:
true
Explanation:
the answer is true
What is the bond order of N2+? Express the bond order numerically. Is N2+ paramagnetic or diamagnetic? paramagnetic diamagnetic neither
Answers
Bond order of N2+ is 2.5. It is a diamagnetic substance.
Bond order is termed as the number of chemical bonds between a pair of the atoms. For example: In case of acetylene the bond order between the two carbon atoms is 3, in diatomic nitrogen the bond order is 3, and the C-H bond order is 1.
The bond order of N2+ is 2.5.
Bond order = 1 / 2[Nb - Na] Where, Nb = no. of electrons in bonding molecular orbital and Na = number of electrons in antibonding molecular orbital.
Bond order = 9-4 / 2
= 2.5
N2+ is diamagnetic in nature because they do not have any unpaired electrons they are having 14 electrons.
To know more about bond order here
https://brainly.com/question/29853110
#SPJ4
Jupiter's moon lo orbits the planet at a distance of 421,700 km. What is the correct way to write this distance in scientific notation?
A. 42.17 x 10^4 km
B. 4.217 x 10^5 km
C. 42.17 x 10-4 km
D. 4.217 x 10-5 km
Answers
Answer:
B) 4.217x10^5km
Explanation:
If a gas exerts a pressure of 3atmospheric pressure at 273 kelvin what will be the pressure if the original temperature is doubled at constant volume
Answers
According to Gay-Lussac's law, if the original temperature is doubled at constant volume the new pressure is 6 atmospheres.
Gay-Lussac's law is defined as a gas law which states that the pressure which is exerted by the gas is directly proportional to temperature and at a constant volume.The law was proposed by the scientist named Joseph Gay-Lussac in the year 1808.
The pressure of the gas at constant volume reduces constantly at a rate as the gas is cooled till it undergoes condensation .
It is given by the formula, P₁/T₁=P₂/T₂ substitution of values in the formula gives P₂=3×546/273=6 atmospheres.
Learn more about Gay-Lussac's law,here:
https://brainly.com/question/30758452
#SPJ1
the diagrams above represent two allotropes of solid phosphorus. which of the following correctly identifies the allotrope with the higher melting point and explains why?
Answers
Allotrope 1 has the higher melting point because it lacks the covalent bonds between phosphorus atoms that are present in allotrope 2.
The Melting Point Differences Between Allotropes of Solid PhosphorusAllotropes of solid phosphorus are different forms of the element which differ in their molecular arrangement and structure. Allotrope 1 is a more stable form and has a higher melting point than that of allotrope 2 due to the absence of covalent bonds between the phosphorus atoms in allotrope 1.
The structure of allotrope 1 is an ordered arrangement in which the phosphorus atoms form a three-dimensional lattice. This lattice structure is held together by strong Van der Waals forces, which are electrostatic attractions between the atoms. This structure is more stable than that of allotrope 2 and has a higher melting point due to the increased strength of the interatomic forces.
In contrast, the structure of allotrope 2 is much less ordered, and the phosphorus atoms are held together by covalent bonds. This structure is not as stable as that of allotrope 1 and has a lower melting point. The covalent bonds between the phosphorus atoms are much weaker than the forces in allotrope 1, and consequently the melting point of allotrope 2 is lower.
This question is incomplete, so I am attaching the image that contains the information needed to answer it.
Learn more about Allotropes :
https://brainly.com/question/13058829
#SPJ4

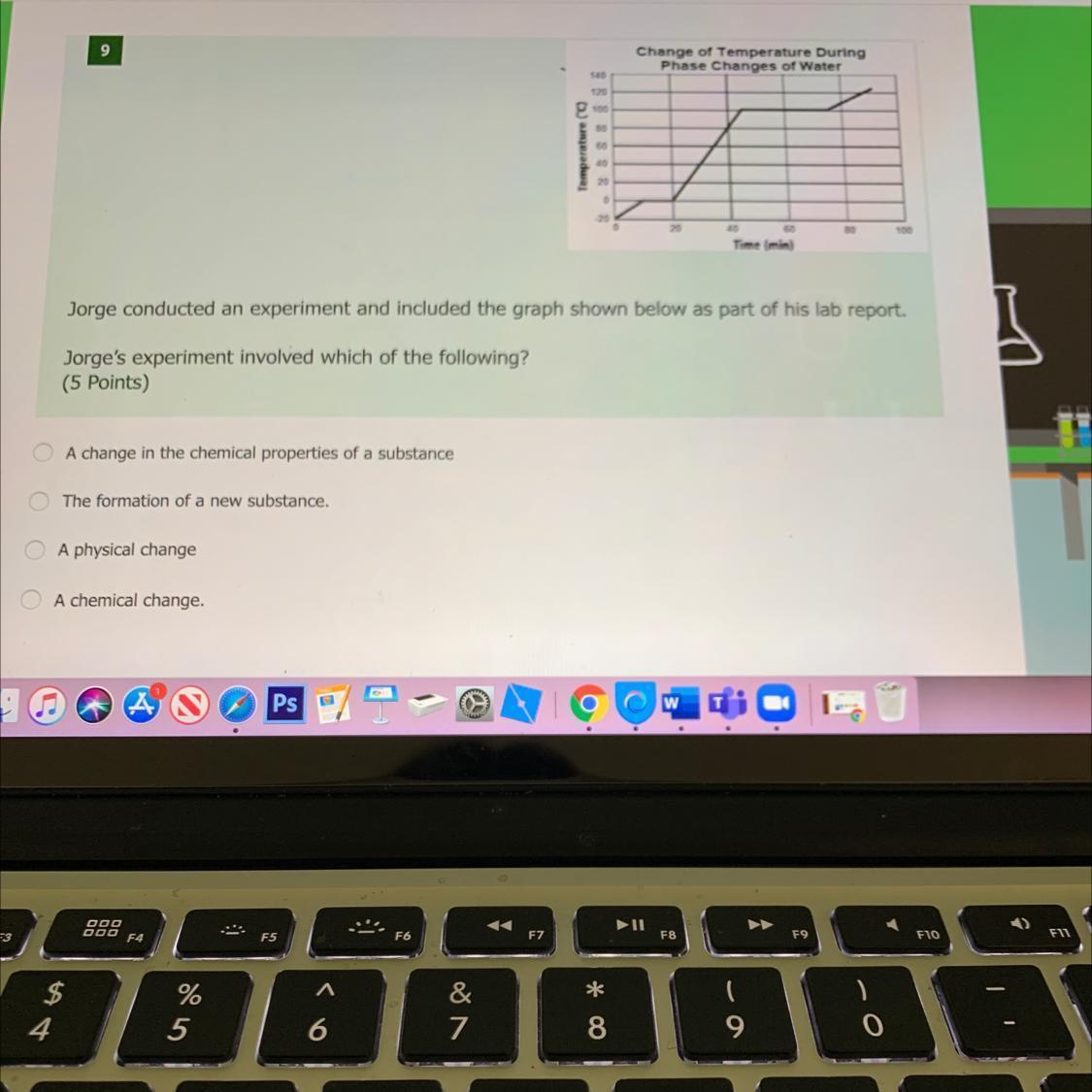
Jorge's experiment involved which of the following?
Answers
Answer:
C. A physical change.
Explanation:
Given the image shown below.
It can be seen that the experiment is about phase changes of a substance. And phase changes of a substance is being done or carried out when the substance has passed through any of the processes such as melting, boiling, freezing, condensing, depositing, or sublimation.
And since these changes in the phase of a substance do not change the substance's chemical nature. It is called a physical change.
Therefore, in this case, Jorge's experiment involved "A physical change."
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
NEED HELP! thank you!
The same element is present before and after a
O chemical change
O physical change
O chemical reaction
O none of these
Answers
Answer:
This is a physical change.
Explanation:
As long as the identity of the substance has not changed, and the element is present before and after, it is a physical change! (Ex: ice melting)
HELP POGGERS!!! I NEED HELP!!!!!!!!!! THIS IS NEEDED IN 10 MINS

Answers
Answer:
(3)
Explanation:
Guaranteed. Look at my comment for linked picture explanation!
Consider the following chemical reaction.
2H2O(l)→2H2(g)+O2(g)2H2O(l)→2H2(g)+O2(g)
Part A
What mass of H2OH2O is required to form 1.3 LL of O2O2 at a temperature of 305 KK and a pressure of 0.901 barbar ?
Express your answer using two significant figures.
Answers
2.0 g \(H_2O\) is required to form 1.3 L of \(O_2\) at a temperature of 305K and a pressure of 0.901 bar.
To solve the problem, we need to use the ideal gas law and stoichiometry of the reaction. Here is the solution:
First, we need to find the number of moles of O2 that will be produced. We can use the ideal gas law, PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in kelvin. Rearranging the equation to solve for n, we get:
\(n = \frac{PV}{RT}\)
Plugging in the values given in the problem, we get:
\(n = \frac{(0.901)*(1.3)}{(0.08206)*(305)} = 0.0561\) mol \(O_2\)
Next, we use the stoichiometry of the reaction to determine the number of moles of \(H_2O\) that are required. For every 1 mol of \(O_2\) that is produced, we need 2 mol of H2O. Therefore:
moles of \(H_2O\) = (2 × moles of \(O_2\)) = (2 × 0.0561 mol) = 0.1122 mol
Finally, we can convert the number of moles of \(H_2O\) to grams using the molar mass of water:
mass of \(H_2O\) = (moles of \(H_2O\)) × (molar mass of \(H_2O\)) = 0.1122 mol × 18.015 g/mol = 2.02 g
Rounding this to two significant figures, the mass of \(H_2O\) required to form 1.3 LL of O2 at the given temperature and pressure is 2.0 g.
learn more about ideal gas law
brainly.com/question/30458409
#SPJ4

Nitrogen gas and hydrogen gas react to produce ammonia according to the following equation.
N2 + 3H2 → 2NH3
Which ratio of components is correct?
A For every 2 moles of nitrogen gas, the reaction requires 3 moles of hydrogen gas.
B For every 3 moles of hydrogen gas, the reaction produces 2 moles of ammonia.
C For every mole of hydrogen gas, the reaction produces 2 moles of ammonia.
D For every mole of nitrogen gas, the reaction produces 1 mole of ammonia.
Answers
Answer:
The answer will be "B"
Explanation:
It is B, because as we can see in the problem, it shows that there are three hydrogen gas, which is H₂. So, it shows that the ratio of Hydrogen gas to Ammonia is 3:2. In the answer, it says for every 3 mol of H₂ 2 moles of ammonia is produce. Therefore, B is correct.
Have a good day, any questions let me know. If you want, you can mark as brainliest if you like the answer!
consider the following image of two buret measurements, the initial and final readings. what is the total volume of liquid delivered in ml?
Answers
The answer cannot be provided without the specific values of the initial and final readings of the burets.
What is the total volume of liquid delivered in milliliters based on the initial and final readings of the burets?To determine the total volume of liquid delivered in milliliters (ml), you need to subtract the initial reading from the final reading of the burets. The difference between these two readings represents the volume of liquid dispensed.
To calculate the total volume, follow these steps:
1. Determine the initial reading of the burets in ml.
2. Determine the final reading of the burets in ml.
3. Subtract the initial reading from the final reading.
The resulting value will give you the total volume of liquid delivered in ml.
Learn more about burets
brainly.com/question/25223760
#SPJ11
Your mommy buys you a helium balloon at the circus. It has a volume of 4.00 liters at STP. What mass of helium, expressed in grams, does this balloon contain?
Answers
Answer:
I believe it will still be 4.00
Explanation:
0.714g of Helium is contained in the balloon
What is STP?STP stands for standard temperature and pressure. STP refers to a specific pressure and temperature used to report on the properties of matter.
According to IUPAC( International Union of Pure and Applied Chemistry), it is defined as -
Temperature of 0 degree celsius (273K)Pressure of 1 atmIt is generally needed to test and compare physical and chemical processes where temperature and pressure plays an important role as they keep on varying from one place to another.
One mole of a gas under STP conditions occupies a volume of 22.4L.
Given,
Volume = 4L
1 mole of a gas occupies 22.4L of volume at STP.
Thus, 1L of a gas occupies = 1 / 22.4 moles
so, 4 L will occupy = 4 / 22.4 moles
= 0.178 moles
Moles = mass / atomic mass
Mass = moles × atomic mass of He
= 0.178 × 4
= 0.714g
Therefore, 0.714g of Helium is contained in the balloon.
Learn more about STP, here:
https://brainly.com/question/29356493
#SPJ3
since this procedure will not work, how would you remove the water of crystallization from a sample of cobalt (ii) chloride hexahydrate?
Answers
To remove the water of crystallization from a sample of cobalt (II) chloride hexahydrate (CoCl₂·6H₂O), the sample needs to be heated gently to drive off the water molecules.
Cobalt (II) chloride hexahydrate contains six water molecules (H₂O) as part of its crystal structure. These water molecules are known as water of crystallization. To remove them, the sample can be heated gently. When heated, the water molecules will undergo evaporation, transitioning from the solid state to the gaseous state. This process is known as dehydration.
Gentle heating is important to avoid decomposition or other unwanted reactions. By applying controlled heat, the water molecules will be removed, leaving behind an anhydrous cobalt (II) chloride solid (CoCl₂) without water molecules.
It is worth noting that the heating process should be carried out carefully, preferably using a drying oven or a desiccator, to ensure controlled and uniform heating throughout the sample. This will facilitate the complete removal of water without causing any unwanted side reactions or damage to the sample.
learn more about dehydration here:
https://brainly.com/question/12261974
#SPJ11
How would you measure the mass and weight of an object?
Answers
Answer:
Weight = mass × earth's gravitational acceleration
Explanation:
Follow the following steps to measure the mass and weight of an object:
Measure the mass of the object using an accurate measuring scale.Multiply the mass of that object with earth's gravitational acceleration which is approximately 9.8 N/kgHow can heat be transferred through a solid
Answers
Answer:
conduction i do believe
When a solution of cesium chloride (CsCl) is subjected to high-speed centrifugation, a stable density gradient is formed.
a. True
b. False
Answers
what is the third quantum number of a 3 s 2 electron in phosphorus, 1 s 2 2 s 2 2 p 6 3 s 2 3 p 3 ?
Answers
The third quantum number (m_l) of a 3s² electron in phosphorus is 0.
The third quantum number, denoted as m_l, represents the magnetic quantum number and describes the orientation of an orbital within a subshell. It can have integer values ranging from -l to +l, where l is the azimuthal quantum number.
In the electron configuration of phosphorus, we see that the 3s subshell is being filled. The azimuthal quantum number (l) for the 3s subshell is 0. Since the electron is in the 3s² subshell, there are two electrons present in the 3s orbital.
For the two electrons in the 3s orbital, they will have opposite spins due to the Pauli exclusion principle. However, the magnetic quantum number (m_l) for both electrons in the 3s orbital will be the same, which is 0.
Therefore, the third quantum number (m_l) of a 3s² electron in phosphorus is 0. This means that both electrons in the 3s orbital have the same orientation within the subshell.
To learn more about quantum number, here
https://brainly.com/question/31955577
#SPJ4
What pillar of sustainability is broken by recycling
electronics in India? Should the US make a law that electronics can
only be recycled in the US?
Answers
The pillar of sustainability broken by recycling electronics in India is environmental sustainability. Implementing a law that restricts electronics recycling to the US would not necessarily be the most effective solution, as it overlooks the complex global dynamics of electronic waste management.
Recycling electronics in India often involves improper disposal methods, such as burning or dismantling without proper safety measures. This leads to environmental pollution, including the release of hazardous substances into the air, soil, and water, thus violating the principle of environmental sustainability.
However, simply mandating that electronics can only be recycled in the US may not be the most optimal solution. Electronic waste is a global issue, and restricting recycling to a single country disregards the fact that electronic products are manufactured and consumed worldwide. A more comprehensive approach to addressing electronic waste would involve international cooperation, strict regulations, and monitoring of recycling practices to ensure they meet environmental standards.
Efforts should focus on improving recycling practices globally, including promoting responsible electronic waste management, developing sustainable recycling infrastructure in multiple countries, and encouraging the adoption of safe and environmentally friendly recycling practices. This approach would foster global sustainability and address the challenges associated with electronic waste disposal more effectively than a geographically limited restriction.
To learn more about sustainability, here
https://brainly.com/question/32771548
#SPJ4
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
How many Joules of energy are there in one photon of orange light whose wavelength is 630nm?
Answers
The energy are there in one photon of orange light whose wavelength is 630nm is \(3.15401\times10^{-19}\) Joules.
W=c/v, c=speed of light, v=frequency
\(6.3 \times 10^-7=3 \times 10^8/v\)
\(v=3 \times 10^8 / 6.3 \times 10^-7\)
\(v=4.76 X 10^14 Hz\)-frequency of the yellow light.
E=hv, h=Planck's constant,
\(E=(4.76 \times 10^14)Hz \times 6.62607×10^-34 J s\)
\(E=3.15401\times10^{-19}\) Joules- the energy of a single photon of yellow light.
What is Planck's constant?Planck's constant or Planck's constant, is a fundamental physical constant of quantum mechanics. The constant gives the ratio of the energy of the photon to its frequency, and for mass-energy equivalent, the ratio of mass to frequency.
In quantum mechanics, energy is exchanged and absorbed in certain amounts called quanta. The Planck constant is a number that defines the amount of energy in these quantities and expresses how small things can be. Learn more about Planck's constant in this infographic.
To learn more about Planck's constant, refer;
https://brainly.com/question/10700482
#SPJ10
place the ions in order from highest to lowest average concentration in seawater.
Answers
Answer:
4w6hj5w6yhu5hytr
Explanation:
sy5rsrs6hyu4sr6y6r
Nora stirs one teaspoon, about 4.2 g, of sugar into her mug, which holds about 0.25 L of tea. What is the concentration of sugar in Nora’s tea?